Tailoring Resorption Rates and Osteogenic Response in Xeno-Hybrid Bone Grafts: The Effect of Added Gelatins
Hao Zhu, Håvard Jostein Haugen*, Giuseppe Perale, Janne Elin ReselandLieert Parreiras Nogueira Antonio Gonzalez Cantalapiedr Fernando Maria Guzon Muñoz,Maria Permuy Mendañ Felie Betge, Ståle Petter Lyngstadaas Jun Xiao*
a Department of Orthopedic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
b Department of Biomaterials, Institute of Clinical Dentistry, University of Oslo, Oslo 0317, Norway
c Industrie Biomediche Insubri SA, Mezzovico-Vira 6805, Switzerland
d Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, Vienna 1200, Austria
e Faculty of Biomedical Sciences, University of Southern Switzerland, Lugano 6900, Switzerland
f Faculty of Veterinary, University of Santiago de Compostela, Lugo 27002, Spain
Keywords:Bone graft Xeno-hybrid Gelatin source Resorption Bone regeneration
ABSTRACT Bone defects resulting from trauma, surgery, congenital malformations, and other factors are among the most common health problems nowadays. Although current strategies such as autografts and allografts are recognized as the most successful treatments for stimulating bone regeneration, limitations such as graft source and complications still exist. SmartBone® is a xeno-hybrid bone graft (made from bovine bone matrix,poly(L-lactic-co-ε-caprolactone),and gelatin)with a positive clinical record for bone regeneration.In this study,the formulation for designing xeno-hybrid bone grafts using gelatins from different sources (bovine- and porcine-derived gelatin, with bone grafts named SBN and SPK, respectively) was investigated, and the biological responses were evaluated in vitro and in vivo. The results demonstrate that gelatins from both bovine and porcine sources can be loaded onto SmartBone® successfully and safely, withstanding the aggressive manufacturing processes. Different bone cell responses were observed in vitro. SBN was found to enhance osteocalcin secretion while SPK was found to upregulate osteopontin from human osteoblasts.In vivo,both bone grafts promoted osteogenesis,but SPK degraded earlier than SBN. Our findings suggest that SBN and SPK provide different yet comparable solutions for optimizing the bone resorption and regeneration balance. These xeno-hybrid bone grafts possess ideal potential for bone defect repairing.
1. Introduction
Bone defects resulting from trauma, surgery, congenital malformations,and other factors are among the most common health problems nowadays [1]. Although bone is a richly vascularized tissue, under some circumstances, such as critical-sized defects,the self-healing ability is insufficient to fully repair the damaged area, and complications such as non-union or pseudarthroses may occur [2]. Implants made of titanium and alloys have exhibited positive clinical results in bone defect treatment. However, most of these materials are non-degradable [3]. Moreover, in severe cases, bone substitutes are not functional enough for large and irregular defect treatment, such as critical bone loss in hip revision, where the restoration of bone stock is of prior importance[4].
A frequently used strategy to treat bone defects is to utilize bone grafts for promoting tissue regeneration. Bone grafts are among the most successful treatments, as they can provide an environment with osteoconductive,osteoinductive,and osteogenic effects that promotes bone healing [5]. Autografts, which are tissues transplanted from one area on an individual to another area on the same individual, are still the ‘‘gold standard” in treating bone defects [6,7]. However, this method has disadvantages such as donor site morbidity and limited supply, and it is difficult in pediatric cases[8,9].Moreover,some patients may experience persistent pain at the donor site for two years after surgery[10].Allografts—that is, bone transplants from different human donors—allow the treatment of larger defects.In fact,impacting bone grafting and structural bone grafting are still recognized as efficient approaches for hip revision [11,12]. Nevertheless, both ethical and regulatory concerns are potential barriers, and the donor source is limited for large-scale manufacturing [13]. Furthermore,potential risks such as immunogenic reactions and the transferal of contagious diseases cannot be neglected.
Therefore, researchers have suggested using alternative bone grafts from different sources,including bone grafts from other species, such as xenografts, and synthetic grafts (e.g., biopolymers,bioceramics, and their composites), to resolve the dilemma [14–19]. A proper bone substitute should resemble the natural bone tissue as much as possible, including the mechanical properties,structural characteristics,and biological functions[8,20].As a bone graft with clinical transformation potential, it should be biologically safe and readily accessible. More importantly, it should have osteoinductive and/or angiogenic potential,no restrictions in graft sizes,a long shelf life,and reasonable cost[21].Synthetic grafts are relatively easier to acquire,and the structures are tunable to some degree. However, they are still limited in terms of biological performance and mechanical properties in comparison with bone grafts. More importantly, implanted grafts generally undergo a process of resorption and remodeling after implantation. An appropriate balance between resorption and volume maintenance should be well tuned to achieve ideal bone remodeling. Consequently,naturally sourced bone grafts are still of great importance and interest because they are associated with a better host response.
Bovine-derived cancellous bone grafts are regarded as having a similar structure to human bone; they are also inexpensive and easily available in large amounts, making them ideal for producing xenografts [22]. However, the essential manufacturing process, including defatting, decellularizing, and deproteinizing,normally affects the toughness of the graft and the overall mechanical performance [23]. Synthetic polymers and biomolecules are available choices for preparing and reinforcing biomaterials [24–26]. Therefore, the idea of xeno-hybrid bone grafts has been introduced to treat bone defects, by combining a natural bone mineral matrix with synthetic materials [20]. A typical product is SmartBone®, which is manufactured by coating the deproteinized bovine bone matrix with a biocompatible polymer coating (poly(L-lactide-co-ε-caprolactone), also called PLCL) and bioactive molecules (arginine–glycine–aspartic acid (RGD)-exposing collagen fragments from animal-derived gelatin) to create a biomaterial that has similar characteristics to natural bone[27–30]. The mechanical properties and biological performance can be improved with the addition of the two aforementioned materials [27,30].
In this study, we investigated the formulation for designing xeno-hybrid bone grafts using gelatin from different sources. The commercially available xeno-hybrid bone graft SmartBone®(manufactured by I.B.I. SA, Switzerland) was used in this study. The manufacturing process of SmartBone®strictly followed that in previous studies [27,30]; however, in this case, the grafts were made using two different sources of gelatin: bovine- and porcinederived gelatin. The two different xeno-hybrid bone grafts were characterized using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and micro-computer tomography(micro-CT).After evaluating their cell responses in vitro with human osteoblasts, we implanted the grafts in pig skulls and assessed their host responses and bone regeneration potential at Weeks 8 and 16 after surgery.
2. Materials and methods
2.1. SmartBone® development
Commercially available xeno-hybrid bone graft SmartBone®was used in this study. This material is mainly composed of a bovine bone-derived mineral matrix, which is improved by reinforcement with a co-polymer coating of PLCL and the addition of RGD-exposing collagen fragments from animal-derived gelatin.The detailed manufacturing process of SmartBone®has been described in previous studies[27,30];however,in the present case,two types of gelatin utilized in the bone graft preparation:bovineand porcine-derived gelatin, and the bone grafts were named SBN and SPK, respectively. Both gelatins are hydrolyzed, with a bloom grade of (120 ± 10) g, manufactured following the standards of the Gelatin Manufacturers of Europe†† https://www.gelatin.org/gme.html., sourced from duly certified food-chain animals, and accompanied by the certifications of European Directorate for the Quality of Medicines. Bovine gelatin was sourced from bone and supplied by Merck KGaA under Ph.Eu.7 compliance (Emprove Pharma-Grade Raw Materials Division, Germany),while porcine gelatin was extracted from hides and skin by Gelita Medical GmbH (Germany). Both gelatins mainly consisted of collagen type I fragments (> 80%) [31], which are a known source of RGD terminals and are commonly used in tissue engineering and the medical devices industry.
2.2. Graft production, samples release, and characterization
SBN and SPK samples with different sizes were produced.Cubic samples (10 mm × 10 mm × 10 mm) and cuboid samples(10 mm × 10 mm × 30 mm) were used in mechanical tests. Samples that were 2 mm in height and 13 mm in diameter were used for in vitro testing, and samples that were 4 mm in height and 16 mm in diameter were used for in vivo evaluation.All the batches were released following the same SmartBone®standard release procedures, under current Good Manufacturing Practice (c-GMP)and ISO13485–2016 compliance (Industrie Biomedicine Insubri SA, Switzerland).
