Systemic lupus erythematosus in a male teenager manifested with cardiogenic shock and extremities infarction
Li-xue Wu ,De-chao Xu ,Ke Sun ,Hao Huang ,Wei-wei Jiang ,Wen-fang Li
1 Department of Emergency and Critical Care Medicine, Changzheng Hospital, Naval Medical University, Shanghai 200003, China
2 Department of Nephrology, Changzheng Hospital, Naval Medical University, Shanghai 200003, China
Systemic lupus erythematosus (SLE) is an autoimmune disease with multisystemic features and a variety of clinical characteristics.Typical clinical manifestations of SLE include alopecia,oral ulcers,cutaneous lesions,arthritis,renal injury,and cardiac damage.Cardiac involvement is one of the common complications of SLE,which is mostly asymptomatic with only approximately 10% of cardiac injury patients experiencing symptoms such as shortness of breath and arrhythmia.However,cardiogenic shock is rare,especially as the initial manifestation of SLE.Herein,we report a case of a 15-year-old male who presented with cardiogenic shock and was eventually diagnosed with SLE.
CASE
A 15-year-old male presented to the emergency department with fever,dyspnea,and acute progressive skin lesions for 10 d.His past medical history was unremarkable except for the presence of maculopapular rashes on the face for one year.On admission,vital signs were body temperature 37.5 ℃,pulse rate 129 beats/min,respiratory rate 23 breaths/min,and blood pressure 75/45 mmHg (1 mmHg=0.133 kPa).Physical examination revealed facial malar erythema accompanied by cutaneous infarction as well as auricle,nailfold and subungual infarctions at the fingertips and toes (Figure 1).In addition,a crescendo-decrescendo 3/6 systolic ejection murmur was auscultated at the apex of the heart and the lower sternum.Laboratory examinations(supplementary Table 1) showed a slight elevation in the white blood cell count (9.8×10/L) and a decline in hemoglobin (102 g/L).The urine dipstick test indicated the absence of proteinuria and hematuria.The C-reactive protein level (14.6 mg/L) and erythrocyte sedimentation rate(17 mm/h) were slightly elevated,and the procalcitonin level(0.133 ng/mL) was normal.Liver function tests revealed significantly elevated total bilirubin (31.2 μmol/L),aspartate transaminase (501 U/L),and alanine transaminase (731 U/L),while hepatitis B and hepatitis C serology tests were both negative.Renal function was normal,with a serum creatinine of 61 μmol/L.D-dimer (5,030 μg/L) and fibrinogen degradation products (22 mg/L) were significantly elevated.Troponin (0.15 ng/mL) and creatine kinase-myocardial band isoenzyme (CK-MB) (25 U/L) were slightly elevated,while brain natriuretic peptide (21,600 pg/mL) was substantially elevated.Electrocardiogram revealed sinus tachycardia,and emergency echocardiography demonstrated a dilated heart chamber,pericardial effusion,severe mitral and tricuspid regurgitation,and a left ventricle ejection fraction of 21%(Figures 2 A and B).Computed tomography of the chest revealed massive pleural effusion (Figures 2 C and D).Thus,a pulse indicator continuous cardiac output (PICCO)monitoring was placed immediately,which showed a cardiac output index of 2.12 L/(min·m) and a systemic vascular resistance index of 2,307 dyn·s·cm·m.All these clinical features indicated a diagnosis of cardiogenic shock in this patient.Dopamine (5 μg/[kg·min]) and dobutamine (5 μg/[kg·min]) were introduced to maintain blood pressure.Furosemide (40 mg/d) and spironolactone (20 mg/d) were administered to improve volume overload on the basis of careful blood pressure monitoring.When the patient’s condition was slightly stable,digoxin (0.125 mg/d) and β-blocker (11.875 mg/d) were administered to improve cardiac function.In addition,low-molecular-weight heparin (2,000 U,twice a day) was given to optimize the patient’s coagulative and fibrinolytic system.Magnesium isoglycyrrhizinate (0.1 g/d) and ornithine aspartate (10 g/d) were also administered for liver function improvement.
