Recent Advances in Printed Thin-Film Batteries
Benoit Clement, Miaoqiang Lyu,*, Eeshan Saneep Kulkarni, Tongen Lin,Yuxiang Hu, Vera Lockett*, Chris Greig, Lianzhou Wang,*
a Nanomaterials Centre,School of Chemical Engineering and Australian Institute for Bioengineering and Nanotechnology,The University of Queensland,Brisbane,QLD 4072,Australia
b Printed Energy Pty. Ltd., Brisbane, QLD 4001, Australia
c Printed Energy Pty. Ltd., Tempe, AZ 85284, USA
d Dow Centre for Sustainable Engineering Innovation, School of Chemical Engineering, The University of Queensland, Brisbane, QLD 4072, Australia
Keywords:Printed battery Electronic device Flexible battery Roll-to-roll printing Monolithic integration
ABSTRACT The rapidly increasing demand for wearable electronic devices has motivated research in low-cost and flexible printed batteries with diverse form factors and architectures. In the past, technological achievements in the field have been emphasized,overlooking the industrial and market requirements.However,different applications require different battery chemistries and formats, that greatly impacts the manufacturing process and competition landscape.These chemistries and formats should therefore be selected carefully to maximize the chances for commercial success. As some of these technologies are starting to be marketed for portable electronics,there is a pressing need to evaluate different printing technologies and compare them in terms of the processing constraints and product requirements of specific electronic devices. By evaluating the intrinsic strengths and current limitations of printed battery technologies,development pathways can be prioritized, and potential bottlenecks can be overcome to accelerate the path to market.
1. Introduction
Printed batteries are emerging as ideal candidates to meet the challenges facing the next generation of small portable electronics,wearables, and Internet-of-Things (IoT) devices. In recent years,printed batteries have sparked great interest in the research community and have been reviewed several times[1–5].Conventional batteries with their rigid casings cannot satisfactorily meet the mechanical requirements of the new generation of flexible devices.Their form factor is limited, and they cannot be integrated monolithically into electronic devices.In contrast,printed batteries show potential for aesthetic versatility, flexibility, and monolithic integration. Multiple printing techniques have been developed over the centuries to pattern ink slurry onto various substrate materials,including organic (e.g., polymers) and inorganic (e.g., metals)materials. Using similar printing methods, electroactive and conductive materials can be layered onto a flexible substrate to produce patterned flexible batteries. Many of the applications that can accommodate printed batteries possess functionalities that are also printable,such as energy-harvesting,displays,and sensors[6–8].Batteries can be potentially printed alongside the other components of an electronic device to produce a so-called monolithically integrated device. The production throughput is amplified if all the components can be printed on the same assembly line by roll-to-roll (R2R) technology. Consequently, printed batteries are often associated with cost-effective manufacturing. Energy densities at sub-millimeter thicknesses that are superior to those of competing technologies such as lithium polymer batteries or ceramic batteries have also been reported [9].
These intrinsic technical advantages are generally accepted,but their demonstration in practice has yet to be reported rigorously.Although the field of printed batteries has already been reviewed,these reports have focused primarily on the development of inks with redox properties and the demonstration of their printability using different printing methods [1–5,10–15]. Comparatively less attention has been paid to tailoring the research toward the technical requirements of the targeted application. In this review, we define the key technical requirements before assessing the potential advantage of printed batteries over the competing technologies. We then review the technical achievements of printed batteries reported to date in terms of their main benefits:①unprecedented form-factor freedom, ②flexibility, ③increased energy density at sub-millimeter thicknesses, ④ cost-effective manufacture, and ⑤monolithic integrability. We conclude with an analysis of the field and offer suggestions for future development.
2. Targeted applications
Storing electrical energy is a challenge for an increasing number of applications that have a range of storage requirements.In the literature, printed batteries are always associated with thin-film applications that have energy requirements below 1 A∙h. These include micro-devices with a footprint of less than 1 cm2and typical power demand in the microwatt to milliwatt range(Table 1)[16–23]. These applications are characterized by the variety in their form factors,ubiquitous deployment,cost-sensitive manufacture,and in some instances,their flexibility,where printed batteries have a strong advantage over competing technologies.
These applications will contain several functionalities,including display, sensing, and wireless transmission. Some of them will have the option to be paired with an energy-harvesting module(i.e., photovoltaic (PV)) [24]. For these devices, the energy storage capacity will be determined by considering the required autonomy of the device and by balancing energy input and output.The energy input and output are intrinsically transient,depending on the harvestable ambient energy available and the duty cycle of the electronic device, respectively. Furthermore, the storage device will need to meet the peak energy demand of the electronic device,creating additional requirements for fast discharge.
Traditionally, capacitors are used for high-power applications.Unlike batteries that store electrical energy chemically in redox reactions, capacitors store electrical energy in an electric field between two conductive surfaces. This allows for faster charge release because the chemical process is comparatively slower and temperature-dependent. Additionally, most capacitor designs are simpler than battery designs and thus potentially more easily transferable to a printing process [25,26]. Although the energy density is lower than that of batteries (Fig. 1) [27], it can be sufficient for small energy demand.Complementary metal–oxide semiconductors (CMOSs), micro-electromechanical systems (MEMSs),and radio-frequency identification(RFID)tags with power requirements in the microwatt to low milliwatt range (Table 1) are good candidates for capacitive energy storage. A 30 μW micropower device would require 0.01 mA of current supplied at 3 V and a storage capacity of 1 mA∙h for 100 h autonomy. If the capacitor’s footprint is smaller than 1 cm2(Table 1), this equals an areal capacitance of more than 1.2 F∙cm-2,which is achievable although already cutting edge [28]. Another limitation of capacitors is their characteristic self-discharge [29,30]. MacKenzie and Ho [9] used the Conway–Ricketts exponential decay model [31] and commercially available information on leakage rates to calculate the halflife of a 1.1 mm thick single-cell supercapacitor charged to 2.3 V.It was found that the supercapacitor’s stored energy dropped by 50% after only 46 h [9].
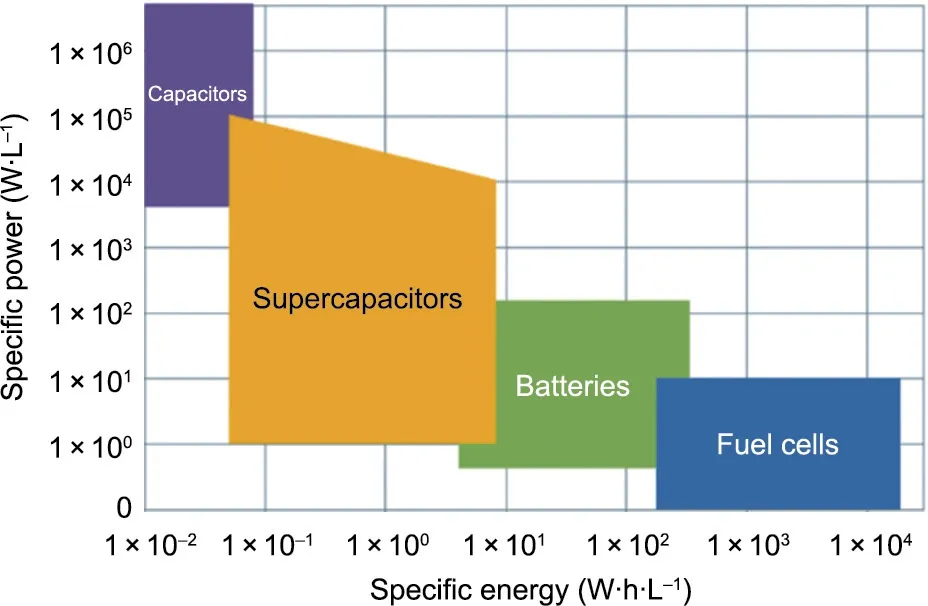
Fig. 1. Energy storage and delivery. Ragone plot of common energy storage technologies. Reproduced from Ref. [27] with permission.
A battery designed to supply energy to the same micropower device would require an areal capacity of more than 1 mA∙h∙cm-2.For a lithium-ion system using lithium iron phosphate(LFP)as the cathode material(specific capacity=160 mA∙h∙g-1)and graphite as the anode material(specific capacity=375 mA∙h∙g-1)concentrated at 90%in the active layer,the electrode loadings would have to be approximately 7 and 3 mg∙cm-2, respectively, which is already achieved routinely in industry with casting methods.In the context of printing batteries, the printing method will need to deposit the thick layer in as few passes as possible. This will reduce the number of printing/drying iterations, thus reducing time and energy consumption, especially if a low-volatility vehicle is used in the ink,which is typically the case to meet printable ink requirements.Because the amount of material deposited in each pass under given constant conditions increases with increasing ink viscosity, it can be seen that even for low-capacity applications, viscous inks are preferable. Printing methods that can handle viscous inks, which include screen (0.5–50.0 Pa∙s-1) and stencil printing (0.1–100.0 Pa∙s-1), should therefore be prioritized, even though their resolution is typically lower than that of dispenser techniques such as inkjet printing (0.001–0.040 Pa∙s-1) or spraying (< 0.150 Pa∙s-1)[13]. For high-resolution printing, which is critical for fabricating micropower devices with small footprints, this could create a conflict.
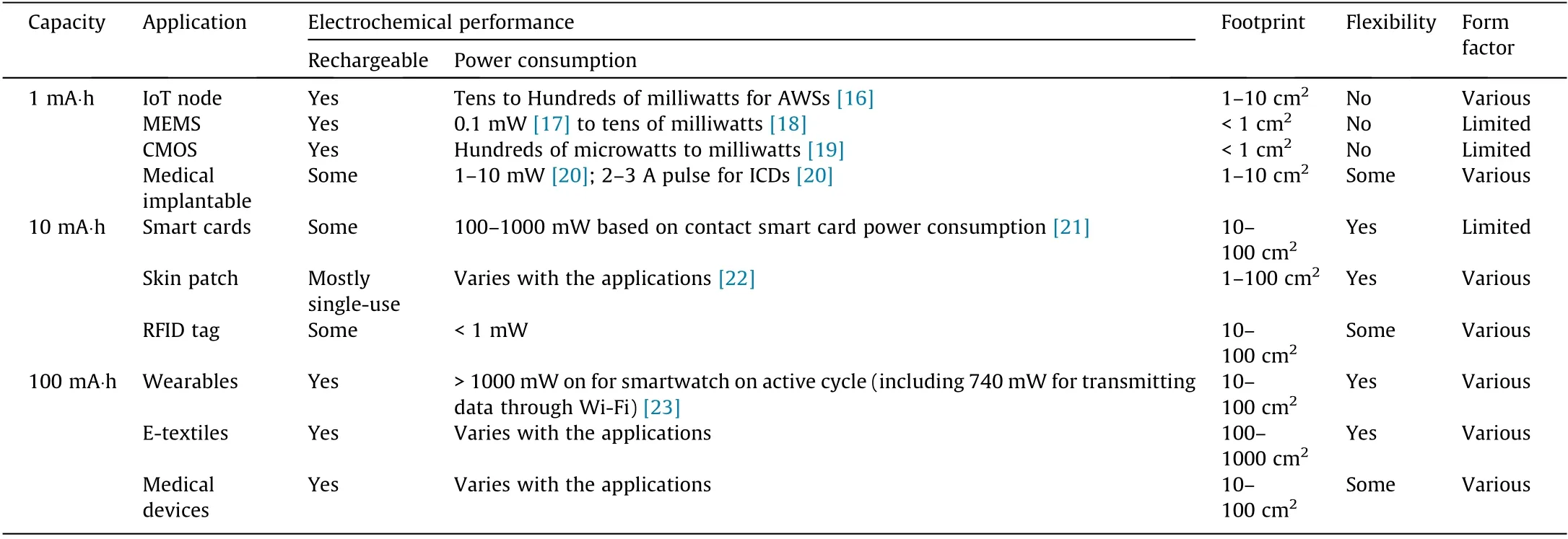
Table 1 Applications and requirements: potential applications of printed batteries and their physical and electrochemical requirements.
For large-area applications with energy requirements in the 10 mA∙h range, including smart cards, skin patches, and RFID tags(Table 1), the energy demands do not scale as fast as the device footprint,and therefore,the areal capacity is typically lower,somewhere between 0.1 and 1 mA∙h∙cm-2. The lower areal capacity is suited to low-viscosity printing techniques for thin-layer deposition, such as inkjet printing, but the larger printed area does not require high-resolution printing. Consequently, low-ink-viscosity printing methods capable of high-resolution printing, which are also typically slower than screen and stencil printing [13,14],may not have a real advantage.
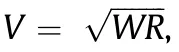
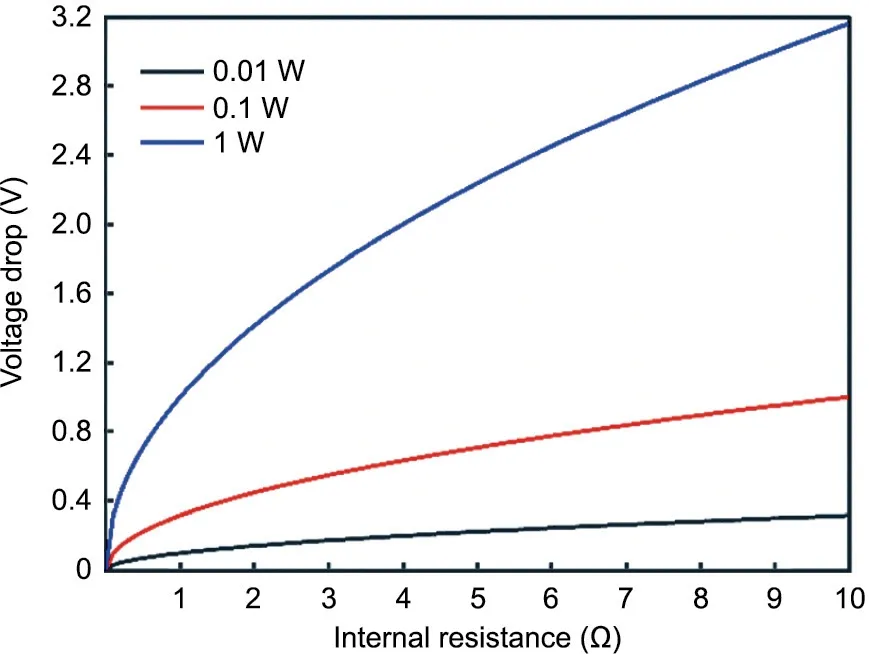
Fig. 2. Internal resistance drop. Voltage drop (V) as a function of the internal resistance (R) of the battery and the power draw (W) from the electronic device.
3. Thin-film battery technologies
There are four main thin-film battery technologies targeting micro-electronic applications and competing for their markets:①printed batteries, ②ceramic batteries, ③lithium polymer batteries, and ④nickel metal hydride (NiMH) button batteries.
