Hepatocellular carcinoma-derived exosomal miRNA-761 regulates the tumor microenvironment by targeting the SOCS2/JAK2/STAT3 pathway
Xiao-hu Zhou ,Hao Xu ,Chang Xu ,Ying-cai Yan ,Lin-shi Zhang ,Qiang Sun ,Wei-lin Wang,Yan-jun Shi
1 Department of Hepatobiliary and Pancreatic Surgery,the Second Affiliated Hospital of Zhejiang University School of Medicine,Hangzhou 310009,China
2 Key Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumor of Zhejiang Province,the Second Affiliated Hospital of Zhejiang University School of Medicine,Hangzhou 310009,China
BACKGROUND: Exosomes and exosomal microRNAs have been implicated in tumor occurrence and metastasis.Our previous study showed that microRNA-761 (miR-761) is overexpressed in hepatocellular carcinoma (HCC) tissues and that its inhibition affects mitochondrial function and inhibits HCC metastasis.The mechanism by which exosomal miR-761 modulates the tumor microenvironment has not been elucidated.METHODS: Exosomal miR-761 was detected in six cell lines.Cell counting kit-8 (CCK-8) and transwell migration assays were performed to determine the function of exosomal miR-761 in HCC cells.The luciferase reporter assay was used to analyze miR-761 target genes in normal fibroblasts(NFs).The inhibitors AZD1480 and C188-9 were employed to determine the role of the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in the transformation of cancer-associated fibroblasts (CAFs).RESULTS: In this study,we characterized the mechanism by which miR-761 reprogrammed the tumor microenvironment.We found that HCC-derived exosomal miR-761 was taken up by NFs.Moreover,HCC exosomes affected the tumor microenvironment by activating NFs via suppressor of cytokine signaling 2 (SOCS2) and the JAK2/STAT3 signaling pathway.CONCLUSIONS: These results demonstrated that exosomal miR-761 modulated the tumor microenvironment via SOCS2/JAK2/STAT3 pathway-dependent activation of CAFs.Our findings may inspire new strategies for HCC prevention and therapy.
KEYWORDS: Exosomes;Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway;microRNA-761;Suppressor of cytokine signaling 2;Tumor microenvironment
INTRODUCTION
Hepatocellular carcinoma (HCC),one of the most common malignant tumors,is characterized by poor prognosis;it has the fifth-highest incidence of all cancers and is the second leading cause of cancerrelated deaths worldwide.Although medical science and technology are rapidly advancing,effective therapeutic options are still limited.Some emergency complications of HCC also seriously affect its prognosis.The clinical symptoms of liver cancer are atypical,including indigestion,epigastria discomfort,and emaciation.However,as more than 80% of HCC develops in cirrhotic livers,some emergency and lifethreatening complications,such as spontaneous rupture of the tumor and upper gastrointestinal (UGI) bleeding,have been observed.Radiofrequency ablation (RFA),surgical resection,transarterial chemoembolization,and liver transplantation may help improve patient survival.However,the exact mechanism of HCC pathogenesis and metastasis remains unknown.
Exosomes are extracellular vesicles containing DNA fragments,mRNAs,microRNAs,and many kinds of proteins.They are 30-150 nm in diameter and may be considered a tool for intercellular communication.Recently,exosomes have been shown to play an important role in tumorigenesis and progression of carcinomaas well as in other diseases.Generally,exosomes produced by cancer cells regulate cancer cells,as well as nonneoplastic cells in the vicinity,through paracrine/autocrine signaling mechanisms.Cancer exosomes transfer malignant characteristics to recipient cells,including activation of invasion and metastasis,resistance to cell death,enhanced proliferation,and drug resistance.Exosomes have also been used in cellfree therapy and as drug delivery vehicles in COVID-19 management.Specific populations of urinary exosomes and proteins can reflect kidney pathology in patients with primary hyperoxaluria type I.The immune cell markers of exosomes even reflect renal calcium/phosphorus physiology.
