Mass Transfer,Gas Holdup,and Kinetic Models of Batch and Continuous Fermentation in a Novel Rectangular Dynamic Membrane Airlift Bioreactor
Gnlu Li,Kequn Chen,Ynpeng Wei,Jinlei Zeng,Yue Yng,Feng He,Hui Li,*,Pingki Ouyng
a College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, China
b Jiangsu Jicui Industrial Biotechnology Research Institute Co., Ltd., Nanjing 211800, China
Keywords:Airlift bioreactor Dynamic membrane Kinetic model Batch fermentation Continuous fermentation
ABSTRACT Compared with conventional cylinder airlift bioreactors (CCABs) that produce coarse bubbles, a novel rectangular dynamic membrane airlift bioreactor (RDMAB) developed in our lab produces fine bubbles to enhance the volumetric oxygen mass transfer coefficient (kLa) and gas holdup, as well as improve the bioprocess in a bioreactor.In this study,we compared mass transfer,gas holdup,and batch and continuous fermentation for RNA production in CCAB and RDMAB. In addition, unstructured kinetic models for microbial growth, substrate utilization, and RNA formation were established. In batch fermentation,biomass, RNA yield, and substrate utilization in the RDMAB were higher than those in the CCAB, which indicates that dynamic membrane aeration produced a high kLa by fine bubbles;a higher kLa is more beneficial to aerobic fermentation.The starting time of continuous fermentation in the RDMAB was 20 h earlier than that in the CCAB, which greatly improved the biological process. During continuous fermentation, maintaining the same dissolved oxygen level and a constant dilution rate, the biomass accumulation and RNA concentration in the RDMAB were 9.71% and 11.15% higher than those in the CCAB, respectively. Finally, the dilution rate of RDMAB was 16.7% higher than that of CCAB during continuous fermentation while maintaining the same air aeration. In summary, RDMAB is more suitable for continuous fermentation processes. Developing new aeration and structural geometry in airlift bioreactors to enhance kLa and gas holdup is becoming increasingly important to improve bioprocesses in a bioreactor.
1. Introduction
Airlift bioreactors are commonly used in bioprocessing to produce a variety of chemicals, enzymes, and medicines because of their unique characteristics, such as ease of operation, low energy consumption, low shear stress, and simple mechanical geometry[1–3]. It has been reported that the internal loop airlift bioreactor is a more suitable fermentation apparatus and process for the growth of Rhodotorula glutinis than the conventional agitation bioreactor [4]. Conventional airlift bioreactors, which use porous plates, perforated pipes, orifices, or static membranes as the aeration unit, produce coarse bubbles, which lead to a low volumetric oxygen mass transfer coefficient (kLa) and low gas utilization efficiency;therefore,they require high aeration rates to meet the oxygen demands of microorganisms[5–7].The production of biomass and lipids by an oleaginous yeast strain and the use of glycerol from biodiesel production as the substrate in agitation and airlift bioreactors has shown that high biomass and lipid yields can be achieved in a 3.0 L airlift bioreactor under uncontrolled pH regimes. However, high aeration rates still cannot meet the required oxygen demand, so pure oxygen needs to be flushed into the airlift bioreactor to maintain the fermentation process [8]. Li et al. [9] developed a new dynamic membrane that produces fine bubbles to enhance the kLa and yield of the product in a bioreactor.Thus, developing new aeration geometries in airlift bioreactors to reduce the bubble size and improve kLa is increasingly important to improve the bioprocess.
Ribonucleic acid (RNA), a very important macromolecule produced during gene expression in living organisms, can be hydrolyzed to yield individual nucleotides [10,11]. RNA and its hydrolysate nucleotides have a variety of physiological functions,such as enhancing immune function and improving self-repair,anti-aging,and antiviral activities[12–14].RNA and its hydrolysate nucleotides are commonly used in the food,feed,agriculture,medicine, and light industries [15–17]. At present, the main industrial RNA-producing species are the ascomycete budding yeasts Saccharomyces cerevisiae and Candida tropicalis(C.tropicalis)[18–20].Fermentation kinetic models are an important technology for the study of microbial growth, substrate utilization, and product formation. Recently, these models have been the focus of research in bioprocessing [21–23]. It has been proposed that unstructured kinetic models can be fitted to describe the principal kinetics involved in ethanol fermentation in a continuous and closed-loop fermentation process with a pervaporation membrane bioreactor,and the models showed good agreement with the experimental data [24]. Unstructured kinetic models in a 50 L airlift bioreactor have been constructed for batch RNA production utilizing the Logistic–Monod model for microbial growth, the Luedeking–Piret model for product formation, and the modified Luedeking–Piret model for substrate uptake [19]. However, fermentation kinetic models of high-oxygen-consuming fermentation processes are rarely reported in airlift bioreactors with a high volumetric gas–liquid mass transfer coefficient.