3.1. Printed batteries
Zinc–manganese has been the chemistry of choice for commercial printed batteries because of its low cost,high safety,and easeof-processing.The first commercial printed battery was developed by the Israeli company ‘‘Power Paper” in 1997 [33]. Nonrechargeable zinc/manganese batteries were integrated into disposable skin patches for medical and cosmetic applications. Blue Spark Technologies (USA) and Enfucell (Finland), founded in 2003 and 2007, respectively, also commercialized integrated products powered by non-rechargeable zinc–manganese printed batteries[33]. More recently, Imprint Energy, a spin-off company from University of California, Berkeley, and Printed Energy (Australia)entered the market with products that were also based on the zinc–manganese chemistry. However, zinc–manganese chemistry is not easily rechargeable, and most research and development effort has been directed toward the development of alternative rechargeable chemistries for non-disposable products, including Ni/MH [34], Li-ion [35–38], radical polymer [39], and zinc–air[40].Although lithium chemistry is an obvious choice for rechargeable and energy-dense storage, the stringent moisture-free requirement associated with its manufacture is not easily adaptable to printing techniques, and the chemistry is often perceived as hazardous, which constitutes an obstacle for portable applications. It is also desirable that all the components of the battery be printable, which can be challenging for some components of some systems such as membrane separators or cathodes of zinc–air batteries. Some notable development efforts on rechargeable printed batteries were carried out by VARTA Microbattery using NiMH chemistry in a project called BatMat [33]. Lithium-ion chemistry was used in a project called green and safe thin-film batteries for flexible cost-efficient energy storage(GREENBAT), which was a collaboration between private and academic partners [33].

Table 2 Energy input and output. Harvestable power source and power requirement of desirable functionalities for small electronic devices (information collected from MacKenzie and Ho [9] unless mentioned otherwise).
3.1.1. Printable current collectors
Conventional batteries use metallic foil for the current collector that also fit the role of substrate.Aluminum is preferred because of its low price and high conductivity, but copper is also used at the graphite anode of lithium-ion batteries (LIBs) because of its enhanced electrochemical stability.A metallic foil current collector can be used as a substrate for printed batteries in a laminated pouch format designed to be plugged externally into the electronic device.However,it is not appropriate for monolithically integrated devices in which the substrate must be shared with the other components of the device. Moreover, metallic current collectors may not be adaptable to flexible applications [41]. Alternative current collector technologies may be necessary to meet the processing and mechanical requirements of printed batteries while maintaining high electrochemical performance. Aluminum thin films are routinely deposited onto flexible polymer substrates using vacuum deposition techniques [42]; aluminum has also been successfully processed into ink [43,44]. Three main approaches are used to deposit a conductive material onto a substrate: ①physical vapor deposition under vacuum, ②particle deposition by casting, and③printing of molten metal droplets [45].
Physical vapor deposition under vacuum is technically more challenging than printing, and it relies on specific environmental conditions that constrain the choice of substrate [45]. It should be noted, however, that this approach is routinely used in industry to produce food packaging, such as by depositing aluminum onto plastic films [42]. Molten metal droplets can be printed but they are restricted to substrates that can withstand high temperatures,which is not the case for common flexible polymer substrates such as polyethylene terephthalate (PET). Alternatively, DuPont’s Kapton®, which is made of heat-resistant polyimide, can be used at temperatures up to 400 °C [46], but its cost is higher than that of PET. In terms of cost, ease-of-processing, and integrability with downstream operations, the most favorable option for processing current collectors for printed batteries is by casting inks containing conductive particles. This process can be carried out at moderate temperatures and it does not disrupt downstream printing; it can therefore be integrated in-line to the R2R process.A range of materials can be processed into conductive inks, including conductive polymers [47,48], carbon [49,50], organic/metallic compounds[51],metal precursors[52,53],and metal nanoparticles(NPs)[54].The ink is produced by dispersing the particles in a solvent,referred to as the‘‘vehicle,”to produce a paste with the desired rheological properties. The highest conductivity is obtained with metal NPs whose resistivity is the same order of magnitude as the metal bulk[45] (e.g., silver: 0.03–0.1 Ω per square [33], 1 square = 9.290304 m2)and several order of magnitude lower than that of carbon materials(i.e.,carbon black:1000–10 000 Ω per square[33])or polymer materials (e.g., poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate)(PEDOT:PSS):100–10 000 Ω per square[33]).
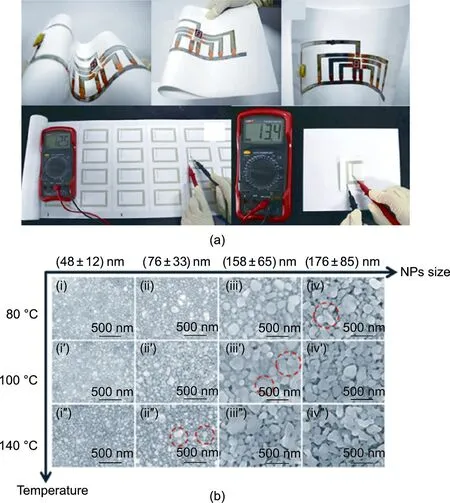
Fig.3. Printed current collector.Screen-printed silver NP ink.(a)Supplying current to light-emitting diode (LED) (top) and to an RFID antenna (bottom). (b) Scanning electron microscopy (SEM) images of samples with different NP sizes sintered at various temperatures. Reproduced from Ref. [56] with permission.
Although the range of metals that can be processed into printable metal NP inks is limited owing to the high reactivity of metallic nanosized particles [45], several metallic inks, including silver,copper,and aluminum,are already commercially available and fitted to various printing techniques,including screen,inkjet,flexography, offset, gravure, and more recently, spraying [45]. Metallic inks may require a high curing temperature post-printing to coalesce the particles and increase the conductivity, which may be incompatible with common polymer substrates such as PET [55].However, the sintering temperature can be reduced by lowering the particle size [45]. Research is being conducted to develop metallic inks with even lower sintering temperatures [56,57]. Mo et al.[56],for instance,developed a nano-silver ink that can be sintered in 10 min at 140°C(Fig.3),which is appropriate for PET substrates.In another study,the PET substrate was thermally stable at 150 °C for 30 min [58]. Carbonaceous materials also require a sintering step post-coating to improve conductivity, but it is conducted at lower temperatures. Wendler et al. [59] heated a carbon black conductive ink to 130 °C for 5 min post-printing to improve the percolation of the conductive particles. Higher resistivity impedes the performance of carbon inks; however, they are less prone to corrosion and therefore more suitable when a protic electrolyte is used,such as in alkaline batteries.It is also possible to protect a metallic current collector with a thin layer of carbon to simultaneously achieve high conductivity and stability in protic systems. To bypass the high-temperature post-printing step entirely, researchers have tried to develop flexible substrates that are already conductive onto which the active material can be cast[60–69] or grown in situ [70–74]. Such flexible substrates include carbon nanotube (CNT) mats, graphene foams, and the like. Such approaches are suitable for a laminated battery format,but cannot be easily implemented for monolithically integrated devices in which the substrate is shared with other components of the electronic device.
3.1.2. Printable electrodes
Casting methods have been used for decades to manufacture electrodes. The original LIBs produced by the Sony Corporation in 1991 were manufactured by repurposed casting equipment used previously for magnetic tapes[75].The battery industry continues to use doctor blading or slot-die coating to deposit viscous slurries onto current collector substrates [76]. Further, the casting process has been transferred successfully to several printing methods,including screen and stencil [35,37,38,77], dispenser [78], spray[36], flexography [79], and transfer [80,81]. The viscous paste is produced by incorporating various powders in a vehicle solvent.The paste includes ① an active material to catalyze the electrochemical reactions, ② conductive additives to facilitate access to and from the active material of the electrons and ions,and ③a binder to hold the structure together on the current collector. Other additives are often added to enhance the stability of the battery. The rheological properties are tuned by varying the binder concentration or sometimes by including a thickener such as cellulose [82] or carbopol [33]. The typical slurry-paste loading onto a current collector used in the industry is in the range of milligrams per square centimeter (mg∙cm-2) to keep the internal resistance below 1 Ω.As discussed earlier,printed batteries cannot rely on stacking or folding electrodes together to increase areal capacity without compromising the aesthetic versatility and monolithic integrability of the battery.To meet the energy requirements of some small electronic devices,loading must be increased to the 10 mg∙cm-2range. This poses two problems: ①How to achieve thick loading without compromising the stability of the layer and its flexibility and ②how to maintain the internal resistance at a reasonable level to meet the power requirements of electronic device applications. Unfortunately, power and energy densities standardized by either the surface area or volume of the entire battery are not commonly reported in the literature,especially the power density; most authors prefer to report the specific capacity instead. Information on the internal resistance and voltage drop under load is also limited.
3.1.3. Printable electrolyte/separator
Although water-based electrolytes used in alkaline NiMH batteries have been processed successfully into ink [83], a printable electrolyte/separator for rechargeable batteries remains the main challenge in the field.The following five approaches are interesting to mention.
(1)In the first approach,the precursors of an acrylic membrane separator are printed first and then cross-linked using chemical or photo-initiators in the presence of an entrapped liquid-phase electrolyte. Azobisisobutyronitrile (AIBN) [84–86], Darocur, and 2-hydroxy-2-methylpropiophenone (HMPP) [87] are commonly used as polymerization initiators.The porous acrylic structure provides the mechanical separation between the stacked electrodes,and the liquid phase provides the ionic conduction. Kim et al.[35] produced a so-called printable solid-state composite electrolyte (SCE) layer using ultraviolet (UV)-curable ethoxylated trimethylolpropane triacrylate (ETPTA) monomers and an HMPP photo-initiator to entrap a high-boiling-point ethylene carbonate(EC)/propylene carbonate (PC) organic solvent-based electrolyte upon UV curing. The ink showed thixotropic properties suitable for stencil printing. In another study by the same group, a fully printed LIB monolithically integrated to a PV module was manufactured using the SCE method [37]. A similar approach was adopted by researchers participating in the European Commission Community Research and Development Information Service (CORDIS)-founded GREENBAT project aiming at developing a printable electrolyte for LIBs. EC- and PC-based electrolytes were entrapped by the polymerization of acrylate monomers of different lengths(ethylene glycol dimethacrylate (EGDMA), 1,4-butanediol dimethacrylate(BDDMA),1,6-hexanedioldimethacrylate(HDDMA)) [84,85]. The non-volatile ionic liquid plasticizer 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide(EMIM-TFSI) [84,86,87] was also investigated as a replacement for the organic solvents. Limiting the evaporation loss by using non-volatile solvents is critical for improving the printing quality of casting-type printing methods (e.g., screen printing, stencil printing, slot-die coating). Another type of acrylate separator membrane, called polymerized high internal phase emulsion(polyHIPE) was also developed during the project [85–87]. Using this approach, surfactants were emulsified with the ink to act as additional porogens. It was found that the pore size of the separator membrane could be controlled by varying the concentration of lithium salt or the concentration of the surfactants(Fig.4)[86,87].
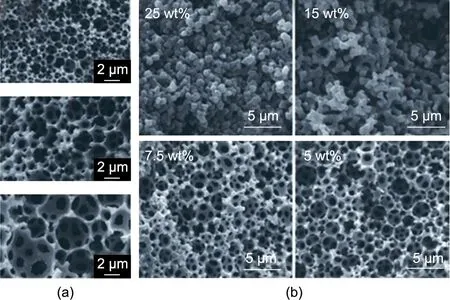
Fig.4. Printed separator. Acrylate-based polyHIPE membrane after polymerization triggered by UV treatment. (a) Varying the lithium salt content: 0, 0.06, and 0.1 mol∙L-1(top to bottom).(b)Varying the surfactant concentration:25 wt%,15 wt%, 7.5 wt%, and 5 wt%. Reproduced from Refs. [86,87] with permission.
(2)In the second approach,the liquid phase is mixed with a preformed polymer or polymer blend. Several polymers have been tried, including polyethylene oxide (PEO) [88–90], polyacrylonitrile (PAN) [88,91], poly(methyl methacrylate) (PMMA) [90,92],poly(vinyl alcohol) (PVA) [93], poly(acrylic acid) (PAA) [77,94,95],polyvinylidene fluoride (PVDF) [91], poly(styrenesulfonate) (PSS)[90,96], polydimethylsiloxane (PDMS) [97], and cellulose [98].More recently, poly(vinylidiene fluoride)–hexafluoropropylene(PVDF–HFP), a derivative of PVDF, was used [79,99,100] because of its high electrolyte-soaking ability and its low crystallinity,which favors ionic conductivity [101]. The mechanical rigidity of the polymer electrolyte necessary to segregate the electrodes is imparted by the rubbery nature of a plasticizer/salt/polymer mixture, which depends on the liquid content and temperature[102]. Physical or chemical crosslinking of the polymer(s) can alternatively be used to increase rigidity[103].Typically,the electrolyte and separator layer must be thick enough to prevent the electrodes from contacting each other,but not so thick that it minimizes internal resistance. Thick-layer printing methods such as screen printing and stencil printing are preferable [33].
(3) The third approach, which is much less represented in the literature,is the jellification of the liquid phase with fumed or colloidal silica to produce a printable silica-based ionogel paste. This approach is borrowed from valve-regulated lead–acid (VRLA) gel battery technology [104] and has been successfully applied to inkjet printing of LIBs [105]. In this instance, the silica particles provide the physical separation between the anode and cathode.
(4) Another approach was patented by Printed Energy, a company that commercializes printed batteries based on the zinc–manganese chemistry.In this approach,the mechanical separation between the electrodes is provided by the frustules of diatoms, a microscopic porous structure of biological origin [106]. Printed Energy has successfully processed this material into printable ink.
(5)The last approach circumvents the need for a separator altogether by adopting a co-planar battery geometry [33]. In this configuration,the ionic diffusion path from one electrode to the other is extended, and consequently, the battery experiences higher internal resistance than the stack configuration,which can be prohibitive for high-power applications.
3.1.4. Practical considerations
Some practical considerations must be examined when selecting the materials and formulating the inks that will be used to print the aforementioned printable layers. The rheology and wettability of the inks,the potential atmospheric contamination,and the grain size of the materials are discussed in this section.