Cancer cells grow in an environment that supports tumor growth;the tumor microenvironment (TME)contains normal fibroblasts (NFs),blood vessels,endothelial cells,immune cells,surrounding extracellular matrix (ECM),and other components.Cancer cells interact with the microenvironment to promote tumor growth and metastasis.Environmental reprogramming includes ECM remodeling,migration,angiogenesis,and the transformation of NFs into cancer-associated fibroblasts (CAFs).Emerging evidence shows that cancer-derived exosomes function as mediators of communication and contribute to TME reprogramming.
In this study,we determined the mechanism by which exosomal microRNA-761 (miR-761) modifies the tumor microenvironment.The findings may provide new insights into the mechanism of HCC metastasis and improve the clinical management of patients with HCC.
METHODS
Cell lines and transfection and reagents
The HCC cell lines LM3,SK-HEP-1,HepG2,LM9,and Huh7 and the healthy liver cell line THLE-2 were maintained at our institute.The cells were cultured as described previously.The human NF cell line WS1 was maintained at our institute.WS1 cells were cultured in Eagle’s minimum essential medium (Gibco,USA)supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich,USA).When the cells reached approximately 70% confluence,exosomes (20 μg/mL)were added to the medium.The morphology of the cells was examined and photographed under a microscope(AMEX1000,Thermo,China).
The miR-761 mimic and negative control (NC)mimic were obtained from GenePharma (China) and used at a concentration of 20 nmol/L.Mimic transfection was performed by using Lipofectamine 3000 (Invitrogen,USA) according to the manufacturer’s protocol.PlasmidmiR-761-sponge and plasmid-NC-sponge transfection were also performed by using Lipofectamine 3000.
AZD1480 (Cat.No.S2162) and C188-9 (Cat.No.S8605) were purchased from Selleck (USA).The concentration of AZD1480 was 5 μmol/L and that of C188-9 was 10 μmol/L.The incubation time for both drugs was 48 h.
Northern blotting
After total RNA extraction from cells and exosomes,approximately 20 μg of RNA was separated in 1.0%agarose gels and transferred to positively charged nylon membranes using a semi-dry transfer kit (BioRad,USA).After 15 min of prehybridization,the blot was incubated overnight withP end-labelled probes at 42 °C. The membranes were washed with a 6×SSPE solution,previously obtained from a 20×SSPE solution (3.6 mol NaCl,0.2 mol NaHPO,20 mmol/L EDTA,pH 7.4),for 30 min at 37 °C,followed by two additional washes at 4 °C.The membranes were placed in a phosphor screen cassette to obtain autoradiography images.
Luciferase reporter assay
A total of 100 ng luciferase reporter plasmid containing either wild-type or mutant 3’-UTR suppressor of cytokine signaling 2 (SOCS2) was co-transfected with NC or miR-761.Lipofectamine 3000 (Invitrogen,USA)was used for transfection according to the manufacturer’s protocol.Forty-eight hours after transfection,WS1 cells were collected and subjected to a dual-luciferase reporter assay (Promega,USA) according to the manufacturer’s instructions.
Statistical analysis
The SPSS version 22.0 (USA) was employed to analyze the data.The quantitative data were expressed as the mean±standard deviation.The experiments were performed in triplicate.The differences between groups were analyzed using Student’s-test;a-value <0.05 was considered statistically significant.
RESULTS
Identification of exosomes and exosomal miR-761
First,we isolated exosomes from five HCC cell lines and one healthy liver cell line and analyzed the expression level of exosomal miR-761 using quantitative real-time polymerase chain reaction (qRT-PCR).As shown in Figure 1A,although the expression level varied,miR-761 was detected in all six cell lines.To characterize the exosomes,the exosomal particulate fractions were examined by transmission electron microscopy.As shown in Figure 1B,the exosomes from Huh7 and LM3 cells appeared as small vesicles.Nanoparticle tracking analysis indicated that the size of exosomes ranged between 50 nm and 150 nm (Figure 1C).Next,northern blotting was performed to further confirm the presence of miR-761 in the exosomes.As shown in Figure 1D,miR-761 was detected in the exosomes of Huh7 cells.Western blotting using the markers CD81 and TSG101 was used to further confirm the presence of exosomes in Huh7 and LM3 cells (Figure 1E).We further confirmed that HCC-derived exosomal miR-761 was taken up by HCC cells,which enhanced the migration of recipient cells by targeting Mitofusin 2 (Mfn2) and promoted EMT.The data were shown in supplementary documents (supplementary Figures 1-4).