In this study,a strain of C.tropicalis that produces large amounts of RNA was used as the producing strain, molasses and glucose served as the carbon sources, and unstructured kinetic models were built to analyze the fermentation process. The volumetric oxygen mass transfer coefficients (kLa) in batch and continuous fermentation for RNA production in a conventional cylinder airlift bioreactor (CCAB) and a rectangular dynamic membrane airlift bioreactor (RDMAB) were compared. Our study provides a theoretical basis for industrial production using fermentation processes in a dynamic membrane airlift bioreactor.
2. Rectangular dynamic membrane airlift bioreactor
The kLa value is predominantly determined by the total gas–liquid interfacial area(a)and the liquid mass transfer coefficient(kL).The value of a is mainly related to the bubble size and gas holdup in the bioreactor [9]. Fine bubbles have a higher kLa than coarse bubbles with the same gas holdup because of the high total gas–liquid interfacial area. The size of the bubbles generated by dynamic membranes is affected by the relative liquid velocity,gas–liquid transmembrane pressure differences, liquid viscosity,and surface tension [25–27]. The higher the relative velocity of the liquid in the membrane pore surface, the smaller the bubble size. The liquid relative velocity on the surface of the dynamic membrane is increased by high speed rotation, which reduces the size of the bubbles[28].Dynamic membranes exhibit increased kLa and improved gas holdup because they form an abundance of uniform fine bubbles [9]. In the present study, a dynamic membrane was used as the aeration unit in the airlift bioreactor(Fig.1).As shown in Fig.1(b),the dynamic membrane had a hollow structure, and the blades were made of a metal membrane with a pore size of 500 nm.Air enters through the air inlet,passes through the hollow shaft, and enters the dynamic membrane hollow area.Subsequently, because of the transmembrane pressure difference,air enters the fluid through the nanopores, forming fine bubbles.
Rectangular airlift bioreactors have improved mixing and mass transfer performance than those of cylinder airlift bioreactors because of the effects of their geometrical and operating parameters on hydrodynamics and mass transfer [29–31]. It has been reported that the hydrodynamic characteristics of a rectangular airlift loop reactor are better for gas holdup and the mass transfer coefficient than those of the cylinder airlift loop reactor [32]. The average kLa of the rectangular airlift loop reactor is approximately 40% greater than that of the cylinder airlift loop reactor [32]. The pre-experiment results showed that the gas holdup and mass transfer characteristics of the rectangular airlift reactor are better than those of the circular airlift reactor, which is consistent with many reported results [31,32]. Therefore, a new rectangular airlift bioreactor coupled with a dynamic membrane was developed,and schematic diagrams of the RDMAB and CCAB are shown in Fig. 1.The air circulation and schematic diagram of the double-layer dynamic membrane in the RDMAB is shown in Fig. 1(c). A double-layer dynamic membrane with a pore size of 500 nm was adopted to ensure almost the same air flux and to control the bubble diameter. Based on the optimization, the dynamic membrane was located at the lowest position inside the draft tube. An air compressor was used to supply air. Table 1 shows the detailed structural dimensions of the RDMAB and CCAB. Both the CCAB as a control and the RDMAB had volumes of 120 L. The volume of the liquid was 100 L.A transparent square glass cell was assembled outside the tank to observe the bubbles.
3. Materials and methods
3.1. Measurement of the volumetric oxygen mass transfer coefficient
The volumetric oxygen mass transfer coefficient (kLa) was determined using a dissolved oxygen (DO) probe (Mettler-Toledo,USA) with a response time of 15 s in the riser and downcomer pipes of the CCAB and RDMAB [9]. Two DO probes were installed in the riser at 0.300 m (position 14) and in the downcomer at 0.200 m(position 8)sections from the lower edge of the rectangular draft tube. The kLa values in the riser and downcomer were measured simultaneously. Briefly, nitrogen was bubbled into distilled water until the DO concentration reached less than 5% oxygen saturation. The nitrogen flow was then interrupted, nitrogen bubbles were released, and air was bubbled into the solution.The increase in the DO concentration was monitored over time until it reached 90% oxygen saturation. All experiments were conducted in triplicate. kLa was calculated using Eqs. (1) and (2):

where cpis the measured DO concentration in the liquid phase(mol∙L-1), c is the actual DO concentration (mol∙L-1), t is the fermentation time (s), c* is the saturated DO concentration (mol∙L-1),τtis the probe response time (s), and kLa is the volumetric oxygen mass transfer coefficient (s-1).
3.2. Measurement of the gas holdup
The overall gas holdup was calculated by measuring the liquid level before and after aeration. All experiments were performed in triplicate.The overall gas holdup εTwas calculated using Eq.(3):

where hLis the liquid level before aeration(m),and hGis the liquid level during aeration (m).