(1)The rheology of the ink paste must be controlled to meet the requirements of the different printing methods. Typically, authors report the viscosity, shear stress, and viscoelastic properties (storage and loss moduli) of the thixotropic ink [35,100,107–109].Other parameters that are important to consider are the ability to wet the substrate [79,110], the vapor pressure of the vehicle[110,111], and the settling rate of the particles in the ink slurry[107,112].The wetting property of the current collector ink is particularly important because it needs to be tailored specifically to the material used to construct the substrate in a monolithically integrated device,which may be imposed on the battery manufacturer by the designer of the electronic device. Subsequent layers need to match the wetting properties of the underlying layer,which is entirely under the control of the battery manufacturer.The risk is then to dissolve the underlying layer when printing a new layer on top, and the inks must be tailored to prevent this[38]. If some layer miscibility is likely to be beneficial to reduce interfacial resistance, an excess amount may have the opposite effect and cause short-circuits. The ink wettability can be tuned by changing the surface tension of the vehicle with additives such as surfactants [110]. Another approach that does not alter the ink chemistry is to develop new substrates for a better wetting. Choi et al. [113], for instance, developed a cellulose nanomat that exhibited advantageous wetting properties and achieved high resolution during inkjet printing of carbon-based(activated carbon(AC)/single-wall carbon nanotube (SWCNT)) and metal-based(Ag nanowire (NW)) current collector inks.
(2)Another important consideration is the contamination of the electrolyte ink by atmospheric species during printing, especially moisture.Nearly all organic solvents and ionic liquids used as plasticizers in polymer electrolyte inks are hygroscopic [114,115]. In LIBs, the presence of water in the electrolyte beyond a few parts per million(ppm)is responsible for the severe reduction of cycling stability [112]. Moisture contamination is also problematic for ionic liquid gel electrolytes used in LIB systems [116]. However,previous reports on printed LIBs have overlooked the role of ambient moisture when processing organic solvents and ionic liquid electrolytes in air[35,38,84–87].It is recommended that the atmosphere be controlled to ensure reproducible performance across research groups.
(3)The particle size of the ink powders must also be considered when selecting a printing technique.Commercially available active material powders are generally coarse, with median particle sizes in the tens of micrometers range to maximize packing density.Such powders cannot be adapted to dispenser techniques with narrow nozzles (e.g., inkjet printing) without further processing that may increase the cost or impact the performance. For instance,Sun et al. [78]used lithium titanium oxide (LTO)and LFP particles with diameters in the nanometer range, which is many orders of magnitude finer than conventional particles, to meet the requirement of their three-dimensional (3D) dispenser printer. Conversely, screen and stencil printing can be adapted to any grain size by changing the mesh or slot opening, and therefore may be better adapted to printing electrodes [33].
3.1.5. Performance and failure modes
The electrochemical performance of thin-film printed batteries depends on the chemistry.The zinc–manganese chemistry is essentially applied in single-use applications,although some companies,including Imprint Energy and Printed Energy, are developing rechargeable zinc–manganese printed batteries. It is challenging to recharge batteries fabricated using zinc–manganese chemistry because of irreversible reactions and dissolution at the manganese cathode,dendrite formation at the zinc anode that has the potential to pierce through the printed separator and short-circuit the battery, and decomposition of the aqueous electrolyte into gaseous hydrogen and oxygen [104]. Printed NiMH batteries are also vulnerable to oxygen and hydrogen evolution, although their nominal voltage is lower than that of zinc–manganese batteries(1.2 V instead of 1.5 V), as indicated by the presence of a pressure release valve in VARTA’s battery designs[117].
The LIB chemistry has already demonstrated great cycling stability when associated with organic solvent-based electrolytes that are stable even at the high nominal voltage of the battery [104].However,as mentioned previously,these electrolytes must be handled in a dry atmosphere, which is not suitable for printing processes.
Another cause of battery failure is mechanical in the case of flexible applications. Mechanical failure during bending can be avoided if a flexible substrate is used and the current collector is printed in place of metallic foils, which are more prone to failure[9]. The adhesion of the ink to the substrate can be further improved by using innovative substrate designs[60–69],although this approach is not always practical, as mentioned previously.
3.2. Ceramic batteries
The Oak Ridge National Laboratory(ORNL)revolutionized ceramic monolithic batteries in the early 2000s by developing a lithium phosphorus oxynitride (LiPON) solid electrolyte manufactured by sputtering under vacuum [118]. Although the ionic conductivity of amorphous LiPON is low, typically 10-5–10-7S∙cm-1[118–120], it is possible to deposit a thin layer a few micrometers thick by sputtering to keep the internal resistance below 1 Ω [121].Other approaches have also been attempted to manufacture ceramic batteries at the laboratory scale, including different physical vapor deposition techniques (thermal evaporation, pulsed laser deposition) and chemical processes (chemical vapor deposition(CVD),sol–gel deposition,electroless deposition,and electrochemical deposition) [122]. The sputtering approach, which is also the most mature one, has a few intrinsic limitations. First, the deposition of the material onto large areas under high vacuum is much slower than printing, even though R2R vacuum deposition processes have been developed recently [123,124]. In addition,temperature-sensitive substrates such as PET require a low sputtering power, leading to slower deposition rates. For instance,Carcia et al.[125]had to lower the radio-frequency(RF)magnetron sputtering power to 100 W to deposit SnO2and In2O3onto a PET substrate at a rate of 20 nm∙min-1, which would be impractical for industrial manufacturing. Secondly, the process is quite different from present practice and thus requires larger initial capital investment, especially if customized equipment is necessary.Thirdly, the process is theoretically more energy-intensive than casting approaches,requiring high energy expenditure on creating and maintaining the vacuum,evaporating the material,and sintering the layers after deposition,thus increasing the associated operating costs. Finally, it should also be noted that even though aluminum deposition onto polymer substrates under vacuum has been adapted successfully to the industrial scale for food packaging applications, the deposition of thick inorganic films used in ceramic batteries is technically more challenging owing to the instability of the layer and its tendency to crack, particularly at high loading [9,126,127]. It should also be noted that thick inorganic layers are intrinsically stiffer than polymer-based ones and thus not necessarily suitable for flexible applications. To increase areal capacity and flexibility, the strategy involves connecting several batteries in packs and modules.ProLogium,a Chinese ceramic battery manufacturer, reported that their ‘‘BiPolar + 3D Structure Solid-State EV Battery Pack,” which is constructed with lithium ceramic cells in series and parallel both vertically and horizontally,achieved a record high energy density of 537 W∙h∙L-1, nearly two time higher than that of the Tesla Module 3 battery pack(272 W∙h∙L-1) [128]. Another ProLogium product, the flexible lithium ceramic battery (FLCB), can be rolled into a cylinder with an energy density greater than 250 W∙h∙L-1[129]. Apple acquired the previous leader in the field of ceramic batteries,Infinite Power Solutions, in 2013 [130], demonstrating the interest of industry in ceramic battery technology.
3.3. Lithium polymer batteries
The process to manufacture lithium polymer batteries is similar to that of conventional hard-case batteries, although the format is a thin-film pouch cell. Unlike monolithic ceramic batteries, the process is similar to current practice and can in principle be transferred to existing manufacturing facilities, reducing the initial capital investment required. The process involves casting a slurry paste onto a metallic current collector with slot-die or bladecoating equipment [76]. The tape is dried and compressed by calendaring to control the electrode thickness and porosity.The electrode paste loading onto the current collector is typically in the milligrams per square centimeter range. The assembly involves winding or stacking three components together: anode, cathode,and separator [131]. Typically, this configuration allows a wide range of capacities to be achieved, from a few milliampere hours to several ampere hours [132–135]. In principle the electrolyte is in a semi-solid state (gel) to improve battery safety, although the term ‘‘lithium polymer battery” is sometimes extended to mean a thin-film pouch LIB with a membrane separator soaked in a liquid electrolyte. The battery is not always designed to be flexible,but the thin aspect ratio can provide flexibility if the casing is soft.The shape of the lithium polymer batteries can be customized to a certain extent, but the form factor is not as customizable as for printed batteries [134,135]. Unlike printed and ceramic batteries,lithium polymer batteries cannot be monolithically integrated into an electronic device but instead must be wired externally (laminated format).
3.4. NiMH button batteries
NiMH button batteries such as the models commercialized by VARTA Microbattery do not exhibit several of the key abilities of the technologies discussed above.The thickness is not on the order of sub-millimeters, even for a ‘‘slim”design [136]. The form factor is limited to a button shape, and the encapsulation is rigid. A feature for venting is added to the design of VARTA’s models to release potential hydrogen gas build-up during operation [136]. This feature prevents their usage in airtight battery compartments and creates design restrictions for electronic device applications.Nonetheless, NiMH button batteries have other advantages that make them a credible alternative for energy storage for small electronic devices.They are energy-dense and intrinsically greener and safer than lithium-ion chemistry as long as a gas venting is supplied. Moreover, these batteries at present have a lower cost per kilowatt hour than the competing technologies [33]. It is worth noting that VARTA Microbattery supported the BatMat project that started in 2007 to develop a printed laminated NiMH battery[33].The project was based on a feasibility study published by Boris Vindus in 2006 [34], and several prototypes and patents were produced. VARTA Microbattery was also a collaborator in the GREENBAT project aimed at developing a printed LIB [33].
3.5. Competitive outlook of printed batteries
There are several typical advantages of printed batteries: flexibility, aesthetic versatility, cost-effective manufacture, monolithic integrability,and high energy density at a sub-millimeter thickness[9]. Table 3 [9,129,132,136–138] and Fig. 5 compare the different technologies available on the thin-film battery markets in terms of these theoretical advantages.
In terms of flexibility, batteries printed onto flexible substrates are less prone to failure under mechanical stress than the flexible lithium polymer batteries, as demonstrated by MacKenzie and Ho [9]. NiMH batteries are manufactured with a hard casing and therefore are intrinsically rigid.Ceramic batteries are also intrinsically stiff, although ProLogium has manufactured flexible ceramic battery packs. It is also interesting to note the emergence of other flexible technologies that are not two-dimensional(2D)thin films,including one-dimensional (1D) cable designs and other selfstanding electrodes, some with energy densities beyond 200 W∙h∙L-1[138,139]. These flexible technologies targeting the wearables market are generally manufactured via vacuumassisted filtration, CVD, sputtering, and other even more complex nano-fabrication processes[140].By comparison,the printing process can in theory achieve higher throughput and lower unit cost.It should be noted,however,that LG Chem is developing a process for the mass production of flexible cable batteries[141]. Readers may refer to the excellent review by Hu and Sun [140] for more information on flexible battery technologies.
In terms of energy density at sub-millimeter thicknesses,Imprint Energy’s printed batteries incorporating the ZincPolyTMprinted electrolyte technology show higher energy densities than other competitive products, according to specifications published in 2015 [9]. However, based on the specifications disclosed on the company’s website[142],the ZincPolyTM8349 3.0 V model only reaches 40 W∙h∙L-1,much lower than ProLogium’s ceramic batteries and LiPol’s lithium polymer batteries, with energy densities over 250 W∙h∙L-1[129] and 100 W∙h∙L-1[132], respectively. Furthermore, Imprint Energy’s ZincPolyTM8349 3.0 V model has an internal resistance one to two orders of magnitude higher than those of typical ceramic and lithium polymer batteries, which is to be expected with a thick-layer electrode of low conductivity manganese dioxide combined with a thick printed separator layer,which is necessary to segregate the electrodes. Internal resistance of such magnitude would induce a voltage drop of more than 1 V for a 100 mW power draw, which would be prohibitive for functionalities such as Wi-Fi transmission (Table 2).
For low-cost manufacture, printed batteries have a clear theoretical advantage over the competition.Technologies based on conventional technologies already show low cost per kilowatt hour because they benefit from a highly optimized and standardized process. However, this standardization might not be adaptable to the future demand for customized battery models to fit diverse form factors of small electronic devices [9]. In such a scenario,the ‘‘one-size-fits-all” approach will need to be replaced by an‘‘on-demand” approach, which favors a printed process that is modular. The target unit price for printed batteries needs to be a few cents to be competitive in the thin-film battery markets.According to Huebner and Krebs[33],the price of printed batteries in 2015 was between 2 and 5 USD depending on the chemistry,and this pricing has yet to be improved.
Printed batteries benefit from an unprecedented form-factor freedom that is superior to all the technologies competing in the thin-film battery markets. Printed batteries also have a unique advantage in terms of monolithic integration into electronic devices that cannot be achieved by lithium polymer or NiMH batteries. If ceramic batteries can be integrated monolithically into electronic devices, the integration can be complicated if battery packs are necessary to reach adequate energy densities, voltages,and flexibility, which constrains the device’s geometry. The high sputtering power necessary for an adequate deposition rate also constrains the material selection.
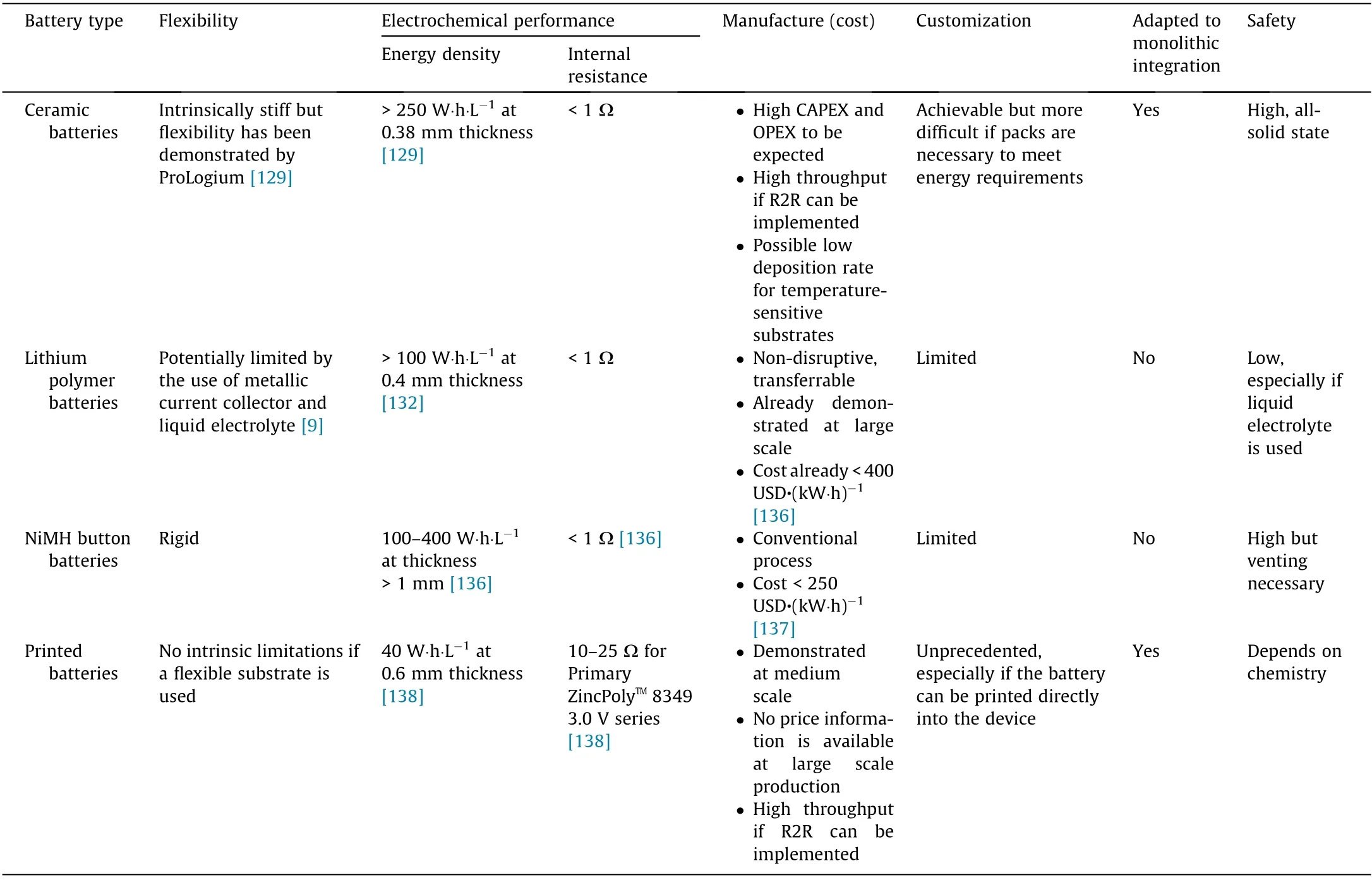
Table 3 Competing technologies. Comparison of the main thin-film battery technologies by technical and manufacturing capabilities.