HCC exosomes transferred miR-761 into fibroblasts and increased cell migration
WS1 cells were incubated with HCC-secreted exosomes.As shown in Figure 2A,exosomes from LM3 cells labelled with PKH26 dye appeared in the cytoplasm of WS1 cells.Moreover,the exosomes increased the level of miR-761 in NFs (Figure 2B).Exosomal miR-761 secreted from both Huh7 and LM3 cells enhanced the proliferation and migration of WS1 cells (Figures 2 C and D).After coculture with HCC exosomes,NFs presented a spindle-like shape,which is recognized as the typical morphology of CAFs (Figure 2E).As shown in Figure 2F,exosomal miR-761 increased the expression of α-smooth muscle actin(α-SMA) and fibroblast activation protein (FAP),which are regarded as CAF markers.In addition,exosomal miR-761 increased the expression of matrix metalloproteinase-2(MMP-2),MMP-3,and MMP-9.These findings suggested that HCC-secreted exosomal miR-761 mediated TME remodeling by inducing the transformation of CAFs and increasing the expression of MMPs.Notably,the expression of Mfn2 was not detected in NFs.
Exosomal miR-761 inhibited SOCS2 expression in WS1 cells
Since Mfn2 was not detected in WS1 cells,we sought to identify the target of miR-761 in these cells.First,we employed the TargetScan program and found a suitable candidate mRNA,SOCS2.Then,we performed a luciferase assay to verify whether SOCS2 was a downstream target of miR-761 (Figure 3A).As shown in Figure 3B,when co-transfected with miR-761,wild-type SOCS2 3’-UTR exhibited a lower translation level than mutated 3’-UTR.When WS1 cells were cultured with LM3 cell-secreted exosomes,SO CS2 expression was downregulated (Figure 3C).Therefore,SOCS2 was the downstream target of miR-761,and HCC exosomes inhibited SOCS2 expression in WS1 cells.

Figure 1. Characterization of exosomes from hepatocellular carcinoma (HCC) cells.A: the expression of exosomal miR-761 in five HCC cell lines and one healthy liver cell line determined by qRT-PCR;B: representative electron micrograph of exosomes isolated from Huh7 and LM3 cells (scale bar: 100 nm);C: size of exosomes in Huh7 and LM3 cells;D: detection of exosomal miR-761 secreted by Huh7 cells in northern blotting,U6 as an internal control;E: exosomes isolated from Huh7 and LM3 cells,expression of CD81 and TSG101 examined by Western blotting using specific antibodies.qRT-PCR: quantitative reverse transcription polymerase chain reaction;*P<0.01;**P<0.001.The black arrow: exosome.
Exosomal miR-761 induced the transformation of CAFs via the SO CS2/JAK2/signal transducer and activator of transcription 3 (STAT3) signaling pathway
Exosomal miR-761 treatment decreased the protein expression of SOCS2 and enhanced the phosphorylation levels of JAK2 and STAT3 (Figure 3D).To investigate whether the transformation of CAFs and the upregulation of MMPs were caused by the activation of the JAK2/STAT3 pathway,we employed a JAK2 inhibitor,AZD1480,and a STAT3 inhibitor,C188-9.As shown in Figure 3E,both AZD1480 and C188-9 partially reversed CAF transformation.The cells treated with both inhibitors appeared rounded.AZD1480 and C188-9 also inhibited exosomal miR-761-induced increases in cell migration and proliferation (Figures 3 F and G).While these inhibitors did not affect SOCS2 expression,both AZD1480 and C188-9 inhibited JAK2 and STAT3 phosphorylation to various extents and inhibited the expression of MMPs,SMA,and FAP (Figure 3H).Collectively,these results indicated that exosomal miR-761 increased the migration of WS1 cells and the transformation of CAFs via the SOCS2/JAK2/STAT3 signaling pathway (supplementary Figure 5).