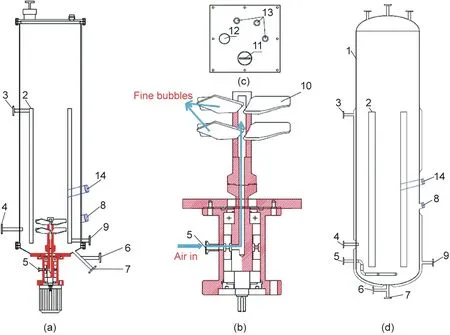
Fig.1. (a)Schematic diagram of the rectangular dynamic membrane airlift bioreactor,(b)the dynamic membrane,(c)the rectangular cover,and(d)the conventional cylinder airlift bioreactor. (1: Bioreactor tank; 2: draft tube; 3: steam inlet or cooling water outlet; 4: sampling outlet; 5: air inlet; 6: continuous fermentation inlet; 7: continuous fermentation outlet;8:thermometer,pH meter,and dissolved oxygen meter(DO)in downcomer;9:condensate water outlet or cooling water inlet;10:dynamic membrane blade; 11: inoculation inlet; 12: tail gas outlet; 13: feeding inlet, and 14: DO meter in riser).
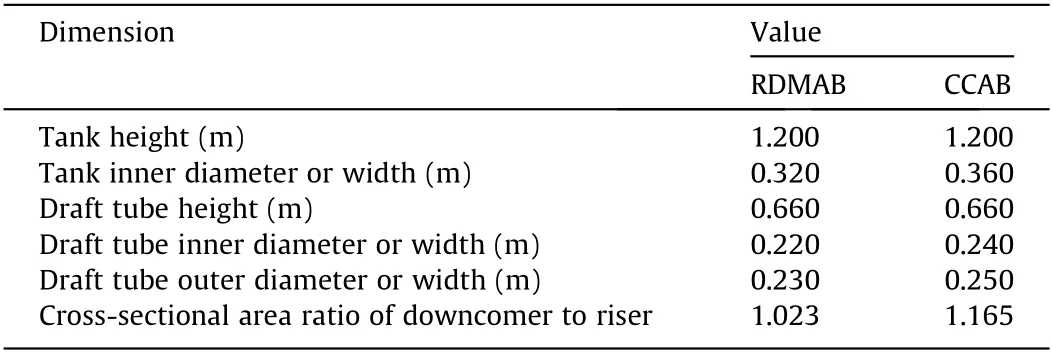
Table 1 Structure dimensions of the RDMAB and the CCAB.
3.3. Measurement of the bubble size
The bubble size distribution was measured by high-speed microscopic photography (Phantom VEO 1310; Vision Research,Inc.,USA)equipped with a Nikon 105 mm F/2.8 micro lens(Nikon,Japan) to produce a controllable field-of-view down to a few millimeters. The images were processed manually by measuring the diameter of approximately 500 randomly selected bubbles.
3.4. Microorganism and culture conditions
The C.tropicalis strain NY6-18,which was used for RNA production in this study,was stored in our laboratory at-80°C in 20%(v/v) glycerol. Molasses containing glucose with 50% mass fraction was used. The strain was cultured on a solid agar plate containing 20 g∙L-1glucose, 20 g∙L-1peptone, 10 g∙L-1yeast extract, and 20 g∙L-1agar for 72 h at 33 °C, after which it was stored at 4 °C.
The primary seed medium consisted of 21 g∙L-1glucose,9 g∙L-1molasses, 10 g∙L-1(NH4)2SO4, 0.5 g∙L-1MgSO4, 3 g∙L-1H3PO4,0.05 g∙L-1ZnSO4, and 0.05 g∙L-1FeSO4. The pH was adjusted to 4.0 with 25% aqueous ammonia, and the medium was sterilized for 15 min at 12 °C in an autoclave (YXQ-LS-75S11; Shanghai Boxun Medical Biological Instrument Corp., China).
The secondary seed medium consisted of 28 g∙L-1glucose,12 g∙L-1molasses, 10 g∙L-1(NH4)2SO4, 0.5 g∙L-1MgSO4, 3 g∙L-1H3PO4, 0.05 g∙L-1ZnSO4, and 0.05 g∙L-1FeSO4. The medium was adjusted to pH 4.0 with 25% aqueous ammonia and sterilized for 15 min at 121 °C in an autoclave.
The fermentation medium consisted of 210 g∙L-1glucose,90 g∙L-1molasses, 100 g∙L-1(NH4)2SO4, 5 g∙L-1MgSO4, 30 g∙L-1H3PO4,0.5 g∙L-1ZnSO4,and 0.5 g∙L-1FeSO4.The pH of the medium was adjusted to 2.0 using 25% aqueous ammonia.