Finally, in terms of safety, the potential advantage of printed batteries depends on the chemistry of the battery and the physical state of the components.Ceramic batteries are all-solid-state,making them intrinsically safer with a wide temperature stability window [129]. The polymer electrolyte of printed batteries and some lithium polymer batteries is jellified, reducing the risk of leakage.NiMH button batteries feature a liquid electrolyte and is therefore intrinsically more prone to leakage, although the rigid casing provides protection against punctures. Overpressure owing to hydrogen gas produced by the decomposition of the aqueous electrolyte can be prevented by incorporating a safety pressure release valve in the design.
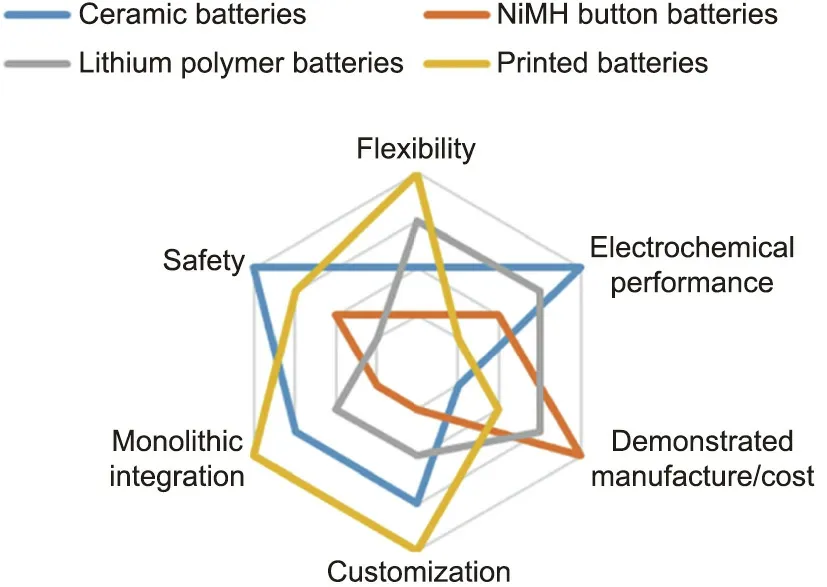
Fig.5. Technical advantages.Strengths and weaknesses of technologies competing in thin-film battery markets.
Overall, printed batteries face greater competition in terms of their laminated, wired-in format than in its monolithically integrated format.The unit price of printed batteries and their performance at this stage are not competitive against lithium polymer batteries or NiMH button batteries. In their monolithically integrated format, printed batteries face competition only from ceramic batteries. In this format, printed batteries benefit from greater form-factor freedom and cost outlook than ceramic batteries.
4. Advances in printed batteries
Fig. 6 presents the major breakthroughs in the field of printed batteries,beginning with the original report from Akuto and Ogata[143] on the fabrication of a printed lead–acid battery to the forecasted demonstration of key technical abilities in the near future[37,78,79,116,144,145]. Table 4 summarizes some of the key reports published in recent years on printed batteries. Lithiumion chemistry is the most represented, but studies on lithium-ion chemistry have focused principally on printed electrodes [35–38,40,59,77–79,82,94–99,116,145–172]. Printing electrolytes is challenging with lithium chemistry because of the stringent process requirements that must be fulfilled to avoid atmospheric contamination of the organic solvent-based electrolyte.Typically,the work conducted in academia has focused on demonstrating the feasibility of processing battery materials into inks and printing batteries using various printing methods. Several groups have attempted to demonstrate some of the benefits claimed for printed batteries,especially flexibility, although overall this has not been the focus.The electrochemical performances reported in the literature are also insufficient for practical applications, with areal capacities typically below 1 mA∙h∙cm-2(Table 4).The power density or internal resistance of the entire device is often omitted in the literature,which makes it difficult to evaluate the potential of the battery to meet the power requirements of electronic device applications.R2R manufacturing is still only a claim at this stage. The development of a printable, air-stable electrolyte ink appears to be a bottleneck, especially for lithium chemistry. Conveniently, the electrolytes of alkaline and NiMH batteries can be processed in air. Although alkaline batteries are well represented in the literature, they are often in a non-rechargeable format because the chemistry is not easily rechargeable, a common shortcoming of multivalent-ion batteries [144].
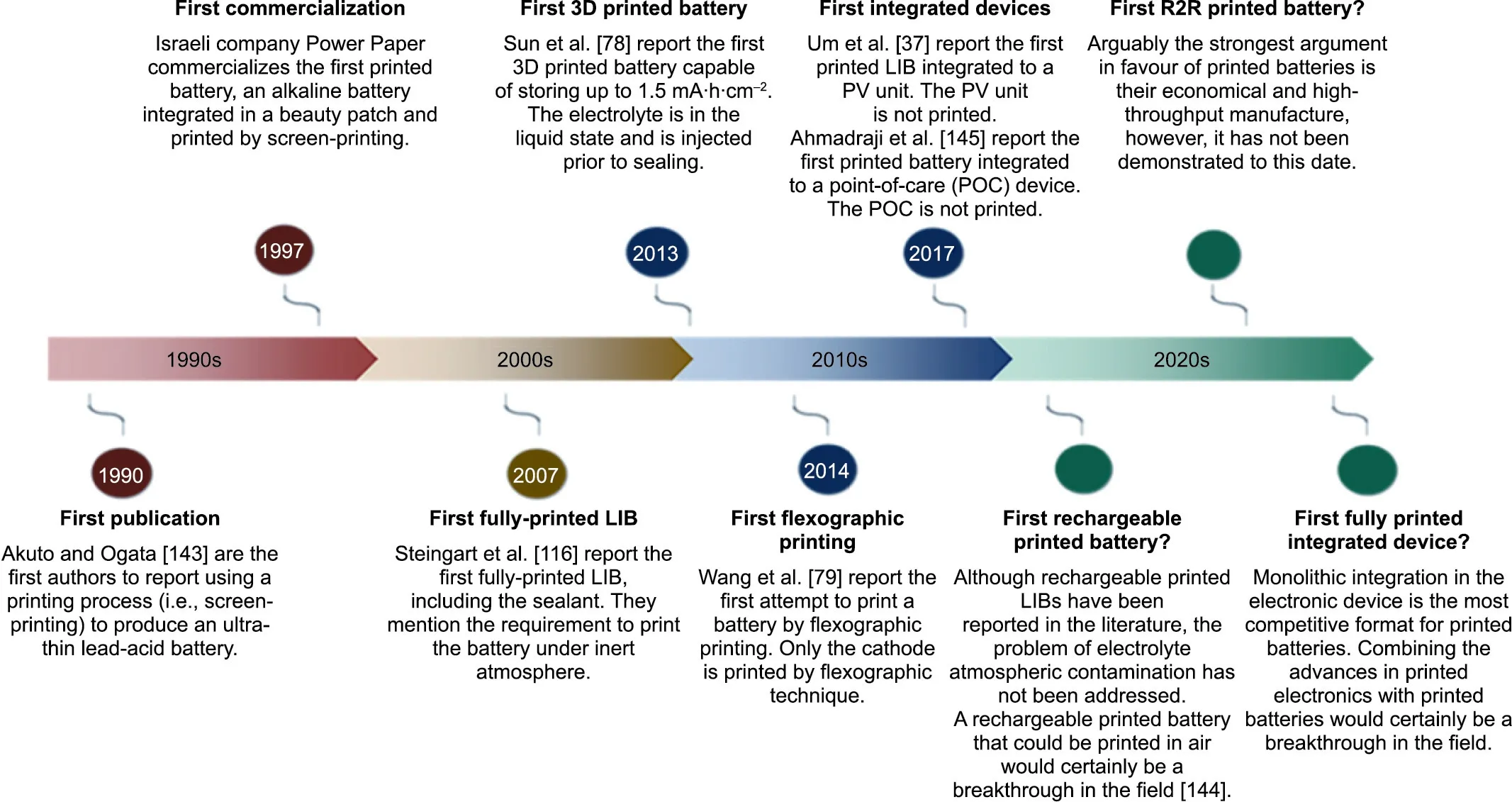
Fig. 6. Milestones in printed battery development. Chronology of the past and potential future breakthroughs in the field of printed batteries.
NiMH chemistry, on the contrary, combines the advantages of being both air-processable and rechargeable. Some breakthrough work has been conducted by Wendler et al. [59] with VARTA Microbattery to process a potash electrolyte into ink and manufacture a fully printed rechargeable battery. The following sections will elaborate on some of the most pivotal work conducted in the field of printed batteries to demonstrate the aesthetic versatility, flexibility, electrochemical performance, high-throughput manufacture, and integrability into electronic devices.
4.1. Aesthetic versatility
As presented in the literature,the aesthetic versatility of printed batteries is mostly limited to geometric shapes. Stencil [35,95],transfer [97], flexographic [79], and dispenser printing[78,99,165,166] techniques have been used successfully to print electrodes or full devices in various geometric and sometimes more complex shapes. Dispenser techniques have been adapted for 3D printing, the additive manufacturing approach with the greatest form-factor freedom. 3D printing is an important emerging field in energy storage[173],but it has little impact in industry because of its low throughput.
Sun et al.[78]3D-printed LTO anode and LFP cathode inks onto gold-coated glass with a dispenser(Fig.7(a)).The process involved a sintering step at 600 °C, which would be prohibitive for organic substrates, including DuPont’s Kapton®. The liquid electrolyte was dispensed onto the printed electrodes before the battery was encapsulated in PMMA and sealed with PDMS gel.Fu et al.[99]also used a dispenser to print a 3D microbattery with LTO and LFP electrodes embedded in reduced graphene oxide for better conductivity (Figs. 7(b) and (c)). The reported electrochemical performance was low, but the battery was fully printed, including the PDMS capsule. Interestingly, the authors freeze-dried the electrodes, an approach that is uncommon in the literature. Other non-printing approaches are also possible for manufacturing 3D batteries,including subtractive methods and molding [174].
Izumi et al. [166] and Gaikwad et al. [167] used a dispenser to manufacture patterned 2D LTO electrodes with increased surface areas (Fig. 7(d)). When tested in a coin-cell format, the batteries demonstrated better rate capabilities than non-patterned electrodes and a relatively low internal resistance of approximately 15 Ω.Hu et al.[169]used a dispenser to print a 3D high-area cathode with a carbon-coated LiMn1-xFexPO4(LMFP) active material(Fig. 7(e)). A capacity retention of 65% from 1C to 100C was achieved, which was ascribed to the innovative structure. Overall,dispenser methods are superior for shaping batteries with complex form factors,but they are also slower than other methods and typically not adapted for thick-layer deposition. Sun et al. [78], for instance, deposited multiple layers (Fig. 7(a)) to achieve an areal capacity of 1.5 mA∙h∙cm-2.
Screen and stencil printing are faster and can handle viscous inks for thick-layer deposition. Kim et al. [35] used a mold during the hardening step to increase the surface area of the electrodes(Fig. 7(f)). They produced batteries in the shape of letters using stencil printing and innovative UV-curable inks for an LTO anode,LFP cathode, and electrolyte (Fig. 7(g)). Berchmans et al. [95] used a stencil-printing approach to manufacture an epidermal device(Fig. 7(h)). The current collector was printed onto an insulating substrate and the active material was electrodeposited, silver for the anode and zinc for the cathode. PDMS and epoxy glue were used for the encapsulation and the sealant, respectively.
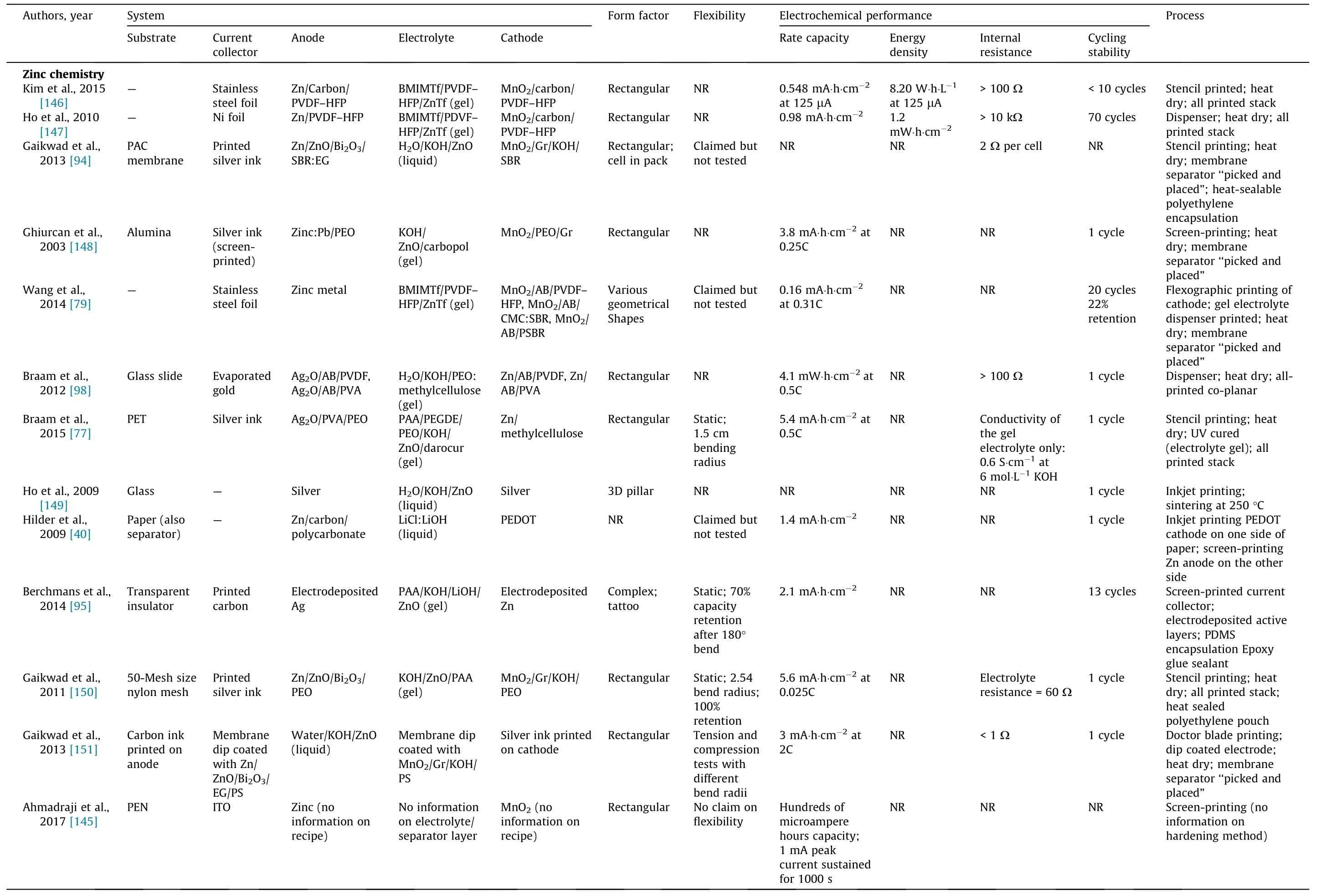
Table 4 Summary of the printed thin-flim batteries with various battery chemistries reported in the literature.