The sample surface was examined by SEM (Hitachi Analytical Table Top SEM TM3030; Hitachi, Japan) with backscattered electron at 15 keV.All samples were sputtered and observed at two different magnifications (100× and 600×; n = 5). Elemental analysis was done on five selective areas of 637 μm × 381 μm with both mapping and quantifiable measurements, with the sample being tilted at 45° (Quantax70; Hitachi). The acquisition time was set to 60 s and the X-ray counts had an average of 1.7 kilocounts per second(kcps).The concentrations of different elements were given in atomic percentages.
Mechanical tests were performed using a Zwicki (ZwickRoell GmbH & Co. KG, Germany) universal testing machine with testXpert (ZwickRoell GmbH & Co. KG) software equipped with a 10 kN calibrated load cell, while compressing the specimen between two parallel plates at a constant speed of 1 mm∙min-1under displacement control. The flexural strength was calculated according to the formula σ = 3FL/2bh2(MPa), where F is the maximum load, L is the length of the support span, b is the width,and h is the thickness of the sample.
Chemical characterization was carried out using attenuated total reflection–Fourier transform infrared spectroscopy (ATR–FTIR; Spectrum 100; Perkin-Elmer Instruments, USA). Five specimens of each material were scanned at the mid-infrared range,with a resolution of 2 cm-1and 64 scans in transmission. In order to provide optimal contact, the ATR crystal cubic sections(3 mm×3 mm×3 mm)of the bone grafts were crushed to powder and placed upon the ATR crystal.Each powder had the same pressure exerted upon it, as controlled by the infrared (IR) software(Perkin-Elmer Instruments). Each spectrum was post data treated with automatic baseline correction and data tuning(QUANT+software; Perkin-Elmer Instruments). The average of five spectra was presented for each sample.
All groups were scanned with the micro-CT system (Bruker microCT1172,Belgium)with an aluminum copper filter.The scanning parameters were set as follows: 104 μA, 95 kV, 0.400° for image rotation, and 7.9 μm in resolution. Sectional images were reconstructed to three-dimensional (3D) models in the software CTvox (Bruker), and snapshots were then obtained. The structural parameters including total porosity, surface/volume ratio, surface density, intersection surface, structure thickness, and structure separation were calculated using the software CT Analysis(Bruker).
2.3. Cell culturing
Commercially available normal human osteoblasts (Lonza,Germany)from three different donors were utilized for cell experiments.Table 1 shows the detailed information of the donors.Cells were cultured in osteoblast growth medium(Promocell,Germany)in a standard cell culturing environment under a 37 °C humidified atmosphere with 5% carbon dioxide (CO2). Cells between Passages 5 and 7 were used for carrying out experiments. The culturing medium was changed every three days for cell expansion.To summarize the detailed experimental process, the bone graft samples were first placed in 24-well plates,with four repetitions,and a suspension of 8×104cells with a volume of 200 μL was dropped onto the surface of each bone graft.After 30 min of incubation,a full volume of the culturing medium was gently added, minimizing the irritation on the attached cells.The medium was changed on Days 2, 5, and 7 of each week, and the whole experiment lasted for 28 days.On Days 2,7,14,21,and 28,which were all two days after the medium change, the medium was collected for later proteins quantification.
2.4. Determination of cell viability
Lactate dehydrogenase(LDH)activity was used to indicate cytotoxicity of the bone grafts to human osteoblasts. Cells cultured in growth media and treated with phosphate buffered saline (PBS)were set as the negative control, and cells treated with 1% Triton X-100 were used as the positive control. The reaction mixture was prepared in advance with a catalyst and dye solution at a ratio of 1:45 (Roche Diagnostics, Germany). After cell attachment and 48 h of culturing, 50 μL of medium was pipetted into another new well plate and mixed with 50 μL of reaction mixture.The samples were incubated at room temperature for 30 min and then read in an enzyme-linked immunosorbent assay (ELISA) reader at 490 nm. The results were presented relative to the control by calculating the optical density (OD) value using the following equation:

2.5. Confocal microscopy
Confocal microscopy was performed on Days 2,7,14,and 28 to observe the cell behaviors. At the designed time points, the samples were washed with PBS three times and fixed in 4%paraformaldehyde for 12 min. After being washed with PBS again,the samples were stained with Alexa 488-labeled phalloidin for 1 h and with 4′,6-diamidino-2-phenylindole (DAPI) for 20 min. The confocal images were taken using laser scanning confocal microscopy (Leica TCS SPE Microsystems Wetzlar GmbH, Germany)and analyzed using Image J software(National Institutes of Health,USA).
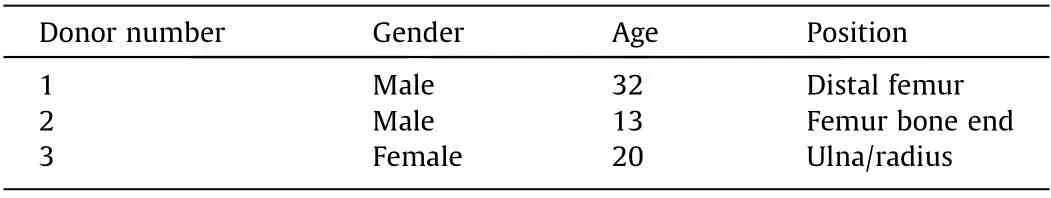
Table 1 Information about the different donors.
2.6. Quantification of specific extracellular proteins
On Days 2,7,14,21,and 28,the culturing medium in each well was collected.Multi-Analyte Profiling(xMAP)of protein levels was performed on the Luminex 200 system (Luminex, USA) for each medium sample. Bone Metabolism Multiplex Assay (Human Bone Magnetic Bead Panel;MILLIPLEX,Germany)was used in this study,which provided the quantification of osteocalcin(OC),osteopontin(OPN),osteoprotegerin(OPG),Dickkopf–related protein-1(DKK-1),sclerostin(SOST),interleukin-6(IL-6),and tumor necrosis factor–α(TNF-α). The acquired fluorescence data were analyzed in xPONENT 3.1 software (Luminex). All processes were completed according to the manufacturer’s protocols.
2.7. Animal surgery
The experimental phase of the study was carried out at the Veterinary Teaching Hospital in the University of Santiago in Lugo,Spain. The study protocol was approved (proceeding code: CEBIOVET (AE-LV-001)) by the Ethical Committee of the Rof Codina Foundation(Lugo,Spain).The investigation was conducted according to Spanish and European Union regulations(European Communities Council Directive 86/609/EEC) on experimental in vivo experimentation. Hybrid pigs from breeding farm, Spain (age: 3–4 months, quarantine period: 21 days), were used for in vivo evaluation. The animals were kept as a group in an area with natural light, air renewal, and regulated temperature, and were fed using a specific granular diet for their species with free water supply.The animals were premedicated with ketamine (10.0 mg∙kg-1),midazolam(0.5 mg∙kg-1),morphine(0.5 mg∙kg-1),and meloxicam(0.2 mg∙kg-1) by intramuscular injection for pain control under veterinary care.The surgical procedure was carried out under general anesthesia induced with propofol(2–4 mg∙kg-1;Abbott Laboratories, UK) by intravenous injection and maintained with iso/sevoflurane (Schering-Plow, Spain). During anesthesia, the electrocardiography, capnography, pulse oximetry, and noninvasive blood pressure were all monitored. The pigs were monitored daily and during the interventions by a veterinarian trained and accredited in the science of laboratory animals (categories B or C,functions a, b, and c). To summarize the detailed surgical procedure, a longitudinal incision was made at the level of the frontal bones and the muscles were reflected.Then,four perforations were made at the level of the frontal and parietal bones under continuous irrigation with sterile saline.The disc samples(4 mm in height and 16 mm in diameter), with four repetitions, were then implanted in the defect site. All the defects were covered with a bovine pericardium membrane (Tutogen Medical GmbH, Germany) and immobilized with five titanium screws, which were also important for accurate guidance in later micro-CT and histology analyses (Figs. 1(a)–(d)). Muscular, subcutaneous, and skin tissue were then gently closed. Antibiotic prophylaxis was administered subcutaneously once a day for one week using amoxicillin(20 mg∙kg-1). Cone beam computer tomography (CBCT) images were obtained immediately after surgery, after 8 weeks, and after 16 weeks. After previous sedation, the animals were sacrificed by an overdose of pentobarbital (40–60 mg∙kg-1) by intravenous injection after 8 and 16 weeks. The skulls were dissected and the bone blocks with the defects under study were obtained, fixed in a 4% paraformaldehyde for 4–7 days at 4 °C.