For seed culture, the strain was transferred from a solid agar plate into an Erlenmeyer flask (500 mL) containing 50 mL of the primary seed medium.The strain was cultured at 33°C with shaking at 150 r∙min-1in a thermostatic incubator(HNY-211B;Tianjin Ounuo Instrument Corp., China) for 18 h. The primary inoculum(10%, v/v) was then transferred to a 15 L airlift bioreactor (Huike Bioengineering, China) containing 9 L of the secondary seed medium. The temperature was controlled at 33 °C, and the pH was maintained at 4.0 by the addition of aqueous ammonia. The aeration rate during incubation was 2.5 volume (air)/volume (culture)per minute (vvm), and peanut oil was used as an antifoam agent.Finally, the secondary inoculum (10%, v/v) was transferred into a 120 L CCAB (Huike Bioengineering) or RDMAB (constructed in our laboratory) containing 80 L of fermentation medium that had been sterilized for 15 min at 121 °C in an autoclave. The temperature was controlled at 33 °C, and the pH was maintained at 4.0 by adding aqueous ammonia.The aeration rate during incubation was 3.0 vvm and the dynamic membrane speed in the RDMAB was 400 r∙min-1.Continuous fermentation conditions were controlled to be the same as those used for batch fermentation. Bioreactor operation was initially conducted in batch fermentation mode until a significant amount of biomass was produced, after which additional fermentation medium(unsterilized)was added to the airlift bioreactor operated in continuous fermentation mode. All experiments were conducted in triplicate.
3.5. Biomass and glucose determination
Samples (5 mL) of the fermentation broth were transferred to dry, weighed centrifuge tubes, which were centrifuged for 15 min at 8000 r∙min-1. The mycelial pellets were washed three times with deionized water, after which the mycelium was dried in an oven at 80°C and weighed,and the biomass(dry cell weight,DCW) was calculated using Eq. (4):

where DCW is the dry cell weight or biomass (g∙L-1), w is the weight of the mycelium(g),and v is the volume of the fermentation broth (L).
Glucose concentration was determined using an automated enzyme analysis system (SBA-40C; Biology Institute of Shandong Academy of Sciences, China).
3.6. RNA assay
Two replicate 2 mL samples of fermentation broth were placed in 5 mL centrifuge tubes and centrifuged at 8000 r∙min-1for 15 min.The mycelial pellets were washed three times with deionized water, and one centrifuge tube was dried and weighed in an oven at 80 °C. Precooled 0.25 mol∙L-1perchloric acid solution(2 mL) was added to another centrifuge tube, which was shaken well and heated in a 70 °C water bath for 20 min, cooled in an ice bath to 25°C,and centrifuged at 8000 r∙min-1for 10 min.Subsequently, a 1 mL sample of the supernatant was placed into a 100 mL volumetric flask and the volume was adjusted to 100 mL with deionized water. The absorption value was measured at a wavelength of 260 nm, and deionized water was used as a blank.The formula for determining the RNA concentration was as follows:
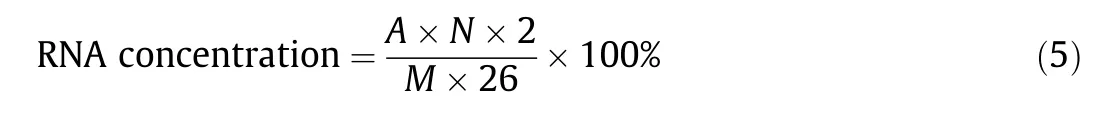
where A is the absorbance value of the sample solution, N is the dilution factor of the sample, 2 is the volume of 0.25 mol∙L-1perchloric acid (mL), M is the dry cell weight of mycelium in 2 mL of fermentation broth (mg), and 26 is the absorbance value of RNA.
3.7. Kinetic models
High substrate concentrations can result in substrate inhibition.To accurately describe the microbial growth kinetics with both substrate-limiting and self-inhibiting factors, a hybrid Logistic–Monod model has been used to analyze microbial growth in the batch fermentation process [33,34]. In our study, a hybrid Logistic–Monod model was used to fit the growth of C. tropicalis using Eq. (6):

where X is the biomass concentration (g∙L-1), t is the fermentation time(s),μmaxis the maximum specific growth rate(h-1),Xmaxis the maximum value of the biomass concentration (g∙L-1), S is the substrate concentration (g∙L-1), and Ksis the saturation coefficient(g∙L-1).
The Luedeking–Piret model is often used to explain the synthesis of metabolites by combining both growth- and non-growthassociated contributions [35,36]. Therefore, RNA production can be described by the Luedeking–Piret model using Eq. (7):

where P is the RNA concentration (g∙L-1), α is the growth correlation coefficient (g∙g-1), and β is the non-growth correlation coefficient (g∙L-1∙h-1).
The substrate utilization rate in the fermentation broth is related to the biomass and product formation rate.There are three aspects to substrate consumption: ①providing energy and nutrients for cell growth,②maintaining cell activity,and ③promoting product formation. A modified Luedeking–Piret model can be used to describe the relationship between substrate utilization and product production, including substrate consumption for maintaining cell growth and substrate transformation[37,38].Therefore,a modified Luedeking–Piret model was employed to explain substrate utilization in C.tropicalis using Eq.(8):

where m is the cell maintenance correlation coefficient (g∙g-1∙h-1),YX/Sis the yield coefficient of biomass on the substrate (g∙g-1), and YP/Sis the yield coefficient of RNA on the substrate (g∙g-1).