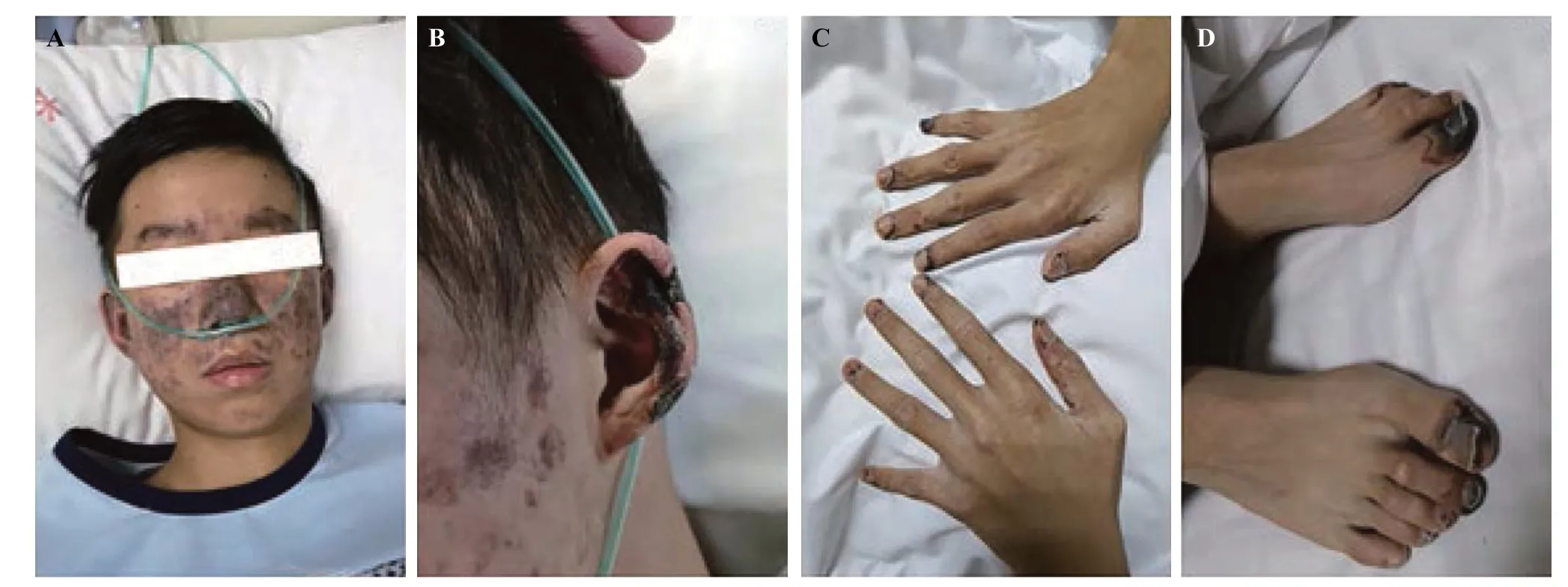
Figure 1. Images of skin lesions.A: lesions on the face;B: lesions in the auricle;C: lesions in the fingertips;D: lesions in the toes.