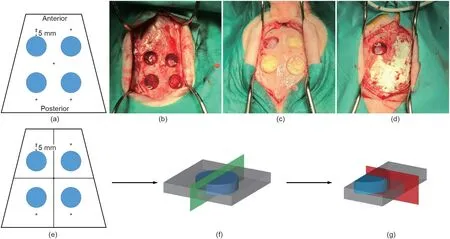
Fig. 1. Animal surgery illustration. (a–d) Surgery design: (a) four full thickness defects were created in the cranium with five titanium screws as reference; (b) defects creation;(c)implantation of samples;and(d)pericardium membrane fixation with titanium screws.(e–g)Histological sectioning method:(e)samples were first cut into four pieces; (f) each piece was halved according to the green plane; and (g) slices were sectioned according to the red plane.
2.8. Micro-CT analysis
The fixed pig skulls were scanned in a micro-CT system with an aluminum copper filter. The scanning parameters were set as follows: 104 μA, 95 kV, 0.400° for image rotation, and 7.9 μm in resolution. After reconstruction, the region of interest (ROI) was relocated with the guidance of the previously implanted titanium screws.A cylindrical ROI of 16 mm in diameter and 4 mm in thickness was made in each sample for quantification. To study the effect of the samples on the space exceeding the defect area,another ROI with an expanded height of 5 mm (2.5 mm on both sides) was created. Morphometrical parameters including bone volume ratio(BV/TV),bone surface/volume ratio(BS/BV),bone surface density (BS/TV), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) were calculated. BV/TV was also calculated for the rings with different distances from the defect center, 1 mm per step in diameter, to build the BV/TV profile of the defect area.
2.9. Histological analysis
The acquired skulls were first cut into four pieces along the cross line with the titanium screws as reference, so that the experimental area was not damaged (Fig. 1(e)). For each piece of bone,a cut was first made through the center of the defect to halve the samples, then lateral sectioning was performed on the halved sample (Fig. 1(f)). The samples were dealt with according to standard hard-tissue sectioning protocol. In brief, samples were dehydrated stepwise with ethanol and embedded in methyl methacrylate (MMA) resin. The section plane was perpendicular to the surface of the sample along the line from the center to the border (Fig. 1(g)). Van Gieson’s staining was performed after sectioning.
2.10. Statistical analysis
Datasets were run for normality tests first. The results were expressed as mean ± standard deviation (SD). For multiple comparisons among groups,one-way analysis of variance(ANOVA)and Tukey’s tests were utilized, while two-way ANOVA and Bonferroni post-tests were applied when the results from time points were collected. Statistical analysis was carried out in Statistical Product and Service Solutions software (SPSS12; IBM SPSS, USA). Significant differences were set at p < 0.05.
3. Results
3.1. Basic characterizations of SBN and SPK
The SEM results showed microstructures of spongy bone for both SBN and SPK, with no morphological significant difference between them. Energy dispersive X-ray spectroscopy (EDX)showed that all the elements, including calcium, oxygen, carbon,nitrogen, and phosphorus, were homogenously distributed(Fig. 2); no significant difference was found in elemental quantification(Table 2).Because nitrogen was only present in the mineralized matrix and PLCL, it can be regarded as a gelatin marker,indicating a homogenous distribution of gelatin on the graft surface.
3.2. Mechanical test of SBN and SPK
The compressive properties of SBN and SPK were evaluated. As shown in Table 3, the maximum compressive stress, compressive modulus, and flexural strength of the SBN and SPK had no significant differences.
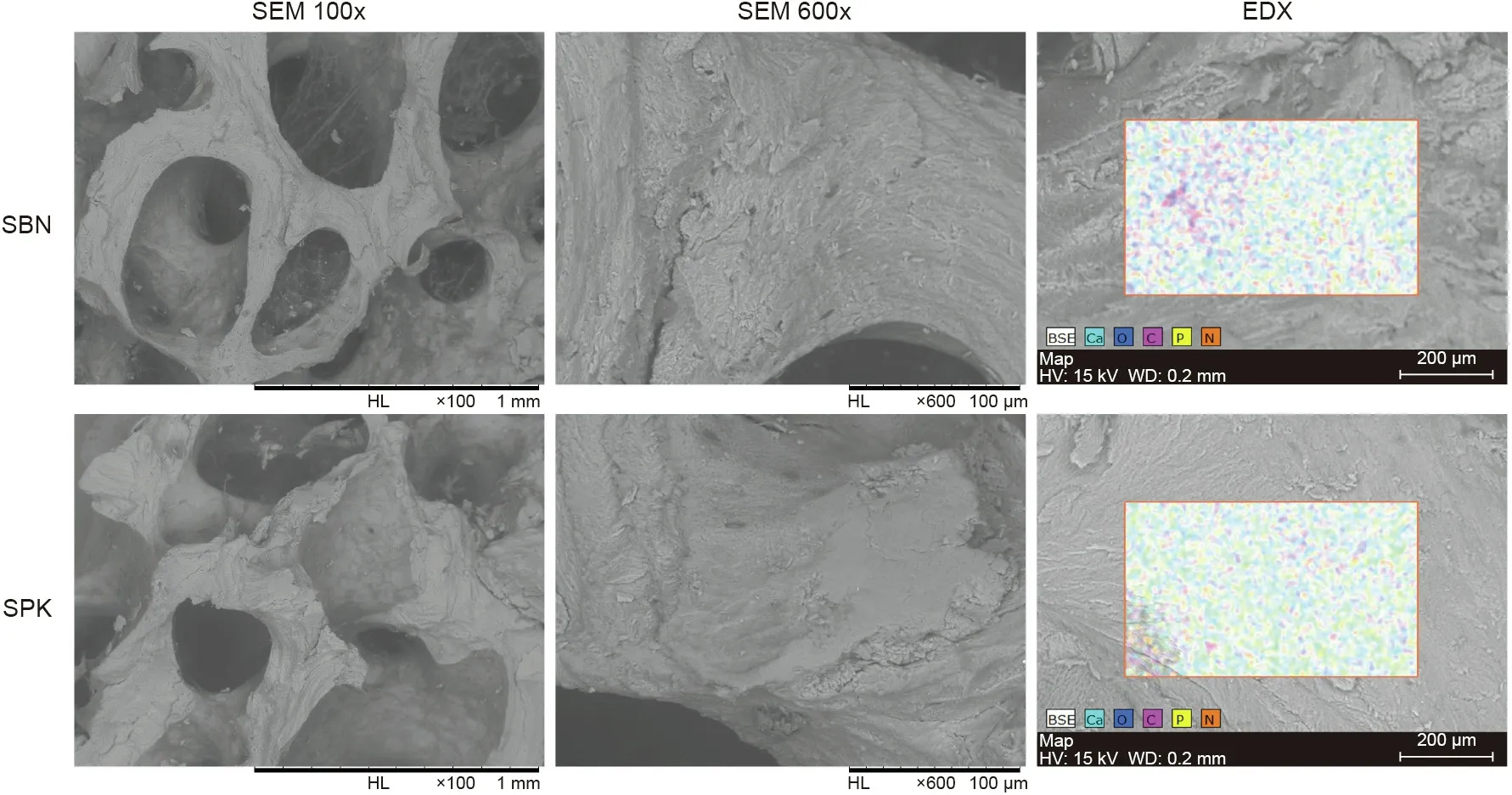
Fig. 2. Representative images of the SEM and EDX results for SPK and SBN. HL: high resolution lens; HV: high voltage; WD: work distance; BSE: backscattered electrons.