4. Results and discussion
4.1. Volumetric oxygen mass transfer coefficient
The kLa value is a key indicator for evaluating bioreactor performance. The kLa values of the riser and downcomer pipes of the CCAB and RDMAB operating at 0, 200, and 400 r∙min-1were compared and are shown in Fig.2.The RDMAB operating at 0,200,and 400 r∙min-1showed higher kLa values than those with the CCAB.The air aeration rate increased continuously, and the value of kLa increased. When the air aeration rate increased to 0.3 m3∙min-1,which is equivalent to 3.0 vvm, the kLa values of the riser in the RDMAB operating at 0, 200, and 400 r∙min-1were 0.091, 0.097,and 0.112 s-1, respectively, while the kLa value of the riser in the CCAB was only 0.07 s-1. As the speed increased, the proportion of fine bubbles increased and the bubble size decreased continuously; the kLa value also increased continuously, which is consistent with previously reported results [9]. The dynamic membrane generates a large number of fine bubbles, which increases the oxygen mass transfer in the airlift bioreactor.
4.2. Gas holdup
The overall gas holdup was determined in an air–water system for CCAB and RDMAB operating at speeds of 0, 200, and 400 r∙min-1. As shown in Fig. 3, increased aeration levels from 0.5 to 3.0 vvm resulted in elevated overall gas holdup levels in both the CCAB and RDMAB operating at speeds of 0, 200, and 400 r∙min-1.In the RDMAB operating at speeds of 0, 200, and 400 r∙min-1, the overall gas holdups were higher than those in the CCAB system under the same conditions. Compared with coarse bubbles, fine bubbles have a lower velocity and longer residence time, which is beneficial for increasing the overall gas holdup [9].
4.3. Bubble size
Fig. 4 shows the bubble size distributions and shapes in the RDMAB operating at a speed of 200 r∙min-1at aeration rates of 0.5, 1.0, 1.5, and 2.0 vvm. The diameter of the bubbles produced by low aeration was small, and the bubble shape in the RDMAB tended to be spherical. The bubble size was evenly distributed and concentrated in a narrow range in the RDMAB when aeration was less than 2.0 vvm. The size distributions of the bubbles in the RDMAB are shown in Fig. 5. The Sauter mean diameter (d32)of the bubbles in the RDMAB at aerations of 