Table 4 (continued)
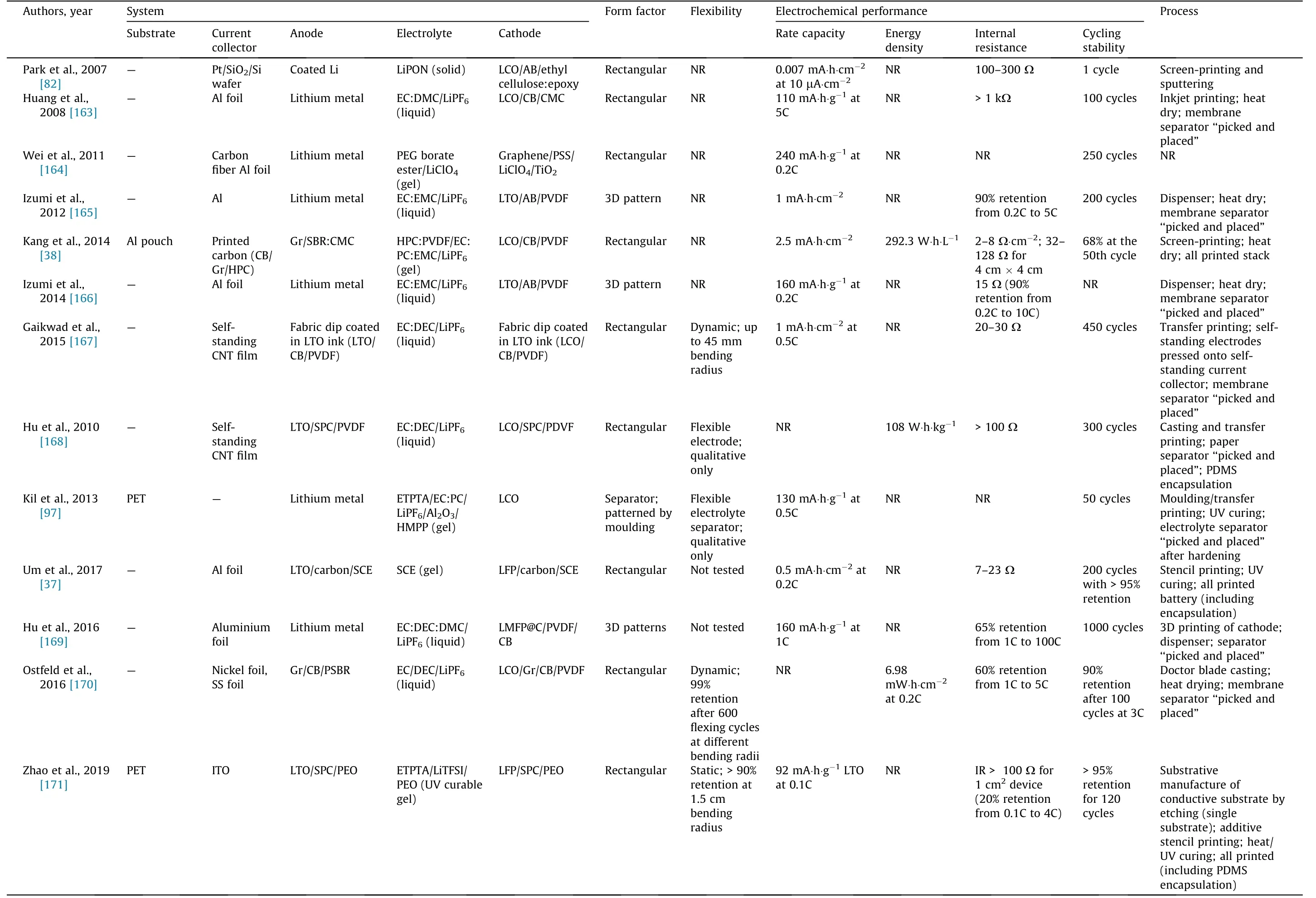
Table 4 (continued)
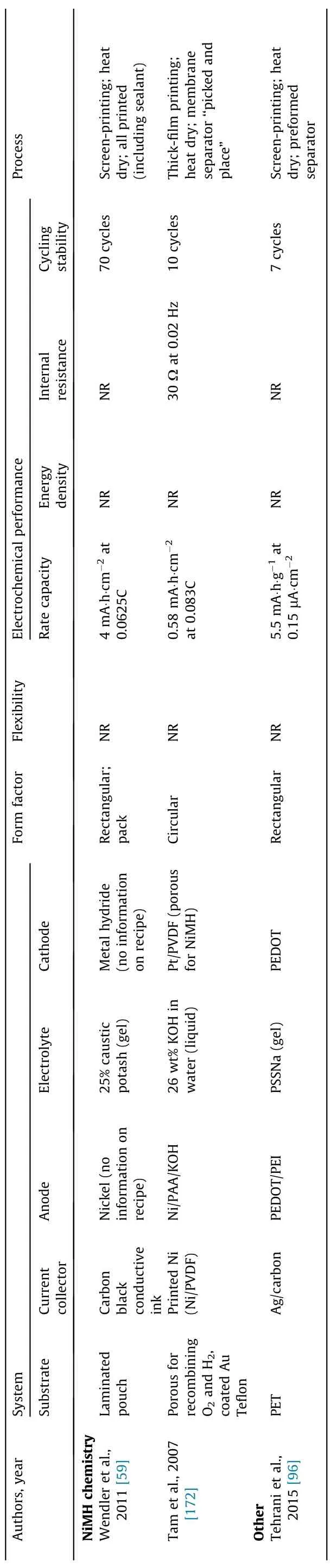
Table 4 (continued)
The inks were based on acrylate monomers polymerized in situ,entrapping the active materials and liquid electrolyte.The reported electrochemical performances were low, and no information was given on the areal capacity.The low performances can be ascribed to the large internal resistance,which was between 10 and 100 Ω.A similar UV-curing approach was used by Kil et al.[97]to mold an electrolyte separator with patterns (Fig. 7(i)). With high potential for R2R manufacture, flexographic printing has also been used to print electrodes in various geometric shapes (Fig. 7(j)). Wang et al. [79] developed various MnO2inks with aqueous and organic solvent vehicles and used flexographic printing to print electrodes onto stainless-steel substrates.The electrodes showed poor capacity retention(22%after 22 cycles)when tested in open-cell format using zinc as the counter electrode and an ionic liquid-based electrolyte,which may have been a consequence of the poor cyclability of the zinc–manganese chemistry and not of the electrode fabrication process.
4.2. Flexibility
The term ‘‘flexibility” is somewhat ambiguous in the literature.It can refer to stretchability, bendability, foldability, and other types of shape deformation. A typical flexible battery experiences flexible stress between three planes: a compression plane, a stretched plane,and a neutral plane sandwiched between the compression and stretched planes (Fig. 8(a)) [149]. Detailed flexibility testing was omitted in flexible battery reports previously[40,79,94] or only qualitative testing was conducted[97,168,157]. Gaikwad et al. [13,94,150,151,167] reported the development of testing protocols for flexible batteries manufactured by printing and dip-coating methods. A flexible zinc–manganese battery manufactured by dip coating and doctor blading was tested under compression and tension stresses at different bending radii.The battery achieved a promising 3 mA∙h∙cm-2areal capacity at a 2C rate, which was ascribed to low internal resistance below 1 Ω [150]. In 2011, Gaikwad et al. [150] developed another flexible zinc–manganese battery that maintained 100% of its initial 5.6 mA∙h∙cm-2capacity when flexed at a 2.54 cm bending radius and discharged at a 0.025C rate (Fig.8(b)) [150]. This battery was manufactured by stencil printing the active material inks onto a 50-mesh-size nylon mesh. In 2015,Gaikwad et al.[167]subjected flexible LIBs to dynamic bending tests. The batteries comprised flexible self-standing electrodes manufactured by dip coating a self-standing CNT film into the active material inks (LTO for the anode and LFP for the cathode).The electrodes were then pressed against a membrane separator and assembled in a pouch cell. The batteries could withstand 100 flexing cycles at bending radii varying from 10 to 45 mm while charging and discharging over a 1 mA∙h∙cm-2capacity at a 0.5C rate (Fig. 8(c)). In collaboration with Gaikwad et al., Ostfeld [170]reported dynamic flexible testing based on Gaikwad’s protocol and casting methods. The bending radius was set between 1 and 3 in (1 in = 2.54 cm) while the device underwent galvanostatic cycling (Fig. 8(d)). The flexible LIB device demonstrated over 95%capacity retention after 200 bending cycles and 20 galvanostatic cycles.
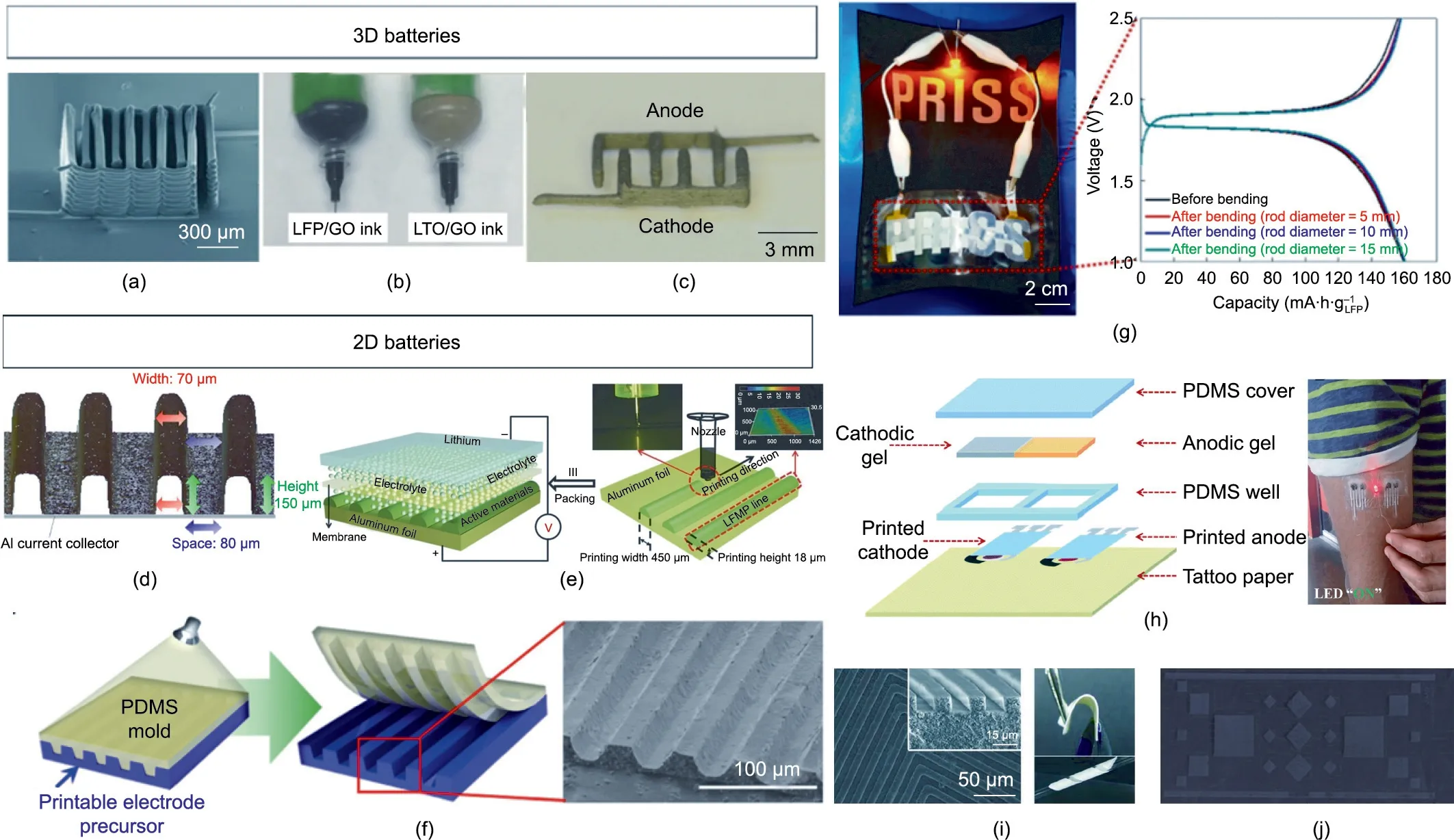
Fig. 7. Aesthetic versatility of printed batteries. (a) SEM image of the printed LTO–LFP electrode with an interdigitated architecture. Reproduced from Ref. [78] with permission.(b)Photo of the LFP/graphene oxide(GO)and LTO/GO inks in syringes.(c)Photo of the interdigitated electrodes.(b,c)Reproduced from Ref.[99]with permission.(d) Micropatterning of the 3D LTO cathode from the laser microscope. Reproduced from Ref. [165] with permission. (e) Schematic of 3D printing process for LMFP nanocrystalline electrode. Reproduced from Ref. [99] with permission. (f) Schematic of the micropatterning process and prepared LFP patterned cathode. (g) Photograph of letter-shaped PRISS cell and corresponding charge/discharge profiles.(f,g)Reproduced from Ref.[35]with permission.(h)Diagram of the fabrication steps for the Ag–Zn cell(left)and demonstration of the tattoo battery on the skin for LED lighting(right).Reproduced from Ref.[95]with permission.(i)SEM images of top and cross-sectional views of a maze-patterned composite gel polymer electrolyte(c-GPE)(left),a photo of the highly bendable c-GPE(right).Reproduced from Ref.[97]with permission.(j)R2R printed MnO2 electrode on stainless-steel film. Reproduced from Ref. [79] with permission.