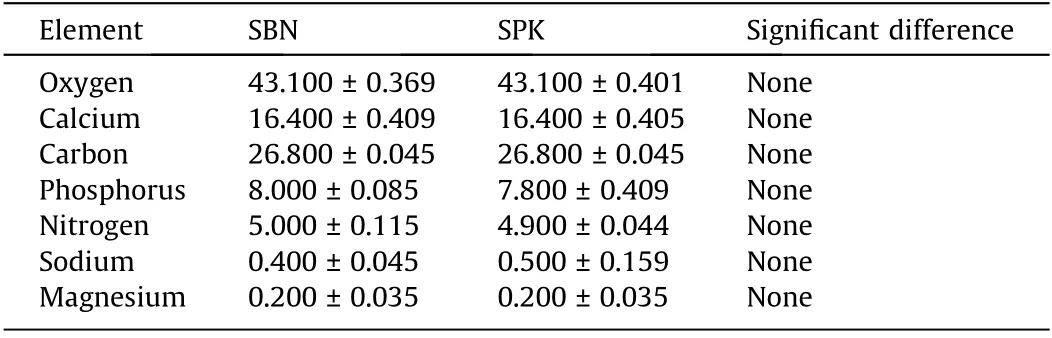
Table 2 Elemental quantification of SBN and SPK groups analyzed by EDX.
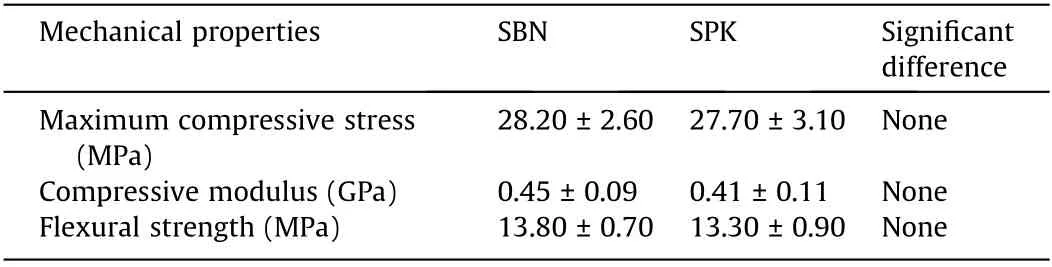
Table 3 Compressive properties of SBN and SPK (n = 6).
3.3. Chemical characterization of SBN and SPK
Fig. 3 presents the average of five different spectra for each sample and reveals no major difference in organic structure between SPK and SBN. The presence of PLCL polymer coating of both SBN and SPK is demonstrated by peaks: 3450 cm-1(–OH groups); 2950 cm-1(asymmetric CH3stretching); 1410, 2030,and 2160 cm-1(carbonyl); and 1640 cm-1(C–O–C). The mineral nature of the matrix is shown by the presence of phosphate groups with peaks at 600 and 1050 cm-1, which are typical of hydroxyapatite.Gelatin is demonstrated by the characteristic protein bands at 1651 cm-1(amide I)and 1551 cm-1(amide II).Therefore,it can be seen that the polymer and gelatin were effectively deposited for both the SPK and SBN bone matrix, and that there were no chemical differences between the tested samples.
3.4. Structural characterization of SBN and SPK
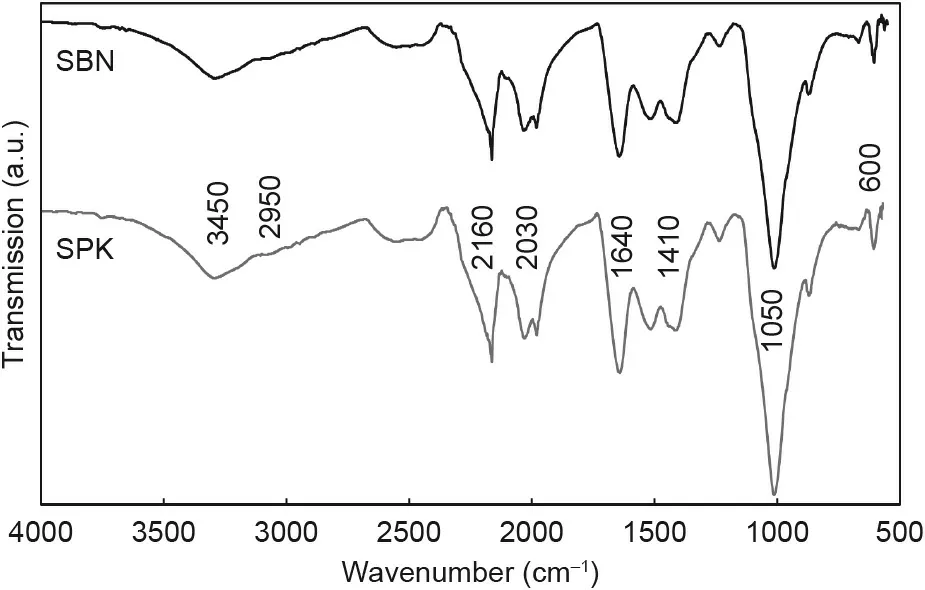
Fig.3. Average FTIR spectra from five samples of SBN and SPK,displaying identical chemical structures. Major infrared peaks have been labeled.
The micro-CT results displayed no structural difference between the groups in terms of their total porosity,surface/volume ratio,surface density,intersection surface,structure thickness,and structure separation (Table 4). The pores inside the bone grafts were accessible through each other, and no closed pores were detected.Since the organic matter and mineralized matrix had different attenuation on the X-ray, we labeled them with different colors for better observation.The organic matter,PLCL,and gelatin were uniformly spread among the pores of the graft matrix, without affecting the open interconnectivity (Fig. 4).
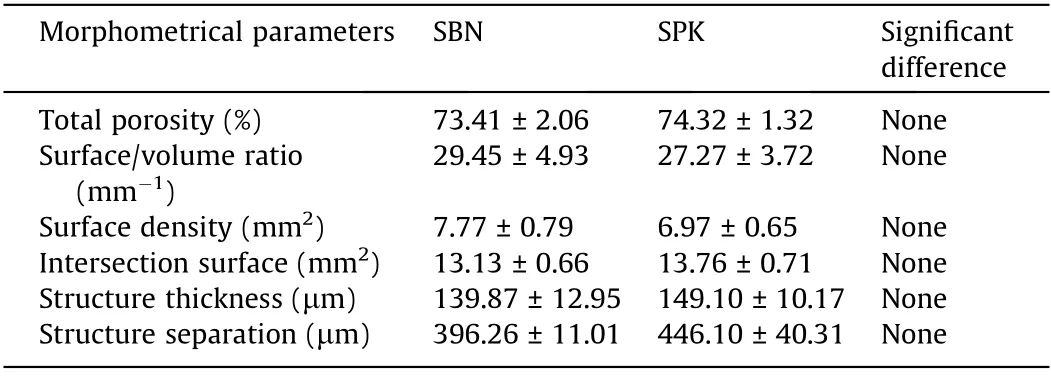
Table 4 Micro-CT analysis of the SBN and SPK groups.