0.5, 1.0, 1.5, 2.0, 2.5,and 3.0 vvm were(0.89±0.40),(0.94±0.47),(1.10±0.54),(1.19±0.72),(1.22±0.90),and(1.25±0.94)mm,respectively,which were obviously smaller than those reported for airlift reactors [39]. It was also found that the bubble diameter increased with increasing aeration. The Sauter mean diameter of the bubbles in the RDMAB at speeds of 0, 200, and 400 r∙min-1were (0.97 ± 0.36),(0.95 ± 0.43), and (0.93 ± 0.39) mm, respectively, which indicates that speed has little effect on the bubble diameter. The main parameter of gas–liquid mass transfer is aeration,and the rotation can enhance the gas–liquid mass transfer by improving the gas–liquid mixing efficiency.
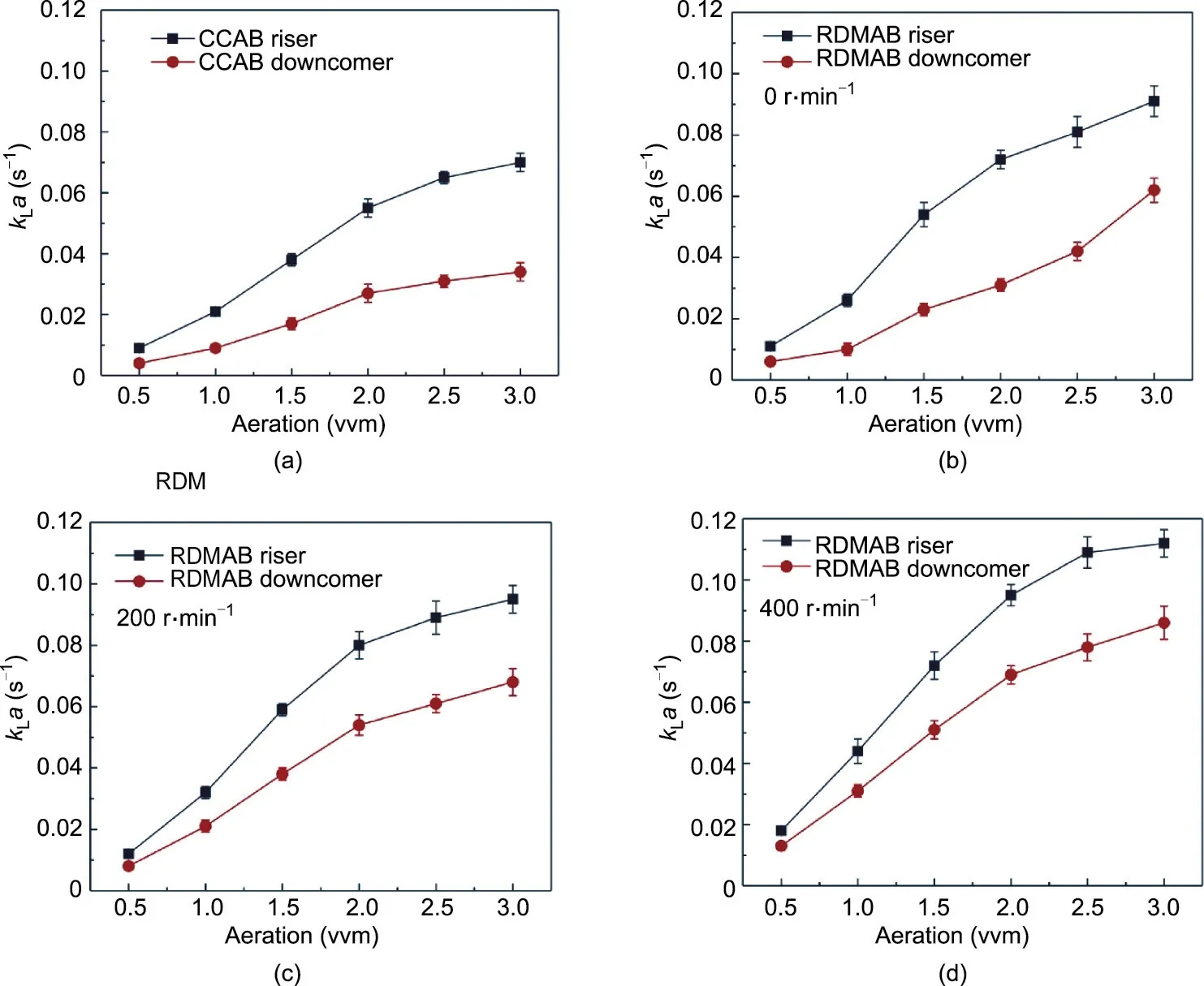
Fig. 2. Comparison of kLa of the riser and downcomer of (a) the CCAB and (b)–(d) the RDMAB operating at speeds of 0, 200, and 400 r∙min-1, respectively.
4.4. Comparison of present data with published results
A variety of data on the hydrodynamics and mass transfer of airlift reactors for Newtonian fluids can be found in the literature.A comparison of our data with literature data is shown in Table 2[39–44].Compared with conventional internal loop airlift reactors,many modified airlift reactors with mass transfer intensification have higher kLa values.Rectangular airlift reactors such as RDMAB and split-rectangle-internal loop airlift bioreactors show higher kLa values than those of conventional circular airlift reactors, even at low aeration levels [43]. The key reason for this phenomenon is that a rectangular structure has a better flow field to promote gas–liquid mass transfer [43]. In addition, a variety of mass transfer intensification methods can significantly enhance gas–liquid mass transfer, such as net draft tubes and helical sieve plates[39,40]. Helical sieve plates cut large bubbles into small bubbles with an enhanced gas–liquid mass transfer rate. As mentioned above, a rectangular dynamic membrane airlift bioreactor has extraordinary advantages and development potential in biological fermentation processes.
4.5. Batch fermentation in the CCAB and RDMAB
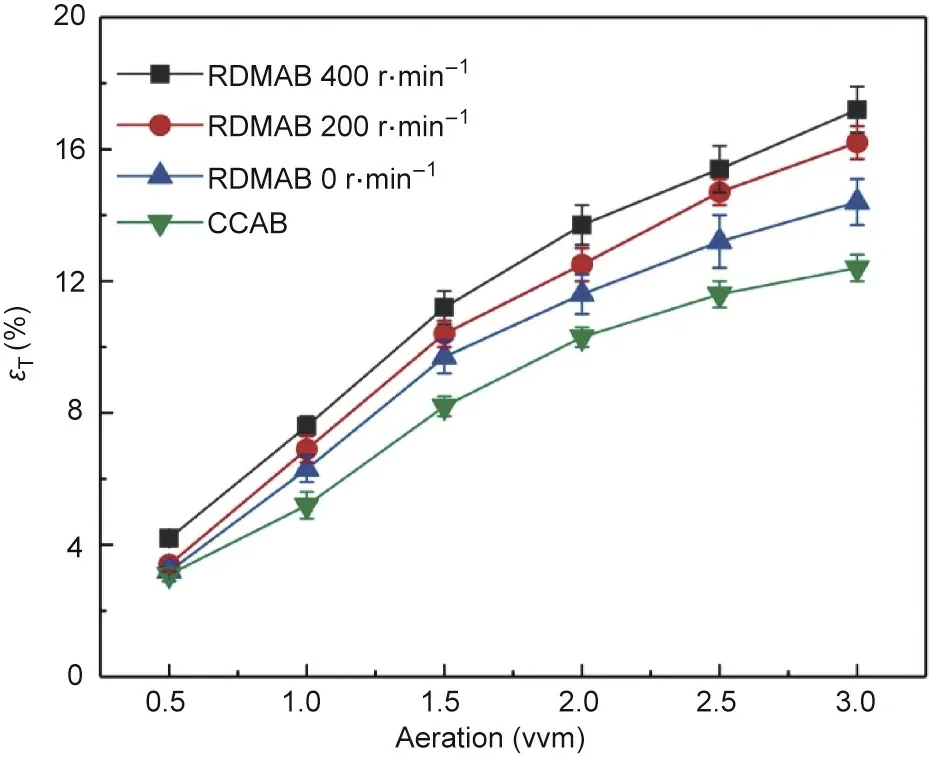
Fig. 3. Comparison of overall gas holdup the CCAB and the RDMAB operating at speeds of 0, 200, and 400 r∙min-1 at different aeration.