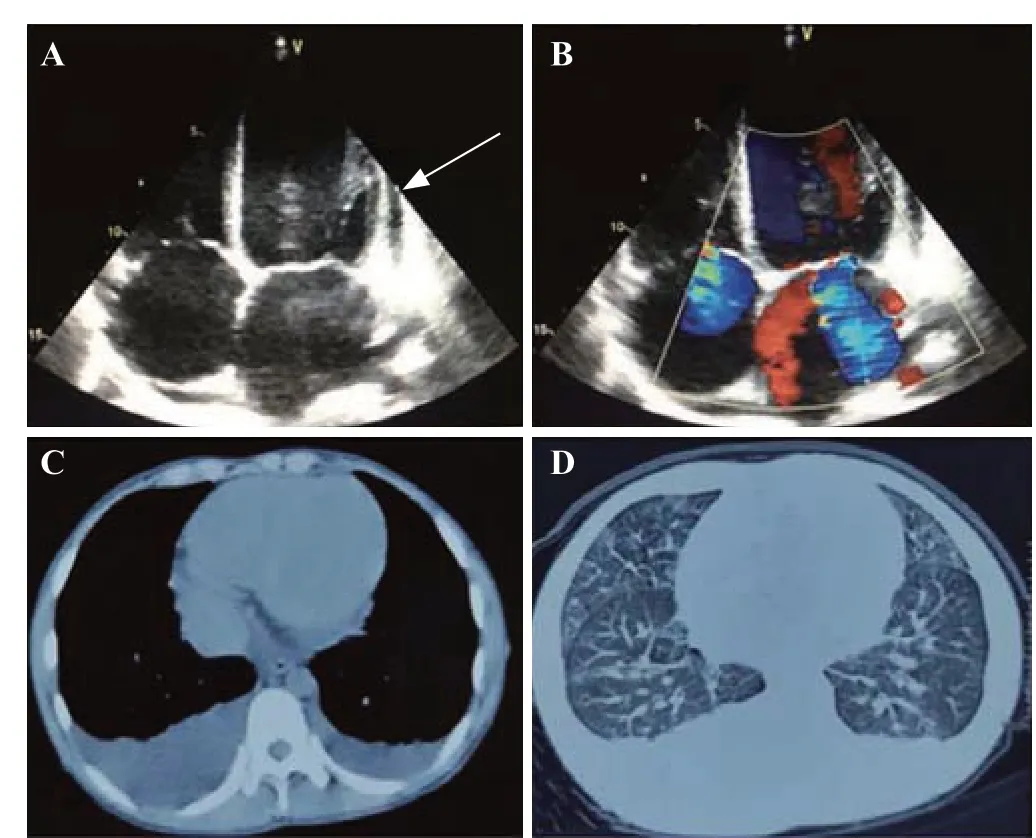
Figure 2. Images of echocardiography and computed tomography.A: echocardiography revealed a dilated chamber and pericardial effusion (white arrow);B: echocardiography showed severe mitral and tricuspid regurgitation;C and D: computed tomography demonstrated a massive pleural effusion.
Two days after admission,serological tests revealed positive antinuclear antibodies,double-stranded DNA antibodies,anticardiolipin antibodies,anti-ribosomal antibodies,and anti-ribonucleoprotein (RNP) antibodies.Meanwhile,serum levels of complement components C3(0.144 g/L) and C4 (0.037 g/L) were significantly decreased.Serological tests of multiple antiviral immunoglobulin M(IgM) antibodies were negative (supplementary Table 1).
The patient was diagnozed with SLE presenting as cardiogenic shock.He received intravenous immunoglobulin 20 g/d for 5 d and methylprednisolone 240 mg/d for 6 d,which was gradually tailored to 40 mg/d.Ten days later,the patient’s blood pressure gradually increased and was maintained at 115/65 mmHg without vasoactive agents.A follow-up echocardiography demonstrated remarkable morphologic and functional improvement of the heart with a left ventricle ejection fraction of 49%.The patient was referred to rheumatology for further treatment with prednisone 50 mg/d and hydroxychloroquine 0.2 g/d.After two years of followup,the patient was stable with normal cardiac function and the absence of SLE relapse in addition to some scar residues at the tip of the nose,auricle,fingers,and toes.
DISCUSSION
SLE is a complex autoimmune disease with multiple organ involvement.Cardiac manifestations include pericarditis,myocarditis,arrhythmia,ischemia,and cardiomyopathy.However,rapid progression of cardiac dysfunction leading to cardiogenic shock is rarely observed in SLE patients.We described the clinical course of a patient with cardiogenic shock due to SLE who achieved clinical remission with immunosuppressive therapy.
In this patient,myocarditis was initially suspected with clinical features such as fever and dyspnea,slight elevation of myocardial enzyme,abnormal cardiac morphology in echocardiography,and cardiogenic shock confirmed by PICCO monitoring.Myocarditis is associated with various etiologies,among which viral infection is one of the most common causes.In this patient,serologic IgM antibodies for a variety of viruses were negative,suggesting no direct evidence supporting the diagnosis of viral myocarditis.The prominent skin lesions and a previous medical history of facial rash indicated the possibility of autoimmune diseases.A comprehensive test for autoantibodies revealed positive antinuclear antibodies and double-stranded DNA antibodies.The extremely low level of complement components and signs of pleural and pericardial effusion,accompanied by the clinical features described above,made the diagnosis of SLE most likely in this patient according to the 2019 classification criteria of the European League against Rheumatism (EULAR) and American College of Rheumatology (ACR).