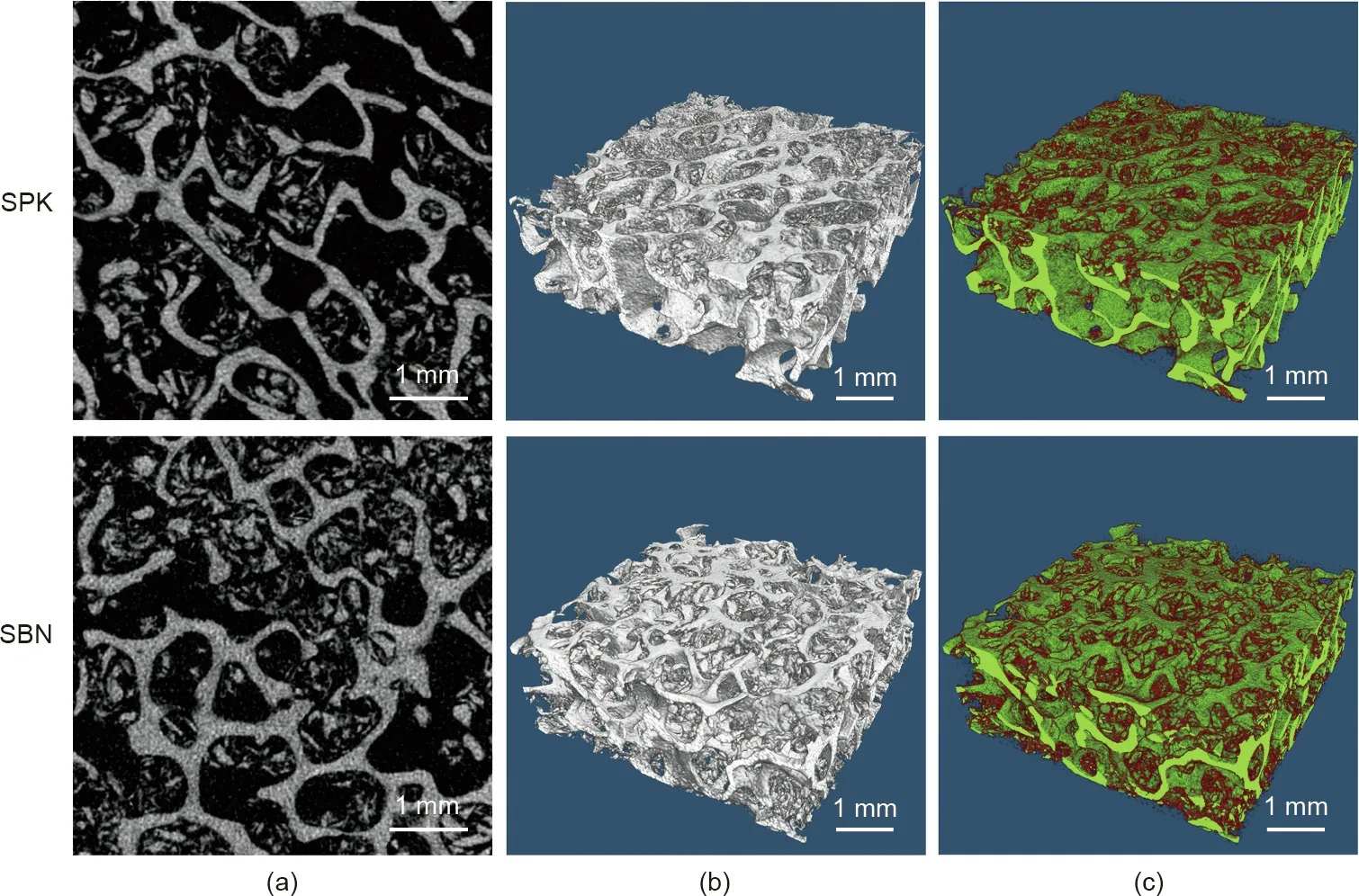
Fig.4. Representative images from the micro-CT analysis.(a)Sectional images;(b)3D reconstructed models;and(c)3D reconstructed models with color labels.The red color indicates organic matter (PLCL and gelatin) and the green color indicates the mineralized matrix.
3.5. Cytotoxicity test of SBN and SPK
No difference was detected in the cytotoxic effects of the two groups with osteoblasts from three different donors. Cell viability was higher in Donors 1 and 3 than in Donor 2 (Fig. 5).
3.6. Cell behavior on SBN and SPK
Cell behavior was observed via laser scanning confocal microscopy. The cytoskeleton was stained with Alexa488-labeled phalloidin (green) and the nuclei was stained with DAPI (blue). In the initial stage, enhanced cell attachment on SPK could be detected after two days for all donors tested, whereas the cells cultured on SBN continued to have round shapes. Compared with the SBN group, strong proliferation effects were detected in the SPK group after 14 days, and a large number of cells were subsequently observed after 28 days. The three donors exhibited similar trends(Fig. 6 and Figs. S1 and S2 in Appendix A). Further quantification in the form of the cell coverage ratio (actin area/graft area) was performed. Significant differences were found in Donor 1 on Days 7,14,and 28;in Donor 2 on Days 2 and 28;and in Donor 3 at each time point (Fig. 7).
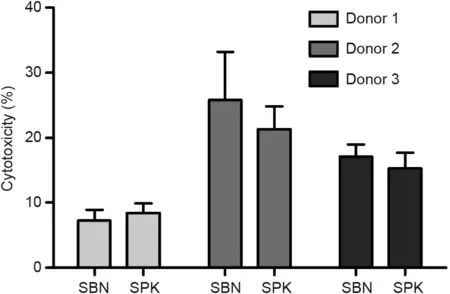
Fig. 5. Cytotoxic effect after 48 h of culturing human osteoblast cells from three different donors on SBN and SPK using an LDH activity assay.
3.7. Extracellular protein quantification of cells cultured on SBN and SPK
In regard to the osteogenic markers, OC generally exhibited higher expression in SBN than in SPK. Fig. 8 shows significant differences in OC on Days 2 and 7 in Donor 1;on Days 2,21,and 28 in Donor 2; and at all time points (except Day 21) in Donor 3. However, the cells cultured on SPK secreted more OPN and OPG compared with the cells cultured on SBN. A significant increase was detected for OPN at later time points,such as after 21 and 28 days in SPK in all three donors.Donors 1 and 2 also had statistical differences in OPN on Day 14. The OPG secretion from cells on SPK was generally higher than that on SBN, but a significant difference could only be observed in Donor 1 on Days 7, 14, and 21; and in Donor 2 on Day 28. For DKK-1 and SOST, few differences were noticed, with the only statistical difference found on Day 2 for SOST in Donor 2.Regarding the secretion of IL-6,significant difference could only be found on Day 7 of culturing in Donor 2 when comparing the two groups. Concerning TNF-α, the secretion was kept at a low level during the whole process for both SBN and SPK. All three donors showed similar trends during the whole experiment (Fig. 8).
3.8. Animal experiments and CBCT evaluation of the healing results
Hybrid pigs were used to create the bone defect model for the in vivo evaluation. CBCT images were obtained immediately after the operation,as well as at 8 and 16 weeks after the surgery.After eight weeks of implantation, the bone defect areas with SBN and SPK were covered with new bone, while the control group remained half unfilled(Fig.9).After 16 weeks of healing,the defect site was still noticeable in the control group,while the SBN and SPK groups displayed signs of full healing.
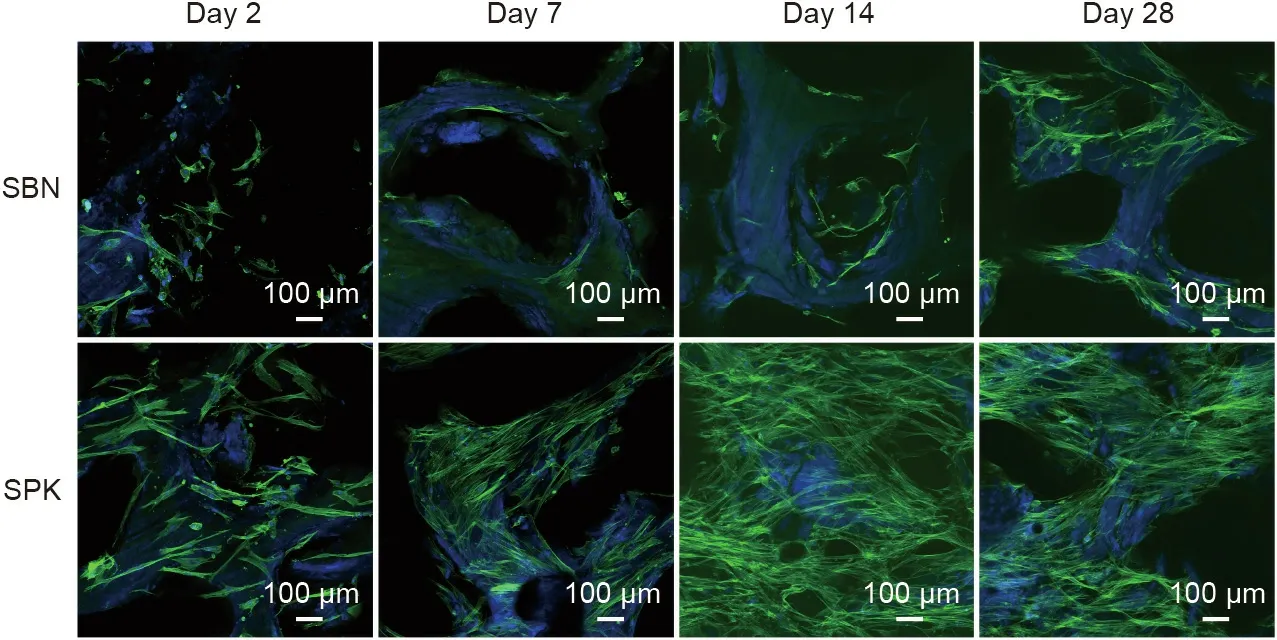
Fig. 6. Representative images of osteoblast cell behavior from Donor 1 taken by laser scanning confocal microscopy on Days 2, 7, 14 and 28.