Static bending tests (also known as fatigue tests) were also reported by several research groups [77,95,146,171]. Braam and Subramanian [77] used stencil printing to manufacture a flexible silver–zinc battery. The battery was printed on a flexible PET substrate onto which was stack-printed a zinc cathode, a UV-curable acrylate-based separator(Fig.8(e)),and a Ag2O anode[77].All curing steps were conducted at a moderate temperature to prevent the PET substrate from melting. The laminated pouch batteries demonstrated 90% capacity retention when statically bent to a 1.5 cm radius and discharged. Berchmans et al. [95] tested the flexibility of an epidermal device with a fatigue test, bending the battery 180° along the latitude and recording its performance before and after bending. The device maintained 70% of its initial capacity after five bending cycles at 180° (Fig. 8(f)). Kim et al.[35] also used a fatigue test with a 7.5 cm bending radius (Fig.8(g)). The LIB device maintained 100% capacity from rest to the bending position. More recently, Zhao and Wu [171] tested the flexibility of an LIB device manufactured by the in-situ polymerization of UV-curable ETPTA monomers onto a flexible and conductive indium tin oxide(ITO)-coated PET substrate.The device was tested with a static protocol using bending radii of 3 and 1.5 cm. When bent at 1.5 cm, the device was able to maintain over 90% of its‘‘horizontal” capacity over more than 200 galvanostatic cycles.
4.3. Electrochemical performance
As discussed in Section 2,printed batteries will need to achieve areal capacities on the order of 1–10 mA∙h∙cm-2to meet practical requirements for energy storage devices and autonomy for the targeted electronic device applications. If a thin-film battery has a thickness of approximately 0.5 mm and needs to deliver the current at 3 V(adapted for silicon circuitry),this equates to an energy density of 6–60 W∙h∙L-1. Unfortunately, information on energy density or areal capacity is not always available in previous reports.Specific energy density in terms of active material utilization is generally reported instead. Although this information is interesting for estimating the efficiency of the battery in terms of active material utilization, it is of little practical value to the electronic device designer who is constrained by space, especially in small electronic devices where the battery occupies a large amount of available space,up to 30%[76].Another important electrochemical criterion is that the power density of the battery device must be large enough to meet the rate demanded by the application device.As mentioned in the previous section, some capabilities such as Wi-Fi transmission require power in the hundreds of milliwatts(Table 2), which can be extremely challenging for printed battery devices. To take the previous example, the voltage output of a 3 V battery under a 100 mW load would drop to 0 V if its internal resistance was greater than 100 Ω according to the expression derived from Ohm’s law, V =√————WR.
The zinc–manganese devices reported by Kim et al.[146]and Ho et al. [147] would not be able to handle such high-power requirements with internal resistances above 100 Ω.Ghiurcan et al.[148]and Braam et al. [77,98] reported areal capacities of more than 4 mA∙h∙cm-2for zinc–manganese batteries, which is sufficient to provide autonomy to small electronic devices.However,the internal resistance of more than 100Ω reported by Braam et al.[98]would be prohibitive, driving the 1.5 V battery voltage output to 0 V if the power draw is higher than 20 mW, such as for organic lightemitting diode(OLED)displays or Bluetooth transmission(Table 2).
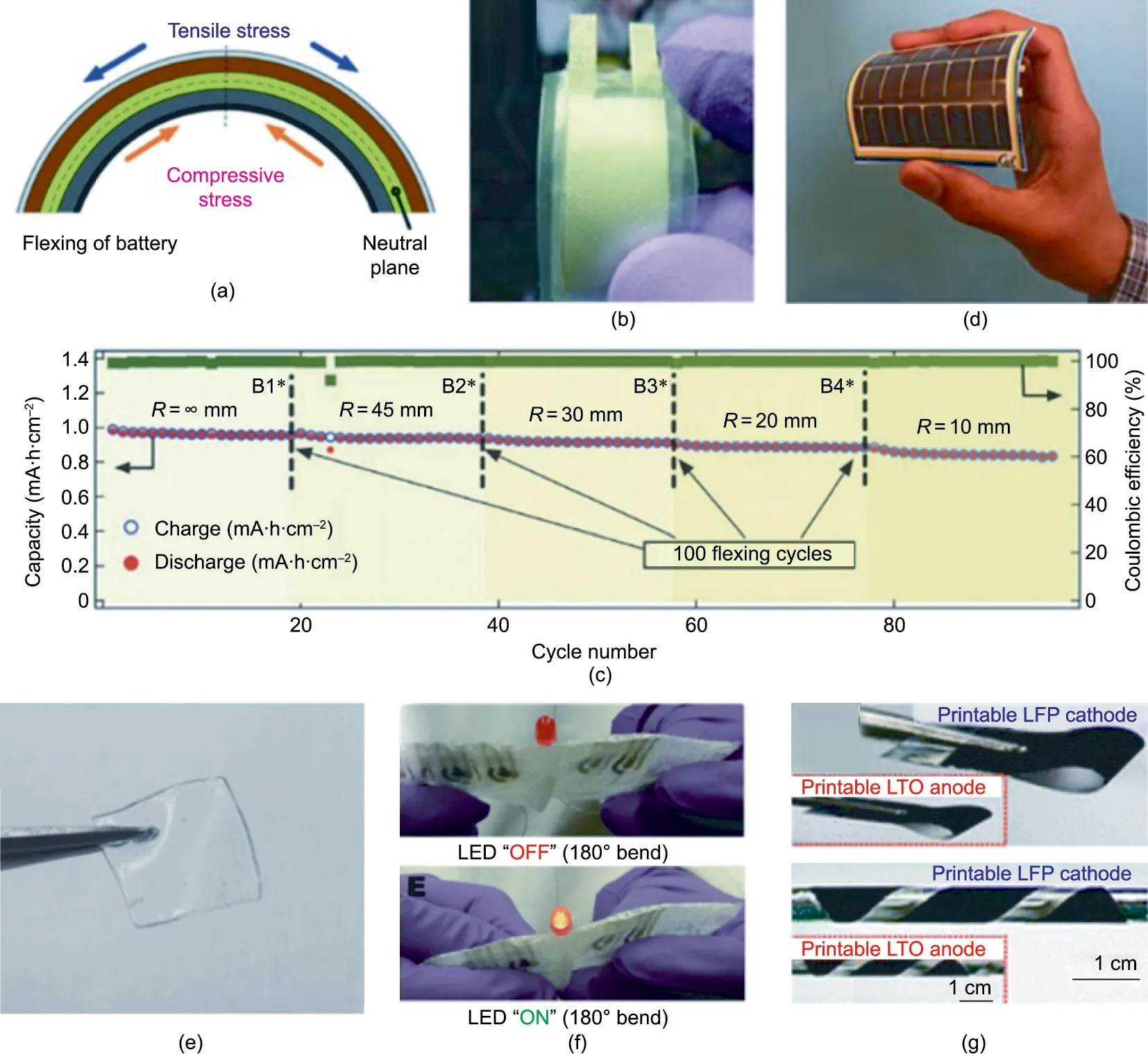
Fig. 8. Flexibility of printed batteries. (a) Schematic illustration of the generated stresses in a flexible battery in the process of flexing. Reproduced from Ref. [151] with permission. (b) Photo of a flexible Zn–MnO2 battery with polyethylene lamination. Reproduced from Ref. [150] with permission. (c) Areal battery capacity under different flexing state. Reproduced from Ref. [167] with permission. (d) Activity-tracking wristband with flexible batteries flexed by a hand. Reproduced from Ref. [168] with permission.(e)Photo of a gel separator based on photo-polymerized PAA.Reproduced from Ref.[77]with permission.(f)Photos of a printable tattoo battery under bending conditions.Reproduced from Ref.[95]with permission.(g)Demonstration of the mechanical flexibility of the printed electrodes.Reproduced from Ref.[35]with permission.
Gaikwad et al. [150] reported the fabrication of two zinc-based non-rechargeable devices assembled with a flexible membrane substrate.In this work,the entire battery stack was stencil printed onto a flexible nylon mesh, including a silver current collector, a zinc anode, and a MnO2cathode (Fig. 9(a)). The printed gel electrolyte displayed conductivity on the order of 10-2S∙cm-1when the concentration of KOH was increased beyond 1 mol∙L-1(Fig. 9(b)). In the second study, the flexible substrate was dip coated in inks containing the active material, zinc for the anode and MnO2for the cathode,before casting the carbon current collector onto the electrodes using doctor blading [151] (Fig. 9(c)). An internal resistance below 1 Ω was achieved, which permitted an areal capacity higher than 2 mA∙h∙cm-2,even at 2C rates and under flexing (Figs. 9(d) and (e)).
The 3D rechargeable battery produced by Sun et al.[78]demonstrated an areal capacity of 1.5 mA∙h∙cm-2over 30 cycles (Figs.10(a) and (b)), which is high enough to meet the energy requirement of micropower devices. It is generally accepted that 3D batteries that are manufactured ‘‘upward” are capable of higher areal capacities compared with thin-film 2D batteries owing to the fact that the active layers are thicker (Figs. 7(a) and (c)).Although the innovative architecture had a large electrode/electrolyte interface and a liquid electrolyte that was typically more ionically conductive than gel electrolyte, the rate capability was low at 80% capacity retention from 1C to 2C, which is indicative of high internal resistance. The high internal resistance can be ascribed to the large gap between the electrodes,which was necessary in this configuration. Izumi et al. [165] also used a dispenser approach to produce a flexible LIB with an areal capacity beyond 1 mA∙h∙cm-2. The high-area LTO electrode (Fig. 10(c)) demonstrated excellent rate capability in a half-cell format, retaining 90% capacity from 0.2C to 5C. Kang et al. [38] demonstrated high areal capacity and energy density values of 2.5 mA∙h∙cm-2and 292 W∙h∙L-1(Fig. 10(d)), respectively, for a laminated LIB device fully printed by screen printing(Fig.10(e)).The gel electrolyte featuring organic solvents EC, PC, and ethyl methyl carbonate (EMC)plasticizers and a mixture of cellulose and PVDF polymer binders achieved high ionic conductivity, reducing the internal resistance to only a few ohms per square centimeter (Fig. 10(f)). However,the cycling stability of the device was low, with only 85%capacity retention after 50 cycles(Fig.10(d)).This may have been owing to moisture contamination of the electrolyte during printing in air.Gaikwad et al.[167]used a similar approach for a zinc battery produced by dip coating to manufacture an LIB device. The battery achieved areal capacities higher than 1 mA∙h∙cm-2and demonstrated moderate internal resistance of tens of ohms, even under flexing (Figs. 10(g) and (h)). Ostfeld et al. [170] reported a flexible battery with an areal capacity of 7 W∙h∙cm-2at 0.2C but with only 60% capacity retention from 1C to 5C (Fig. 10(i)), which is indicative of high internal resistance. The battery was manufactured by doctor blading onto nickel and stainless-steel foils to produce graphite and an LCO electrode,respectively,which was combined into a fully laminated pouch battery filled with a liquid electrolyte.Using the NiNH system, Wendler et al. [59] fabricated a fully printed and laminated pouch battery with an areal capacity of 4 mA∙h∙cm-2at a 0.0625C rate. The researchers processed a 25%KOH potash electrolyte into ink and used screen printing for all the layers. Unexpectedly, the capacity more than tripled when the charging and discharging rates were quadrupled.
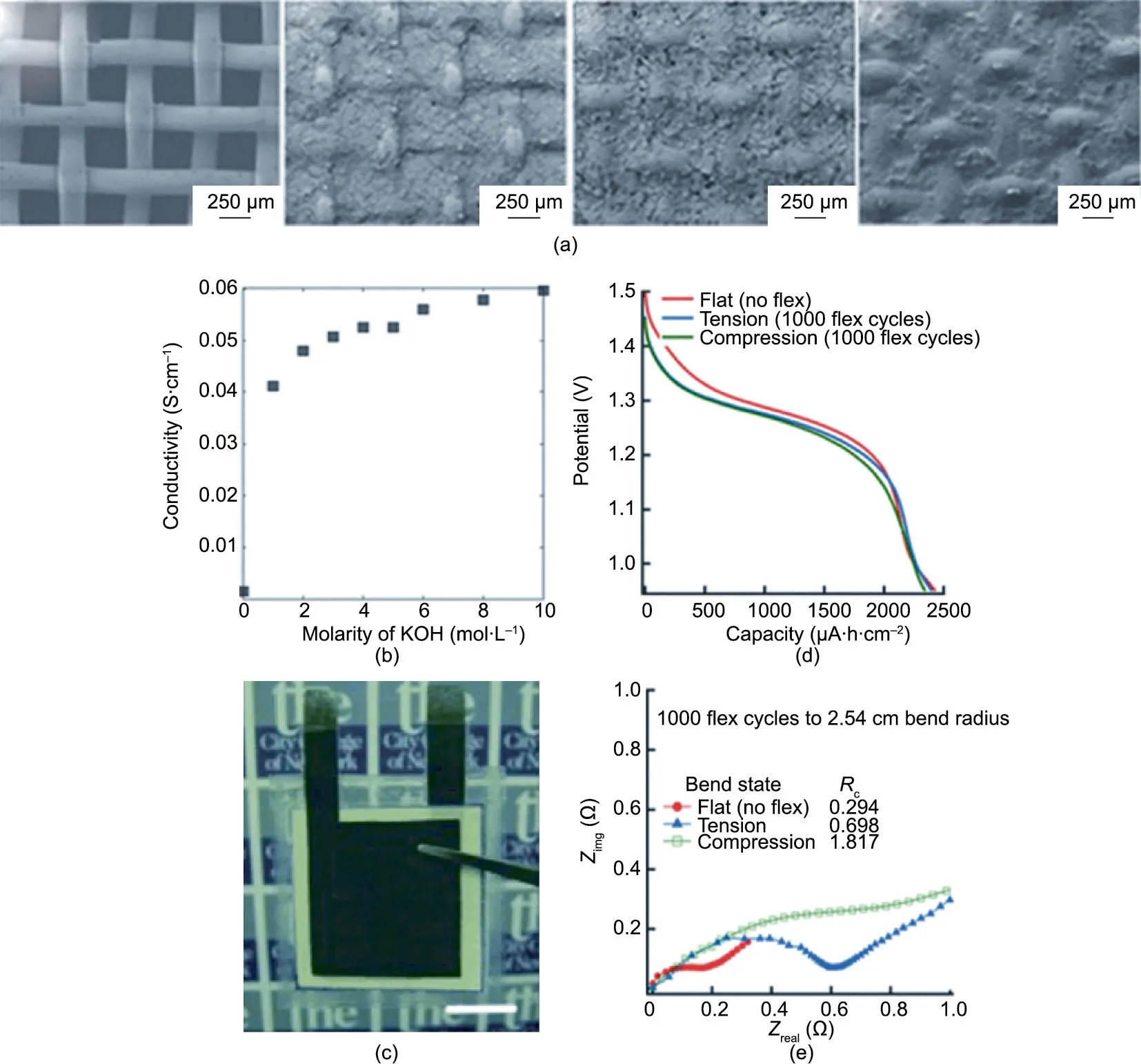
Fig.9. Electrochemical performances of zinc–manganese printed batteries.(a)SEM images of the nylon mesh-supported electrodes.From left to right:nylon mesh,Zn on the mesh electrode,MnO2 on the mesh electrode,and silver current collector on the mesh electrode.(b)Conductivity of the PAA-based gel electrolyte in KOH.(a,b)Reproduced from Ref.[150]with permission.(c)Photo of a flexible Zn–MnO2 battery with a scale bar of 1 cm.Comparison of an unflexed battery and a battery subjected to 1000 bending cycles (bending radius: 2.54 cm). (d) Discharge curves and (e) electrochemical impedance spectroscopy (EIS) curves. (c–e) Reproduced from Ref. [151] with permission.