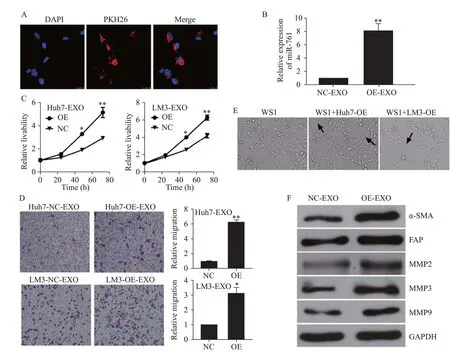
Figure 2. HCC exosomes transferred miR-761 into fibroblasts and enhanced their migration.A: exosomes from LM3 cells taken up by fibroblasts,exosomes labelled with PKH26 dye (red),and nuclei labelled with 4’,6-diamidino-2-phenylindole (blue);Scale bar: 25 μm;B: quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis of miR-761 expression in WS1 cells after co-culture with HCC exosomes for 48 h;C: WS1 cell proliferation determined by CCK-8 assay after treatment with exosomes secreted by Huh7 or LM3 cells;D: representative images of the migration assay after WS1 cells co-cultured with HCC exosomes;E: WS1 cell morphology after treatment with HCC exosomes;F: Western blotting analysis of MMP proteins in fibroblasts after co-culture with HCC exosomes.α-SMA: α-smooth muscle actin;FAP: fibroblast activation protein;MMP-2: matrix metalloproteinase-2;MMP-3: matrix metalloproteinase-3;MMP-9: matrix metalloproteinase-9;GAPDH: glyceraldehyde-3-phosphate dehydrogenase.*P<0.01;**P<0.001.NC: negative control;EXO: exosome.The black arrow: spindle-like NFs.
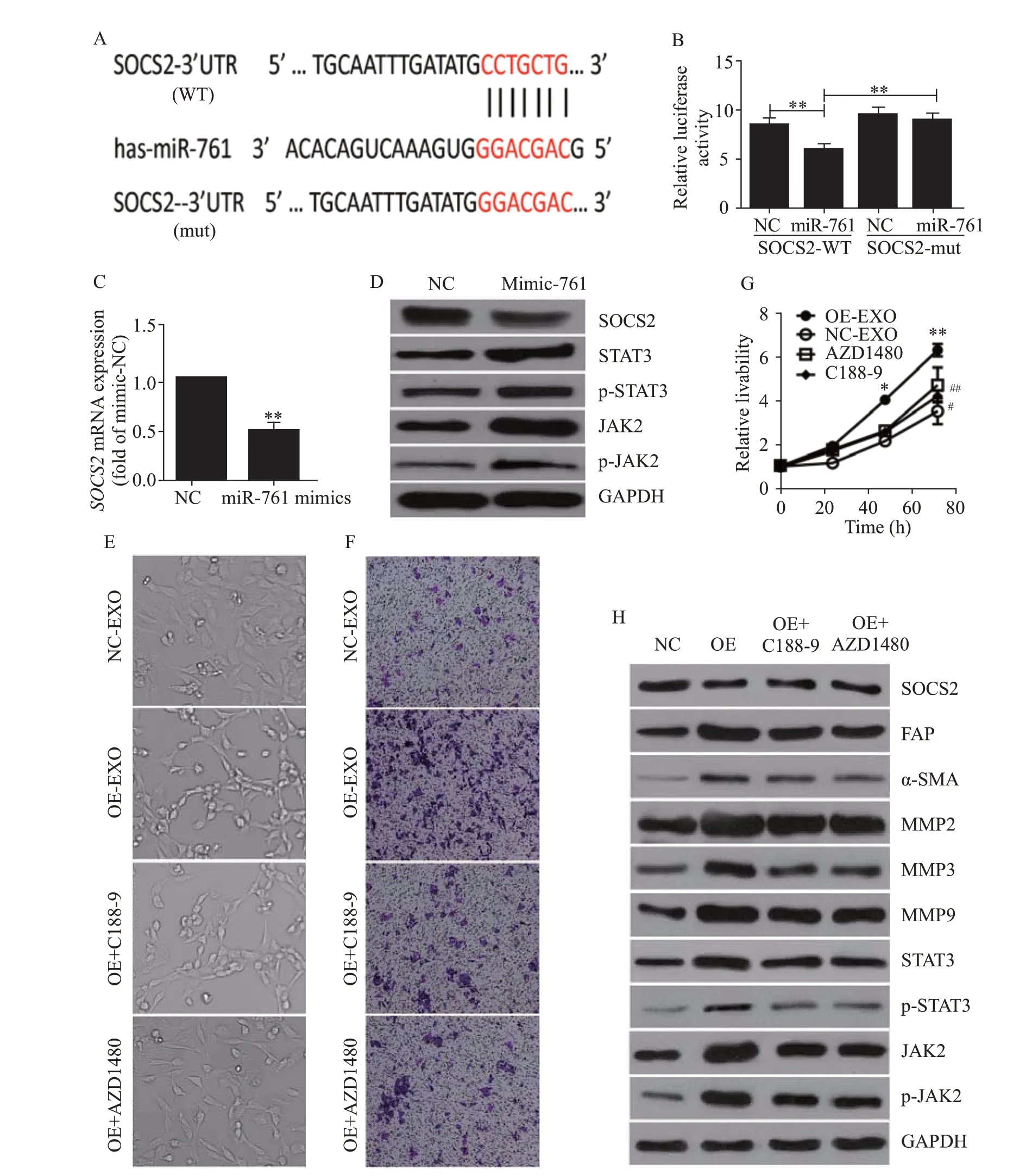
Figure 3. Exosomal miR-761 induced the transformation of cancer-associated fibroblasts via the SOCS2/JAK2/STAT3 signaling pathway.A: the predicted miR-761 binding site within the SOCS2 3’-UTR and its mutated version;B: luciferase assay performed in WS1 cells after cotransfection with miR-761 or NC mimics and reporter vectors carrying wild-type or mutant SOCS2 3’-UTR;C: SOCS2 expression after transfection with NC or miR-761 mimics detected by quantitative reverse transcriptase-polymerase chain reaction;D: Western blotting to determine SOC S2 expression and JAK2/STAT3 signaling pathway;E: morphology of WS1 cells;F: representative images of migration assay;G: WS1 cell proliferation detection using CCK-8 assay;H: Western blotting detection of SOCS2,JAK2,STAT3,and MMPs expression.WS1 cells were treated with LM3-secreted exosomes and JAK2 inhibitor (AZD1480),or STAT3 inhibitor (C188-9).SOCS2: suppressor of cytokine signaling 2;FAP: fibroblast activation protein;α-SMA: α-smooth muscle actin;MMP-2: matrix metalloproteinase-2;MMP-3: matrix metalloproteinase-3;MMP-9: matrix metalloproteinase-9;STAT3: signal transducer and activator of transcription 3;JAK2: Janus kinase 2;GAPDH: glyceraldehyde-3-phosphate dehydrogenase.Compared with negative control grouip,*P<0.01,**P<0.001;compared with negative control grouip,#P<0.05,##P<0.01.