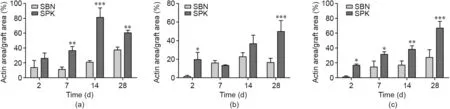
Fig. 7. Quantification of the cell coverage ratio of the bone grafts, i.e., actin area/graft area. (a) Donor 1; (b) Donor 2; and (c) Donor 3. *p < 0.05; **p < 0.01; ***p < 0.001.
3.9. Micro-CT evaluation after in vivo experiments
Upon examining the reconstructed models, it was noticeable that, after eight weeks of healing, the SBN samples were not fully degraded, while the residue grafts in the defects filled with SPK samples were undetectable, indicating complete resorption. After 16 weeks of healing, the defects filled with SBN and SPK could not be detected due to new bone formation at the site (Fig. 10).
A cylindrical ROI of 16 mm in diameter and 4 mm in thickness was made in each sample for quantification (Fig. 11). To study the effect of the samples on the space exceeding the defect area,another ROI with an expanded height of 5 mm (2.5 mm on both sides) was created (Figs. 11(a) and (b)). After 16 weeks of healing,significant differences were observed in BV/TV between the groups. The SBN group exhibited the highest BV/TV, with a significant difference compared with the control and SPK groups.The SPK group also exhibited a significantly higher BV/TV compared with the control group (Fig. 11(e)). No significant difference was detected in terms of BS/BV(Fig.11(f)).A significant difference was also detected in BS/TV when comparing the SPK group with the control group after eight weeks of healing, and when comparing both treated groups with the control group after 16 weeks of healing (Fig. 11(g)). Regarding trabecular parameters, the SPK group was statistically lower in terms of both Tb.Th and Tb.Sp when compared with the control group after eight weeks of healing (Figs. 11(h) and (i)). The SBN group also exhibited the lowest Tb.Sp after 16 weeks of healing, with significant differences compared with the control and SPK groups (Fig. 11(i)).
Concerning the analysis of the extended ROI with 5 mm of height, although both the SBN and SPK groups demonstrated increased BV/TV levels after 16 weeks of healing, a significant difference was only detected when comparing the SBN and control groups (Fig. 11(j)). The results of BS/BV, BS/TV, Tb.Th, and Tb.Sp generally showed trends similar to the results from the ROI with 4 mm of height (Figs. 11(k)–(n)).
When observing the BV/TV variation profile,it was seen that the SBN and SPK groups generally exhibited better bone volume compared with the control group.After eight weeks of healing,the SBN group had higher bone volume than the SPK group near the defect boundary, while the SPK group promoted more bone formation than the SBN group close to the center. After 16 weeks of healing,both the SBN and SPK groups exhibited evenly distributed BV/TV, with higher values for the SBN group than the SPK group(Figs. 11(c) and (d)).
3.10. Histological evaluation of the defect area
After eight weeks of healing,new bone formation was detected in the SBN and SPK groups,with full coverage of bone tissue on the defect area close to the bottom side (brain side). Residue particles from the bone graft could still be detected on the top(Fig.12).After 16 weeks of healing, a full thickness of newly formed bone tissue was observed in the SPK group, with total resorption of the bone grafts. However, SBN did not display as well as SPK in terms of degradation. In the control group, bone tissue was also formed,albeit not as much as in the treated groups. In the SPK group, the bone structure maturation was from the center to the border, as the trabeculae close to the center was generally thicker than that close to the border,with a noticeable boundary between the newly formed bone tissue and the defect margin.
4. Discussion
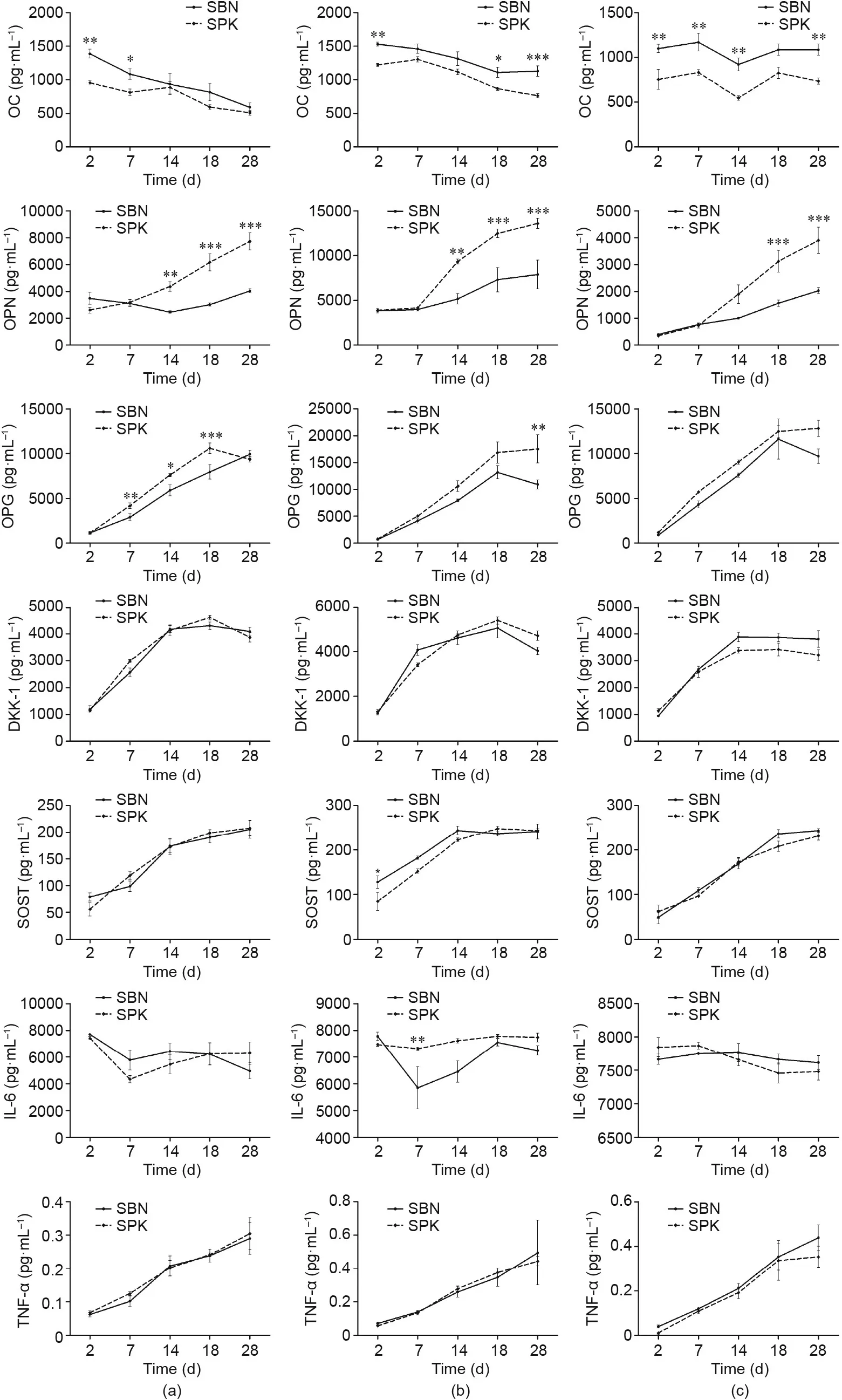
Fig. 8. Quantification of specific extracellular proteins. (a) Donor 1; (b) Donor 2; and (c) Donor 3. *p < 0.05; **p < 0.01; ***p < 0.001.