The biomass concentration, RNA concentration, sugar concentration, and DO level for C. tropicalis cultured in the CCAB and RDMAB were obtained in batch fermentation mode,and the results are shown in Fig. 6. During batch fermentation, the biomass level and RNA concentration in the RDMAB were higher than those in the CCAB, which indicates that biomass growth and product synthesis were promoted in the RDMAB because of an adequate oxygen supply. The RNA concentration in the fermentation broth seems to parallel microbial growth in CCAB and RDMAB. The biomass and RNA concentrations increased constantly, while the sugar concentration decreased constantly. The rate of sugar consumption in the RDMAB was significantly higher than that in the CCAB, which demonstrates that sugar was more efficiently converted into both products and biomass. A high DO level can promote product accumulation in a high-oxygen-consumption and high-density fermentation process.Variation in the DO level affects biomass concentration, lipid accumulation, and composition [45].High RNA production by C.tropicalis is a high-oxygen-consumption bioprocess.RDMAB provides a high DO level or kLa to improve RNA accumulation. Finally, the maximum RNA concentration and biomass production in the RDMAB after 120 h of fermentation were 14.10 and 84.02 g∙L-1respectively, which were much higher than those attained in the CCAB at 13.0 and 73.5 g∙L-1, respectively.
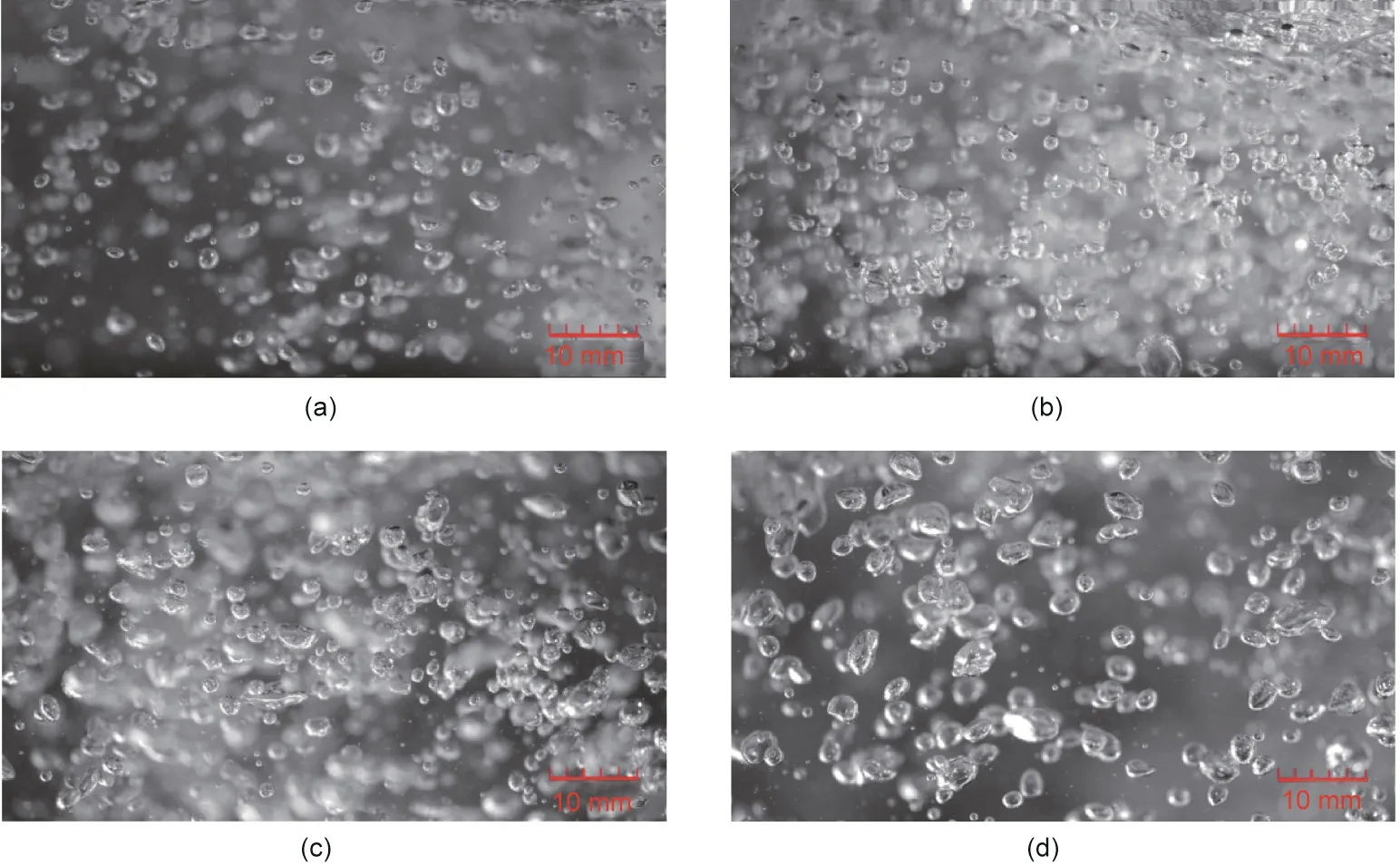
Fig. 4. Bubble size distributions and shapes in the RDMAB operating at speed of 200 r∙min-1 at aeration of (a) 0.5, (b) 1.0, (c) 1.5, and (d) 2.0 vvm.