Zhao and Wu [171] recently reported an original design for printing an LIB based on an LTO anode and an LCO cathode. The design combined subtractive etching of an ITO current collector that then serviced both the anode and the cathode. This approach was somewhat similar to the ceramic thin-film battery design in the sense that the anode/electrolyte/cathode stack was supported by a single current collector substrate and not two, as is the case for most printed batteries. The printable electrolyte containing UV-curable acrylate ETPTA monomers, a PEO/succinonitrile (SN)plasticizer, and lithium bis(trifluoromethanesulfonyl)imide(LiTFSI)salt achieved a maximum conductivity of 2.5×10-3S∙cm-1at room temperature. The compositions of the electrode inks included a PEO binder and an ethanol vehicle that is generally considered too volatile for printing.Tested in half-cell format,the electrodes showed high resistance in the 100 Ω range, despite the addition of super P carbon to increase the electronic conductivity.In a fully stencil-printed format,the technology showed a low rate capability, with only 20% capacity retention of the initial specific capacity of the 90 mA∙h∙g-1LTO from 0.1C to 4C, which can be ascribed to high internal resistance. The decision to report the specific energy density instead of the volumetric energy density makes it difficult to evaluate the technology in terms of the technical constraints for potential applications.
4.4. Roll-to-roll manufacturing
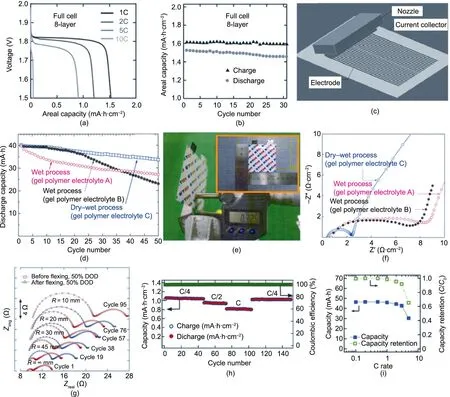
Fig.10. Electrochemical performances of lithium-ion printed batteries.Printed interdigitated batteries based on the LTO–LFP electrodes.(a)Voltage–areal capacity curves for 8-layer interdigitated batteries based on LTO–LFP electrodes. (b) Cycling stability and areal capacity of 8-layer interdigitated batteries based on LTO–LFP electrodes.Reproduced from Ref.[78]with permission.(c)Schematic of the printing setup of 3D micropatterning.Reproduced from Ref.[144]with permission.Pouch-type printed LIBs with different gel polymer electrolytes:(d)cycling performance and(e)photo of pouch-type thin-film LIB.(f)Impedance spectra after initial charging and discharging.(d–f)Reproduced from Ref. [38] with permission. Impedance spectra of (g) a flexible LIB based on the LCO electrodes and (h) areal capacity and cycling number. DOD: depth of discharge.(g,h)Reproduced from Ref.[167]with permission.(i)Discharge capacity and capacity retention of the flexible LIB based on LCO electrodes.Reproduced from Ref.[170] with permission.
Although many authors identify R2R manufacturability as one of the main advantages of printed batteries,no report could be found of batteries produced entirely by R2R, and only a few devices reported in the literature had all fabrication steps adapted to R2R with all electrochemical components printed,including the encapsulation and sealant.To the best of our knowledge,only Wang et al.[79]reported a printed electrode using an R2R printer(Fig. 11(a)),in which the authors developed a MnO2ink adapted to R2R flexographic printing. The researchers tested different binders and binder couples, including PVDF–HFP, a carboxymethyl cellulose:styrene-butadiene rubber(CMC:SBR),and a polystyrene butadiene rubber (PSBR), and they found that the latter provided the best printing performance. Several cathodes were printed on stainlesssteel foil(Fig.11(b))and tested in an open-cell format with an ionic liquid-based gel electrolyte,a membrane separator,and zinc metal for the counter electrode.Although the authors could demonstrate the concept, the electrochemical performance was limited. There was a significant drop in capacity after only a few cycles that was most certainly owing to the lack of cyclability of the zinc–manganese chemistry.Kim et al.[146]and Ho et al.[147]produced fully printed stacks using the zinc–manganese chemistry with a PVDF–HFP binder in both electrodes and in the gel electrolyte that also featured ionic liquid 1-butyl-3-methylimidazolium trifluoromethanesulfonate (BMIMTf) and zinc salt ZnOtf2. The printed stack was then tested in coin-cell and open-cell formats but not in a fully printed format. Co-planar geometry is easier to manufacture than stacked geometry,and it does not require a separator,although such batteries typically suffer from higher internal resistance because of the extended distance between the electrodes.Braam et al.[98]manufactured a fully printed co-planar battery based on the zinc–silver chemistry.Two different binders,PVA and PVDF,were tested for the electrodes,PEO was used as a plasticizer,and methylcellulose was used as a thickener in the caustic gel electrolyte.The cells manufactured with PVA binder showed a marginal increase in capacity,but the internal resistance was similar to that of PVDF. Although the active and electrolyte layers were all printed by dispenser printing,the gold current collector was manufactured by the physical vapor deposition technique.Despite a large internal resistance higher than 100 Ω,the batteries achieved a maximum areal energy density of 4.1 mW∙h∙cm-2at 0.5C. Braam and Subramanian[77]produced an all-printed zinc–silver battery with potential for R2R production using stencil printing and a stack geometry (Fig. 11(c)). A PVA binder was used for the Ag2O anode and a methylcellulose binder for the zinc cathode. Unlike coplanar geometry, which does not need a separator, a UV-curable gel electrolyte containing an acrylate binder was stencil printed to provide physical separation between the electrodes once hardened. The internal resistance of the entire device was not reported, but the conductivity of the gel electrolyte was high, 0.6 S∙cm-1for a 6 mol∙L-1KOH concentration.
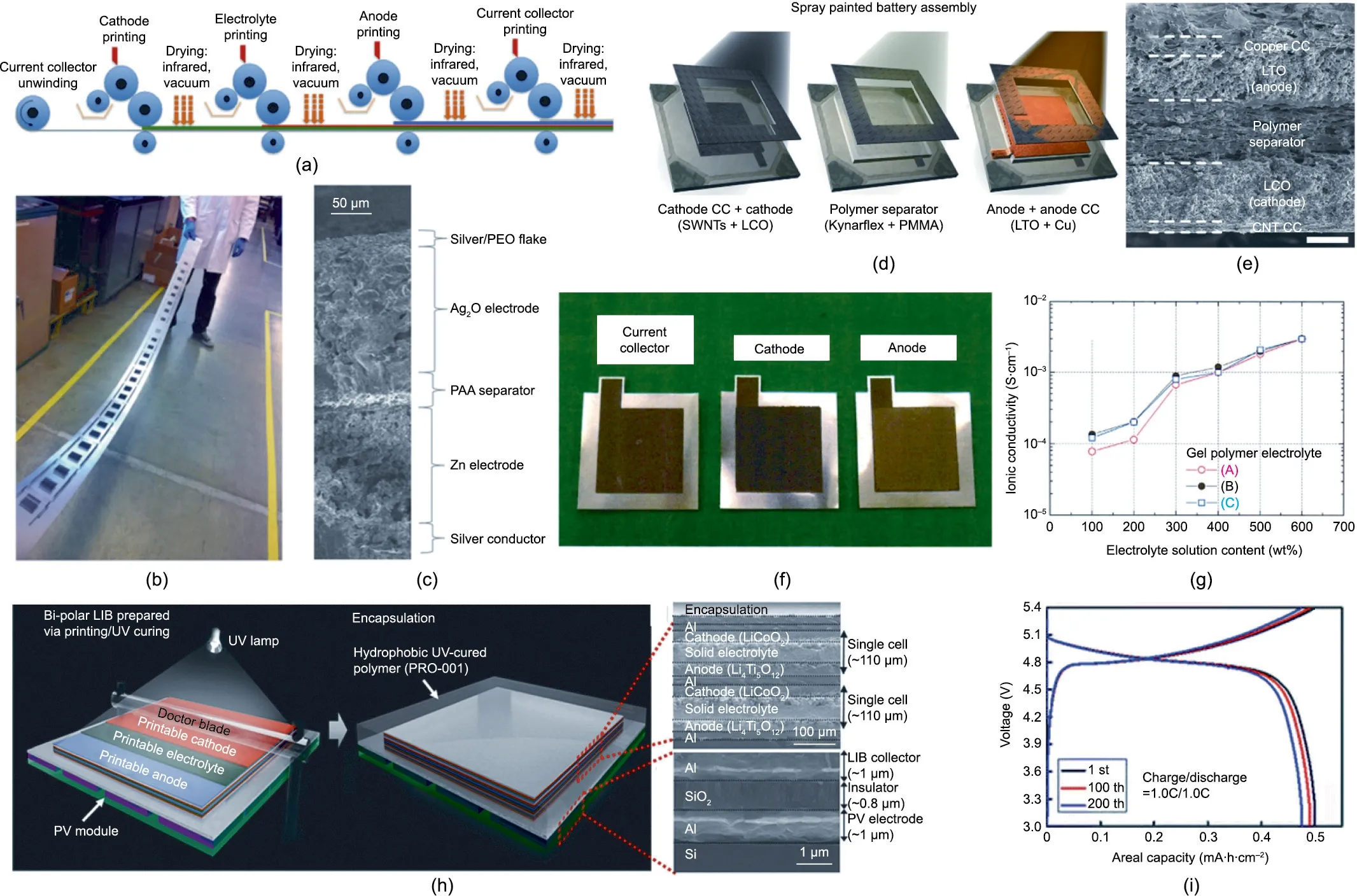
Fig. 11. Potential for R2R manufacture of printed batteries. (a) Schematic illustration of multi-station flexographic printing technique. (b) Photograph of the flexographic printed batteries on a stainless-steel foil.(a,b)Reproduced from Ref.[79]with permission.(c)SEM image of a fully stencil-printed battery stack.Reproduced from Ref.[77]with permission.(d)Conceptual procedure of spray printing for battery fabrication.CC:current collector.(e)SEM image of a spray printed battery.(d,e)Reproduced from Ref.[36]with permission.(f)Photos of the screen-printed current collector,cathode,and anode.(g)Room-temperature ionic conductivities of the gel polymer electrolytes.(f,g)Reproduced from Ref.[38]with permission.(h)Scheme of the printing processes of the solid-state SiPV–LIB device.(i)Galvanostatic charge/discharge curves of a printed LIB battery in an integrated device as a function of the cycling number. (h, i) Reproduced from Ref. [37] with permission.
Ahmadraji et al. [145] manufactured an integrated device with two functionalities(display and sensor)and a battery fully printed by the screen-printing method. The manufacture of the device itself necessitated additional methods including inkjet printing,photolithography, and lamination that are also compatible with R2R manufacture.
Using the lithium chemistry, Kim et al. [35] stencil printed an LTO anode, a UV-curable acrylate-based gel electrolyte, and an LFP cathode in sequence to fabricate a fully printed stack,compatible with R2R manufacture. The laminated battery showed moderate internal resistance, but it was unstable, likely owing to moisture contamination of the organic solvents used in the electrolyte and processed in air. Singh et al. [36] used spray painting to manufacture a fully printed stack that included an LTO anode, an LCO cathode, and a heat-curable gel electrolyte composed of proprietary Kynarflex®-280, PMMA binder, and SiO2ceramic filler in an air-processable dimethylformamide (DMF)/acetone vehicle(Figs. 11(d) and (e)). The authors omitted to mention the addition of lithium salt in the electrolyte, although the ionic conductivity was measured to be 1.24×10-3S∙cm-1.Kang et al.[38]produced a fully printed LIB stack by screen printing(Fig. 11(f)),but,similar to Kim et al. [35], the gel electrolyte was processed in air despite the fact that it contained the hygroscopic organic solvents EC, PC,and EMC. Another concern is that, unlike Kim et al. [35], the gel electrolyte was heat cured,which poses the problem of plasticizer evaporation losses that would impact the gel electrolyte conductivity (Fig. 11(g)).
Some researchers have also attempted to print the encapsulation [37,99,171] or the encapsulation sealant [59,116] for even greater potential for R2R manufacture. Um et al. [37] used a UVcurable hydrophobic polymer to encapsulate an all-printed stack(Fig. 11(h)) fabricated using a method similar to that of Kim et al.[35] and therefore also prone to air-contamination of the hygroscopic organic solvents in the UV-curable gel electrolyte.However,unlike Kim et al. [35], the monolithically integrated LIB device achieved satisfactory cycling stability, with more than 95% capacity retention over 200 cycles (Fig. 11(i)). More recently, Zhao and Wu [171] used the same ETPTA-based UV-curable electrolyte approach as Kim and Um, but they replaced the air-sensitive organic solvents with PEO and liquid SN as the plasticizers.Although this approach circumvented the problem of moisture contamination reinforced using a highly hydrophobic SN vehicle in the gel electrolyte, the ionic conductivity was also lower at 2.5 × 10-3S∙cm-1. This led to high internal resistance and a low rate capability, with only 20% capacity retention from 0.1C to 4C,despite the high interfacial area created by the original cell geometry. A cured PDMS film was used to encapsulate the device. Fu et al. [99] also used a cured PDMS film to encapsulate an all-3Dprinted battery stack, although the encapsulation was most certainly not printed because it was operated in a glove box prior to liquid electrolyte injection to avoid contamination from the air.Steingart et al. [116] used a dispenser printer placed in a glove box to avoid moisture contamination.A fully printed LIB stack featuring a PVDF–HFP binder and an ionic liquid-based gel electrolyte was encapsulated and the sealant printed using the same dispenser,but they did not disclose the type of sealant used.Wendler et al. [59]also developed a printable sealing technology for a fully printed NiMH battery.