DISCUSSION
Spontaneous tumor rupture is a life-threatening complication of HCC;its incidence ranges from 12% to 15%in Asia.Approximately 10% of patients with HCC die of rupture per year.Tumor rupture necessitates acute intervention,including transcatheter arterial embolization(TAE),hepatic resection,and RFA.Although various methods have been proposed to manage HCC rupture,the outcomes of HCC rupture are still poor.The incidence of tumor ruptures in HCC has decreased in recent years owing to the early detection and new treatments of HCC.Another life-threatening HCC complication is UGI bleeding.As most patients with HCC have liver cirrhosis and portal hypertension,UGI bleeding is the most common acute complication.The overall incidence of UGI bleeding in patients with HCC is between 15% and 30%;in some cases,UGI bleeding is the main cause of death.Early upper endoscopy is recommended in most patients with UGI bleeding.Exploring the mechanism will contribute to effectively preventing and treating HCC and decreasing the incidence of acute life-threatening complications.
Our former study has shown that miR-761 targets the 3’-UTR of thegene and inhibits its expression.mediates the fusion of the mitochondrial outer membrane.Our current results indicated that HCCderived exosomes increased the expression of miR-761 and inhibited the expression ofin HCC cells,increasing mitochondrial fission.HCC-derived exosomes enhanced the migration of recipient HCC cells,which could partially be due to increased mitochondrial fission.A similar result has shown that the downregulation ofimpairs mitochondrial fusion,contributes to mitochondrial network fragmentation,and promotes metastasis.Mitochondrial fragmentation may contribute to the cancer phenotype,for example,by increasing mitotic fission,decreasing intramitochondrial calcium waves,or promoting glycolysis.