SBN establishes a microstructure similar to that of healthy human spongy bone, and the polymeric reinforcement of SBN provides better osteointegration compared with a bovine-based xenograft [30,32]. When analyzing the polymer coating on SPK,we confirmed that the microstructure was comparable to that of SBN.In addition,the gelatin was homogenously distributed,as uniform spread was observed during EDX analysis. This is a statistically homogeneous coating structure, which is meant to mimic natural tissues that do not display strictly homogenous structures[33].
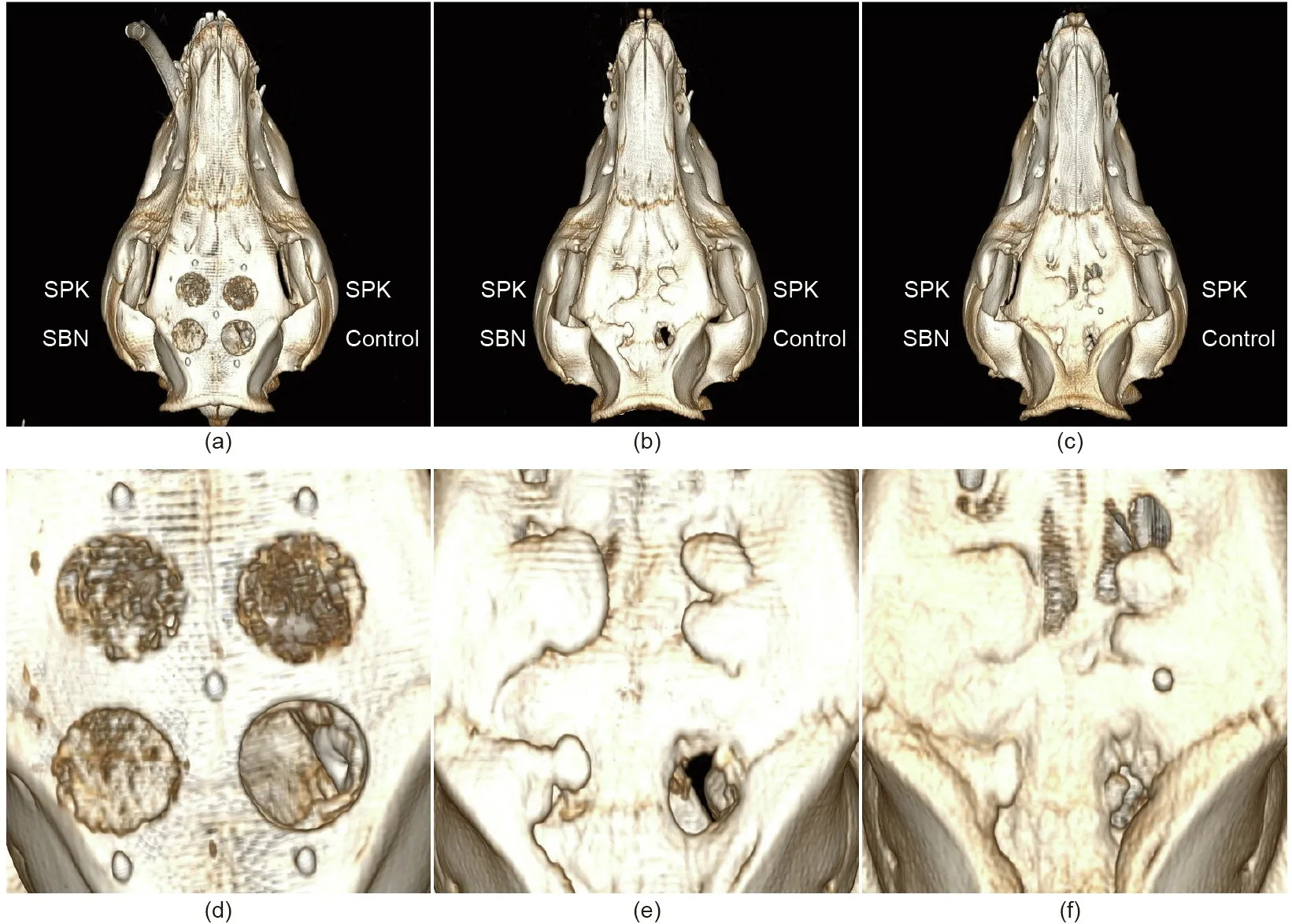
Fig. 9. Representative images of CBCT analysis at different time points. (a, d) Immediately after surgery; (b, e) 8 weeks after surgery; and (c, f) 16 weeks after surgery.
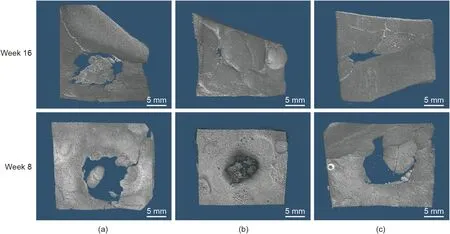
Fig. 10. Representative images of micro-CT 3D reconstruction of the defect area in (a) the control group, (b) the SBN group, and (c) the SPK group after 8 and 16 weeks of healing.
Both SBN and SPK exhibited satisfying structural features, as confirmed by micro-CT analysis. The addition of different types of gelatin did not affect the pore structure of the bone grafts. Both SBN and SPK had ideal porosity and pore size, with all pores open and accessible through interconnection. The coating made of biopolymers and gelatin was statistically homogenously distributed among the pores without affecting the connectivity. This combination provided an ideal environment for cells to attach,allowing for cell migration and proliferation.
Changing the gelatin source did not significantly affect the cytotoxicity of the grafts,although the donors differed in their viability.It was observed that SPK had a more favorable microenvironment for osteoblasts to adhere and proliferate than SBN, not only at the initial stage but also at the later time points. The quantification results further proved that a high percentage of the SPK surface was covered with cells.
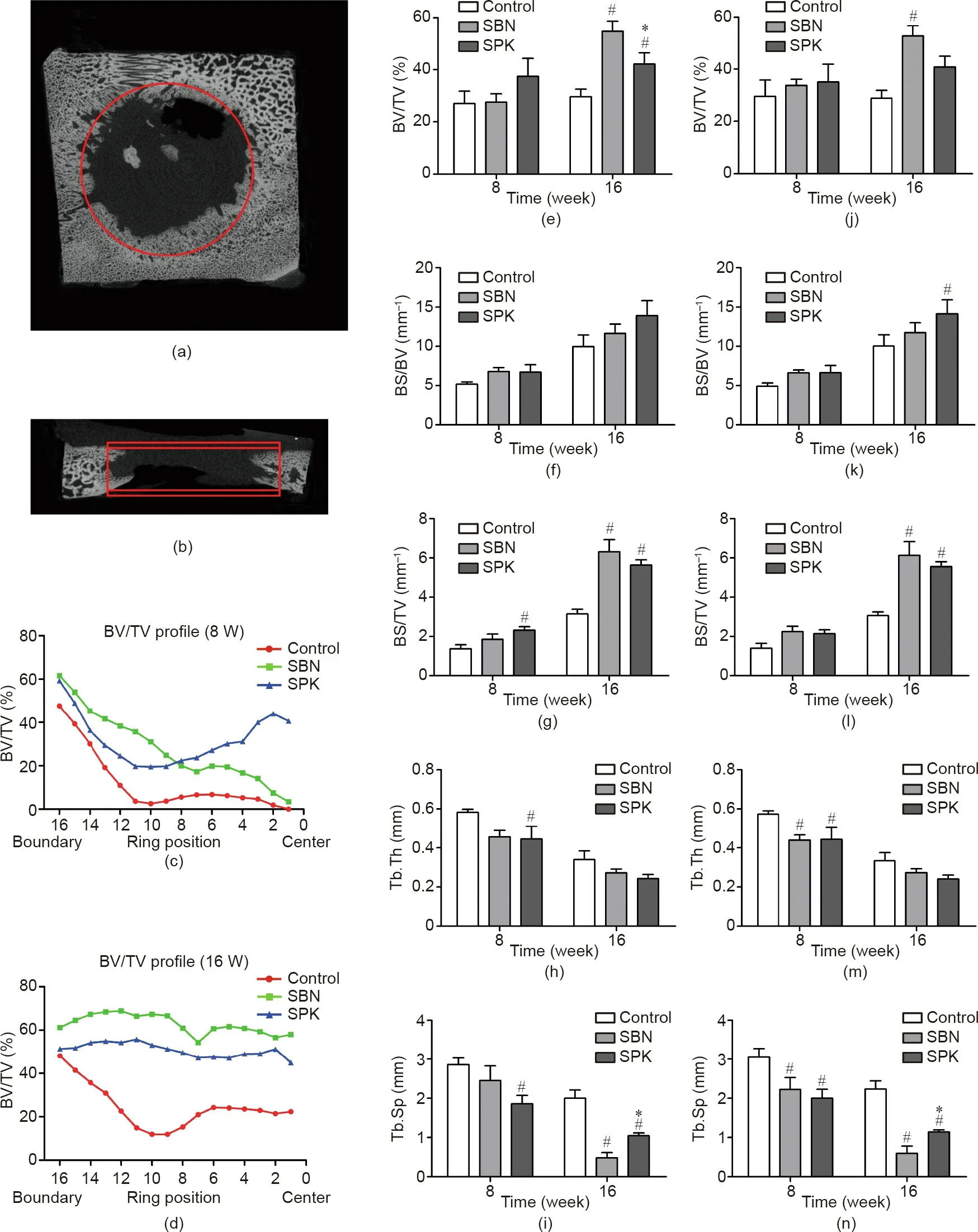
Fig. 11. Quantification analysis of new bone formation in the defect area. (a, b) Illustrations of ROI. ROI was set as a cylinder, with two different heights (4 and 5 mm);(c,d)representative BV/TV variation profile from the boundary to the center of the defect area for a height of 4 mm;(e–i)quantification results based on the 4 mm height ROI;and (j–n) quantification results based on the 5 mm height. *p < 0.05 when comparing SPK with SBN, and #p < 0.05 when comparing SPK with the control.