Reports on R2R printing are also scarce in the industry. Printed Energy recently developed a production-scale R2R printing facility,the very first of its kind.Imprint Energy has also reported upscaling their means of production.The industry-supported GREENBAT project, which aims to develop a printed LIB, published one report related to R2R manufacturing. They observed adequate multipass loading, printing resolution, and screen-printing processability with a Valtion Teknillinen Tutkimuskeskus (VTT) modular R2R rotary koater (ROKO) machine (Fig. 12) [175].
4.5. Monolithic integration
Integrated devices have been the object of intensive research and development effort in industry, but not so much in academia.Power Paper,the first company to commercialize printed batteries in 1997 [33] filed 21 patents on integrated devices, all of them related to skin and beauty patches. More recently, Nth Degree Technologies and its spin-off company Printed Energy have been developing integrated devices, including printed batteries powering printed RFID antennae that are currently being tested on a live scale (Figs. 13(a)–(c)). Nth Degree Technologies holds several patents on printed LED displays (Fig. 13(d)), and printed PV holds much promise for more integrated products with Printed Energy’s printed batteries in the future.
Despite the clear advantages of printed batteries over the competing technologies regarding integrated devices, advantages that are already being leveraged by industry,only two academic groups have reported integrated devices to date to the best of our knowledge[37,145].Um et al.[37]printed an LIB device integrated with an inorganic silicon-based PV device that was not printed but rather manufactured by vacuum and thermal deposition techniques. Although the PV module was not printed in this instance,both inorganic [176] and organic [177] printed PV technologies are already mature and could be used instead of vacuum deposition techniques. Printed OPV technology in particular has shown great potential for R2R processing [177]. Consequently, a fully printed integrated device can be envisioned in the near future. It is promising that the integrated silicon-based PV and LIB system(SiPV–LIB) fabricated by Um et al. [37] demonstrated adequate energy and power densities to meet the electric vehicle(EV)target goal fixed by the US Department of Energy(DOE)when exposed to sunlight (Figs. 13(e) and (f)). The device performance can be ascribed to moderate internal resistance, especially under illumination (Fig. 13(g)).
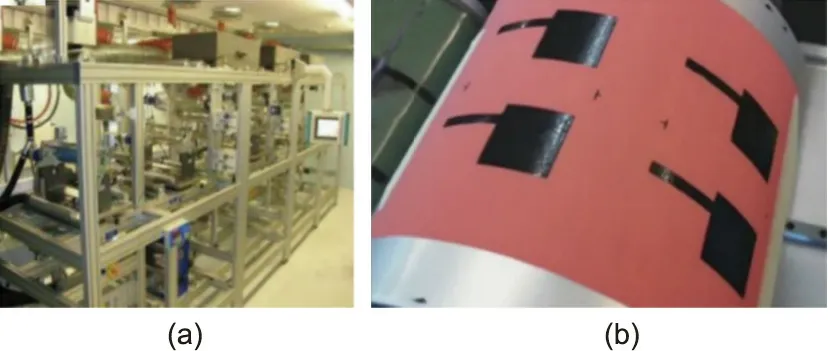
Fig.12. CORDIS GREENBAT project for the development of a flexible printed LIB.(a)VTT modular R2R ROKO machine. (b) Ink printed onto copper metallic foil.Copyright 2011, European Commission CORDIS.
Ahmadraji et al. [145] targeted point-of-care (POC) diagnostic test applications, which are well adapted to printed batteries because ①all the components can be printed (sensor, display,and battery), ②the test is single-use, and therefore the nonrechargeable but highly printable zinc–manganese chemistry can be used, and ③the zinc–manganese chemistry is adapted to the moderate energy storage and power requirements of the device.In the study, 24 fully printed devices were produced (Figs. 13(h)and (i)). The functionalities (sensor and display) and the energy storage (battery) were all screen printed. The assembly required additional methods, including inkjet printing, photolithography,and lamination,all of which are compatible with R2R manufacture.The battery was composed of three single cells connected in series to deliver the necessary voltage required to drive the conventional silicon circuitry(>3 V).The moderate energy storage requirement(in the range of hundreds of microampere hours) was achieved with moderate active layer loading(10–80 μm thickness)for short ionic paths, thus securing low internal resistance, which allowed the device to sustain a high peak current of 1 mA.The device accurately identified the serum concentration of H2O2and cholesterol during a titration experiment.
Zhao and Wu [171] developed a device with the potential for monolithic integration by using a mixture of subtractive and additive manufacturing methods to produce a flexible printed battery device. The researchers etched an ITO-coated PET substrate with an acidic solution to create a right-angle groove pattern. Stencil printing was used to sequentially cast an LTO-based anode material, an ETPTA-based gel electrolyte, and an LFP-based cathode material within the grooves delimited by etching. The key was to fill the groove with the electronically insulating gel electrolyte to insulate the anode and cathode current collectors. By using this approach, only one current collector/substrate was used instead of two, which simplified the fabrication process using a design reminiscent of that of ceramic LIBs.The device demonstrated several of the potential advantages of printed batteries, including flexibility and potential for R2R fabrication and monolithic integration. Unfortunately, the electrochemical performance was reported in terms of specific capacity instead of areal capacity and energy density,and consequently it is difficult to assess whether the energy storage meets the requirement of small electronic devices.
Finally,it is of interest to compare recent developments in integrated printed batteries with those of integrated ceramic batteries because both technologies are competing in the integrated device market.Koo et al.[80]integrated a ceramic LIB fabricated by sputtering under vacuum and featuring a LiPON solid electrolyte and an OLED display fabricated by spin-coating and thermal evaporation methods on a flexible ITO conductive substrate(Figs.13(j)and(k)).The device achieved an energy density of 2.2 mW∙h∙cm-3at a rate of 46.5 μA∙cm-2(0.5C).The high voltage of 3.9 V is suitable to service the silicon circuitry without connecting several batteries in series. Assuming the dimensions of the card to be 80 mm×50 mm×1 mm,Koo’s battery is capable of storing 10 mW∙h and delivering close to 2 mA current (0.5C equivalent) for 30 min.However,there are more energy and power densities than that calculated from the specifications provided by Ahmadraji et al.[145].
5. Challenges and future outlook
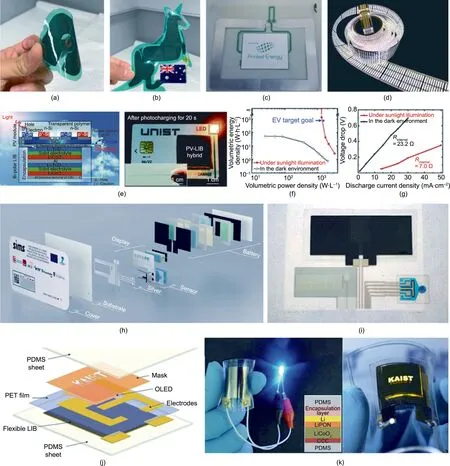
Fig. 13. Potential for monolithic integration of printed batteries. Printed batteries from Printed Energy: (a) printed flexible thin-film batteries,(b) kangaroo-shaped printed batteries, and(c) printed battery integrated with an RFID antenna. Copyright, Printed Energy Pty. Ltd. (d)R2R printed LED lights with potential for integration with printed batteries.Copyright 2020,Nth Degree Technologies.Monolithically integrated SiPV–LIB:(e)internal device structure and operational schematic(left)and photo of the SiPV–LIB-embedded smartcard (right), (f) Ragone plots of the devices under light and in the dark with the EV target goal marked by the star in the plot, and (g) comparison of internal resistances of LIB in SiPV–LIB devices under light and in the dark.(e–g)Reproduced from Ref.[37]with permission.(h)Schematic of an integrated sensor system with complete layer stack and(i)integrated printed sensor(bottom right),display(bottom left),and battery(top).(h,i)Reproduced from Ref.[145]with permission.(j)Schematic illustration of an LIB and OLED integrated system and (k)picture of the integrated device that is turning on an LED (left)and the integrated system with OLED(right). CCC:cathode current collector. (j, k) Reproduced from Ref. [80] with permission.
The printing approach bears great potential for the mass production of batteries with various form factors and characteristics,especially those monolithically integrated into electronic devices.The technology targets applications with moderate energy storage requirements (below 10 mA∙h), cost-sensitive production, often with a variety of form factors, and in some instances mechanical flexibility. The mild operating conditions associated with printing are well suited to handling materials used for flexible devices.The printing process is highly modular and customizable, and benefits from expertise that has been accumulated for centuries.This is a crucial advantage over standardized batteries to meet the ever more specific constraints of the new generation of electronic devices. Implementing a new battery design is facilitated greatly by printing,and the unit price can be kept competitive even for low production volumes related to customized models with R2R manufacture.
These new electronic devices include IoT nodes and other ubiquitous functional components that will sense, record, and exchange information among themselves and with the surroundings.Many of these functionalities can already be printed and integrated monolithically with the battery into the electronic devices.This particularity, although not unique to printed batteries, is not accessible to most of the technologies competing in the thin-film battery markets. Ceramic batteries that can also be monolithically integrated will likely be the main technology competing with printed batteries. Assuming comparable technical performance,the technology with the most promising outlook will be the one with the lowest manufacturing cost and the highest manufacturability. Printed batteries have the potential to be manufactured en-masse by R2R processing and can accommodate a range of nominal voltages, currents, and capacities by tailoring the battery chemistry,geometries,number of battery units,and configurations to meet the specifications of the electronic device. By comparison,the vacuum processes that are used to manufacture ceramic batteries are likely to be more expensive to run and maintain; however, it should be noted that examples of profitable, large-scale vacuum manufacturing processes exist in the industry, even if these processes concern much less complex products such as food packaging. Ahmadraji et al. [145] estimated the price of a POC monolithically integrated diagnosis device that can measure the concentration of cholesterol and H2O2in blood serum.The authors calculated the unit cost of a prototype device manufactured on a laboratory scale to be 5 USD,similar to the price of POC strips available in a pharmacy (between 2 and 3 USD) that still necessitate a separate but reusable meter. They estimated that the unit price can be reduced to 1.5 USD or less on a production scale, sold at 5 USD, and still be competitive, a 230% markup. The healthcare sector has been an appealing target for printed batteries.
Printing integrated devices presents many challenges.The complexity of the physical integration increases exponentially with the number of components that must be compatible in terms of construction material and process requirements. To maximize processability, the integrated device should be manufactured with the minimum number of steps/layers. Each additional step increases the risk of process failure and the costs involved with mitigating this risk.Producing integrated devices can help simplify logistics if all the components are manufactured on-site on the same assembly line.
A more fundamental challenge for printed batteries may involve demonstrating a greater range of electrochemical performance.To the best of our knowledge,all printed batteries commercialized to date have been non-rechargeable zinc–manganese batteries. As for other multivalent ion chemistries, the zinc–manganese reactions are challenging to cycle reversibly.Printed batteries based on rechargeable chemistries, including NiMH and lithium-ion, have been reported in the literature, but no commercial products have been released yet to the best of our knowledge.For both chemistries, the key challenge is the development of printable electrolytes. The NiMH electrolyte is corrosive and can pose compatibility issues with the other materials used in printing.Another formidable challenge facing the use of NiMH chemistry is the requirement for a pressure relief valve,which is common to the design of NiMH batteries using aqueous electrolytes that can decompose into gaseous hydrogen and oxygen, presenting the grave possibility of a bursting hazard. For R2R compatibility, this valve will need to be printable. Lithium-ion chemistry is highly sensitive to moisture contamination,and thus,the electrolyte must be handled in a dry atmosphere. Some reports in the literature have overlooked this fundamental requirement.
One alternative solution would be to develop a printable,rechargeable zinc–manganese battery. In addition to increased safety and raw material availability compared to LIBs, the zinc–manganese chemistry could also be competitive in terms of electrochemical performance. The theoretical capacity of MnO2based on a two-electron discharge mechanism is 617 mA∙h∙g-1[178](3100 mA∙h∙cm-3), and the theoretical capacity of metallic zinc is 820 mA∙h∙g-1[179](5900 mA∙h∙cm-3). Assuming ideally balanced electrode loading (no limiting electrode) and using the equation reported by Ulvestad[180], this equates to 352 mA∙h∙g-1for a full cell without any inactive material,equivalent to 528 W∙h∙kg-1for a nominal voltage of 1.5 V. Assuming 30% v/v of inactive material—which is high even in reference to Ulvestad’s figures that apply to LIBs and typically necessitate greater encapsulation to avoid moisture contamination—and assuming that this equates to 20%w/w because the active component is entirely metal-based and therefore denser than the inactive component that is a mixture of inorganic and organic materials, the energy density at the battery level could be as high as 420 W∙h∙kg-1—similar to Ulvestad’s figures for future LIBs based on a liquid electrolyte and a lithium metal anode.
Acknowledgments
Financial support from the Cooperative Research Centres Projects (CRC-P) grant and Australian Research Council through its Linkage and Laureate Fellowship programs are acknowledged. Dr.Miaoqiang Lyu acknowledges financial support from Advance Queensland Industry Research Fellowships (AQIRF) organized by the Queensland government, Australia. Benoit Clement acknowledges financial support from the Research Training Program scholarship provided by the Australian government and the Research Higher Degree Top-up scholarship provided by the CRC-P.The support from the Dow Centre for Sustainable Engineering Innovation and the University of Queensland is kindly acknowledged. The authors acknowledge the contributions of Dr. William Ray, Chief Technical Advisor at Printed Energy, Dr. Mu Xiao, Researcher in the Wang group at the University of Queensland, and Mr. Rodger Whitby, CEO of Printed Energy.
Compliance with ethics guidelines
Benoit Clement, Miaoqiang Lyu, Eeshan Sandeep Kulkarni,Tongen Lin, Yuxiang Hu, Vera Lockett, Chris Greig, and Lianzhou Wang declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Electric Air Taxis Create Megadeal Buzz
- Tissue Engineering and Regulatory Science
- Factors Predicting Progression to Severe COVID-19: A Competing Risk Survival Analysis of 1753 Patients in Community Isolation in Wuhan,China
- A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses
- Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers
- Past and Future Changes in Climate and Water Resources in the Lancang–Mekong River Basin: Current Understanding and Future Research Directions