The EMT process is considered a key step by which tumor cells gain the capacity to invade and metastasize.The main features of EMT are the loss of epithelial markers(such as E-cadherin) and the gain of mesenchymal markers(such as N-cadherin).In this study,we demonstrated that HCC-derived exosomes increased the expression of N-cadherin.However,the expression of E-cadherin was not significantly affected.The increase in N-cadherin plays an important role in cancer metastasisand may enhance cell migration even without a decrease in E-cadherin.EMT promotion may also contribute to the mechanism by which HCC-derived exosomes induce the metastasis of recipient HCC cells.
To adapt to their microenvironment and survive,cancer cells not only undergo intracellular changes but also alter the TME.Indeed,a modulatory effect of exosomes and exosomal microRNAs on the TME has been previously reported.Cancer cell-secreted exosomes that mediate the transformation of NFs to CAFs have a key role in tumor progression and metastasis.CAFs support tumor metastasis by promoting tumor angiogenesis,remodeling the ECM structure,secreting MMPs,and regulating the inflammatory microenvironment.In this study,we demonstrated that HCC-derived exosomes were internalized by NFs and enhanced their migration.HCC exosomes modulated the microenvironment by inducing the transformation of NFs to CAFs and by increasing the expression of MMPs.
Interestingly,we could not detectexpression in NFs by Western blotting or qRT-PCR.As mentioned before,miR-761 has different targets in distinct cancer cells.Here,we found thatmRNA was a target of miR-761 in NF cells.SOC S2 is a member of the SOCS protein family.SOCS family members are classic negative regulators of the JAK/STAT signaling pathway and include SOCS1-7 and cytokine-inducible SH2 domain-containing protein.Some studies have shown that the inactivation of SOCS proteins has a substantial impact on tumorigenesis and cancer development.A previous study has demonstrated that exosomal miRNA-155-5p,secreted by melanoma,induces the proangiogenic transformation of cancerrelated fibroblasts through the SOCS1/JAK2/STAT3 pathway.In this study,we found that exosomal miR-761,secreted by HCC cells,targetedin WS-1 cells,leading to the continuous activation of the JAK2/STAT3 pathway and therefore of CAFs.
CONCLUSIONS
This study reveals the existence of exosomal miR-761 in HCC cells.miR-761 can be transferred to NFs via exosomes.SOCS2 was identified as the downstream target of miR-761 in NFs,and exosomal miR-761 was found to activate CAFs and modulate the TME by targeting the SOCS2 and SOCS2/JAK2/STAT3 pathways.Our study identifies a new molecular mechanism by which HCC cells and NFs communicate to promote tumor metastasis.Our findings may inspire new strategies for HCC prevention and therapy.
This work was supported by the National Natural Science Foundation of China (82072203),and Natural Science Foundation of Zhejiang Province (LQ19H160025).
All specimens were obtained with informed consent.
The authors declare that they have no competing interests.
XHZ and HX contributed equally to the manuscript.XHZ,HX,WLW,and YJS designed the study.XHZ and CX performed the majority of the experiments and analyzed the data.YCY and LSZ performed confocal analyses.QS performed northern blotting and analyzed the data.All authors contributed to manuscript writing and approved the submitted version.
 World Journal of Emergency Medicine2022年5期
World Journal of Emergency Medicine2022年5期
- World Journal of Emergency Medicine的其它文章
- Intestinal microcirculation dysfunction in sepsis:pathophysiology,clinical monitoring,and therapeutic interventions
- Timing of brain computed tomography for predicting neurological prognosis in comatose cardiac arrest survivors: a retrospective observational study
- Development and evaluation of a predictive nomogram for survival in heat stroke patients: a retrospective cohort study
- Analysis of imaging characteristics of blunt traumatic aortic dissection: an 8-year experience
- Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial
- Arctigenin attenuates paraquat-induced human lung epithelial A549 cell injury by suppressing ROS/p38 mitogen-activated protein kinases-mediated apoptosis