Symptomatic lupus myocarditis,characterized by left ventricular dysfunction and acute heart failure,is a lifethreatening complication.It occurs in nearly 10% of SLE patients,although a studyon autopsy suggests that the majority of SLE patients have subclinical myocarditis.The gold standard for the diagnosis of myocarditis is endomyocardial biopsy.Nevertheless,the low sensitivity and possible complications of biopsy have limited its application in clinical practice.Cardiac magnetic resonance imaging is the most sensitive noninvasive diagnostic technique for myocarditis.However,it was refused by the patient on admission due to his critical situation.A previous study reported that anti-RNP and anticardiolipin antibodies were associated with lupus myocarditis,which was positive in 62% and 33% of patients,respectively.In this case,positive anti-RNP and anticardiolipin antibodies were detected,which might support the diagnosis of SLEassociated myocarditis.Moreover,facial rash and toe necrosis presented in this patient were generally recognized as typical symptoms of systemic vasculitis.Antianutrophil cytoplasmic antibody (ANCA)-associated vasculitis was ruled out due to a negative ANCA test.Small vessel vasculitis is the most common type,which is characterized by cutaneous lesions that are also present in this patient.In addition,liver dysfunction in this patient also supported the diagnosis of SLE,since a previous study reported that liver dysfunction occurred in approximately 50% of patients with SLE,which may present as hepatomegaly,jaundice,and elevation of liver enzyme levels.In this patient,liver congestion caused by rapidly progressing heart failure might also partially contribute to liver dysfunction.
Current treatment of acute lupus myocarditis is largely empirical,and corticosteroids serve as an important option.In this patient,the significant improvement after large doses of intravenous immunoglobulin and methylprednisolone reflected the need for early diagnosis of lupus myocarditis.Additionally,supportive therapy to improve cardiac function is also indispensable.In critically ill cases,the utilization of cardiac resynchronization therapy (CRT),intra-aortic balloon pump (IABP),and extracorporeal membrane oxygenation (ECMO)may help patients during the acute phase and stabilize them for better treatment opportunities.For intractable diseases,other immunosuppressive treatments,such as cyclophosphamide,azathioprine,and mycophenolate,could be considered.Meanwhile,biological agents have shown promising clinical outcomes and may also be helpful for the treatment of lupus myocarditis,especially in cases where large doses of corticosteroids are ineffective.
CONCLUSION
This case report emphasizes that SLE could be a possible diagnosis in patients presenting with myocarditis and cutaneous lesions.Early diagnosis and adequate treatment with immunosuppressive therapy combined with cardiac supporting treatment may bring promising outcomes.
This study was supported by Shanghai Sailing Program(19YF1447500);the National Natural Science Foundation of China (82000627) to De-chao Xu;the Three-Year Project of Action for Shanghai Public Health System (GWV-10.1-XK24) to Wen-fang Li.
Not needed.
They have no conflicts of interest.
LXW and DCX contributed equally to this work.LXW and DCX wrote the draft of this paper and collected the data.WFL proposed the study.KS and HH collected the data.WWJ participated in the clinical management.All authors approved the final version.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World Journal of Emergency Medicine2022年5期
World Journal of Emergency Medicine2022年5期
- World Journal of Emergency Medicine的其它文章
- Intestinal microcirculation dysfunction in sepsis:pathophysiology,clinical monitoring,and therapeutic interventions
- Timing of brain computed tomography for predicting neurological prognosis in comatose cardiac arrest survivors: a retrospective observational study
- Development and evaluation of a predictive nomogram for survival in heat stroke patients: a retrospective cohort study
- Analysis of imaging characteristics of blunt traumatic aortic dissection: an 8-year experience
- Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial
- Arctigenin attenuates paraquat-induced human lung epithelial A549 cell injury by suppressing ROS/p38 mitogen-activated protein kinases-mediated apoptosis