The Luminex results provided detailed information about how SBN and SPK influence the osteoblast phenotypes. OC is one of the most abundant proteins in bone and is produced exclusively by osteoblasts[34–37].SBN,with a bovine-derived gelatin coating,facilitated the secretion of OC.OC was identified as being involved in the process of mineralization rather than matrix production;therefore,the cells on SBN were more in a state of depositing minerals than in a state of proliferation.OPN is a secreted phosphoprotein that was first identified in the mineralized matrix of bone;it is related to immune modulation and wound healing[38–43].OPN is also a structural molecule in the bone matrix linking organic–mineral interfaces and contributing to the structural integrity and toughness of bone [44]. Osteoblasts from all the donors had high secretion of OPN on the SPK bone grafts,indicating that these cells underwent a process of tissue repairing and regeneration. On the other hand, since OPN is also an RGD-containing molecule [45–47],enhanced secretion of OPN in the SPK group could further promote cell migration, adhesion, and proliferation, reinforcing the bioactivity of the SPK bone graft compared with the SBN bone graft. The secretion of DKK-1 and SOST showed barely any difference during the whole process in all donors; thus, osteogenesis was not inhibited for either SBN or SPK.
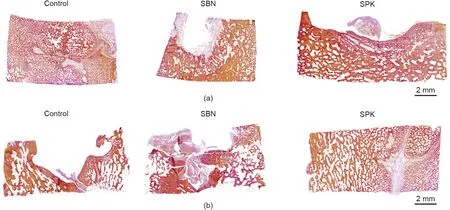
Fig. 12. Representative images of Van Gieson’s staining of all three groups. (a) After 8 weeks of healing and (b) after 16 weeks of healing.
OPG is a decoy receptor for RANKL, as it interrupts the interaction of receptor activator for nuclear factor-κB ligand/receptor activator for nuclear factor-κB(RANKL/RANK)and thereby inhibits both the differentiation and the function of osteoclasts [48–51].Here, osteoblasts seeded on the SPK surface generally displayed higher expression of OPG than those seeded on the SBN surface,indicating that osteoclast activity was potentially inhibited once SPK was implanted in the bone defect area,which favored the process of bone repair.IL-6 and TNF-α are also important mediators of osteoclast genesis [52,53]. Although TNF-α increased during the whole process, there was no difference between the two groups in any of the donors,and the concentration remained at a low level.The level of IL-6 was relatively stable in all donors during the whole experiment, except for that of Donor 2, which decreased after seven days in the SBN group.These results indicate that both SBN and SPK had a slight influence on osteoclast activation.
The animal experiment further exhibited the osteogenic capabilities of both SBN and SPK. Bone ingrowth and defect healing were observed after 8 and 16 weeks of healing using CBCT. Using micro-CT analysis, it was found that both SBN and SPK could promote new bone formation, with SBN generating higher bone volume than SPK. When we expanded the ROI area to 5 mm in height,the bone structure parameters showed hardly any decrease,indicating that the effect was not only limited to the bone graft area, but also expanded to the surrounding environment. The BV/TV variation profile further demonstrated that SPK had an earlier effect on osteogenesis than SBN at the early time point, and that SBN had greater bone formation than SPK when given 16 weeks of healing. After 16 weeks of healing, the newly formed bone exhibited a homogenous structure for both the SBN and SPK groups. Histological evaluation further demonstrated that after eight weeks of healing, the SBN and SPK groups were not fully resorbed, while after 16 weeks of healing, SPK was fully degraded and the defect area was filled with the newly regenerated bone tissue, which had a relatively mature structure.
The bone grafts generally underwent a process of resorption and remodeling after implantation. An appropriate balance between resorption and volume maintenance is important for achieving ideal bone remodeling. Thus, an ideal bone graft is expected to be replaced by bone and remodeled at a tailored absorption rate [20,54,55]. In this study, the SBN and SPK groups showed different degradation speeds. The SBN had lower resorption speed compared with SPK, but both had full resorption and bone formation after 16 weeks of healing.Although the young pigs had relatively more activity during this process, which might accelerate the process of resorption, the regeneration speed was still in accordance with the degradation speed. Histology results also confirmed this finding, in which mature bone tissue was formed from the center to the border.Therefore,SBN and SPK provided different solutions for resorption and regeneration balance.
5. Conclusions
In this study,we investigated the bone regeneration potential of different sources of gelatin. To evaluate their osteogenic potential,we embedded them into a well-established xeno-hybrid bone graft, SmartBone®. We demonstrated that gelatins from both bovine and porcine sources can be loaded onto bone grafts successfully and safely, and can withstand the heavy manufacturing processes including the use of aggressive solvents and sterilization.The results from this study verified the enhanced bone cells’response using in vitro tests and demonstrated osteogenesis using in vivo experiments. SBN was found to enhance osteocalcin secretion, while SPK was found to upregulate osteopontin from human osteoblasts. Both bone grafts promoted osteogenesis, but SPK degraded earlier than SBN. Overall, we have demonstrated that these xeno-hybrid bone grafts possess an ideal balance of resorption and regeneration for bone defect repairing.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2018YFB1105500), the Research Council of Norway (FRINATEK; 231530), and the exchange project between Research Council of Norway and China Scholarship Council (276617). IBI generously donated all samples for testing.
Compliance with ethics guidelines
Hao Zhu, Håvard Jostein Haugen, Giuseppe Perale, Janne Elin Reseland, Liebert Parreiras Nogueira, Antonio Gonzalez Cantalapiedra, Fernando Maria Guzon Muñoz, Maria Permuy Mendaña, Felice Betge, Ståle Petter Lyngstadaas, and Jun Xiao declare that they have no conflict of interest or financial conflicts to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.01.010.
- Engineering的其它文章
- Electric Air Taxis Create Megadeal Buzz
- Tissue Engineering and Regulatory Science
- Factors Predicting Progression to Severe COVID-19: A Competing Risk Survival Analysis of 1753 Patients in Community Isolation in Wuhan,China
- A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses
- Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers
- Past and Future Changes in Climate and Water Resources in the Lancang–Mekong River Basin: Current Understanding and Future Research Directions