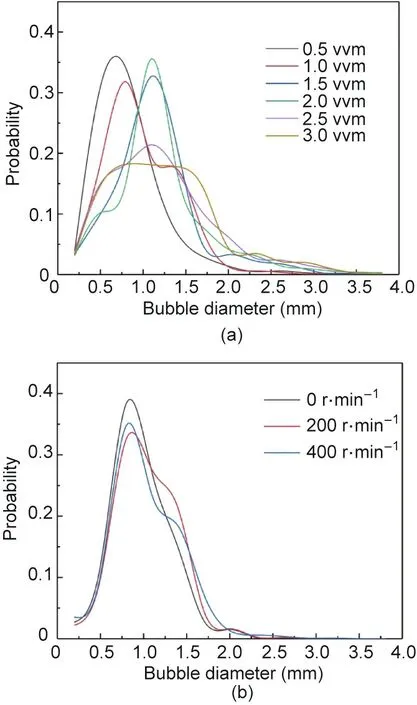
Fig. 5. Size distribution of bubbles in RDMAB operating at (a) aeration of 0.5–3.0 vvm when speed of 200 r∙min-1 and (b) speeds of 0, 200, and 400 r∙min-1 when aeration of 1.0 vvm.
The starting time of continuous fermentation is very important to continuously and efficiently produce fermentation products,and the starting time of continuous fermentation can be determined by the microbial growth curve in batch fermentation mode. In general, different bioreactors may have different optimal starting times for continuous fermentation.Under batch fermentation conditions, the oxygen uptake rate increases rapidly, and the biomass level reaches 40%–80% of the maximum. This point in time can be considered as the starting time for continuous fermentation [46].Fig. 6(a) shows that, in the CCAB, the oxygen uptake rate was the highest and the DO level reached the lowest point at 70 h of fermentation time,and the biomass level reached 68.4%of maximum.Therefore,70 h was chosen as the starting time for continuous fermentation in the CCAB.Fig.6(b)shows that in the RDMAB,the oxygen uptake rate was the highest and the DO was the lowest at 50 h of fermentation, and the DCW (biomass) reached 71.4% of maximum.Therefore,50 h was chosen as the starting time for continuous fermentation in the RDMAB. Figs. 6(a) and (b) shows that the starting time for continuous fermentation in the RDMAB was 20 h earlier than that in the CCAB. The kLa in the RDMAB was higher than that in the CCAB,and the microorganism(C.tropicalis)had a higher biological activity, faster growth rate, and slower senescence rate under growth conditions with a sufficient supply of oxygen.
4.6. Kinetic models of batch fermentation in the CCAB and RDMAB
The fitting curves for biomass concentration, RNA concentration, and sugar concentration during batch fermentation in the CCAB and RDMAB are shown in Fig. 7. The Logistic–Monod,Luedeking–Piret, and modified Luedeking–Piret parameters are summarized in Table 3. The correlation coefficients (R2) of microbial growth in the CCAB and RDMAB were 0.9496 and 0.9527,respectively (Fig. 7(a)). Our results show that the experimental data agreed well with the prediction model. The microbial growth curves in the CCAB and RDMAB are typical sigmoidal curves. The growth curves can be structured as a lag phase,exponential growth phase,decelerating growth phase,and stationary phase[19,47].In addition, we found that the Xmax, Ks, and μmaxin the RDMAB were 81.04 g∙L-1,5.097 g∙L-1,and 0.10680 h-1respectively,considerably higher than those in the CCAB (74.55 g∙L-1, 2.954 g∙L-1, and 0.07061 h-1, respectively), which shows that a high kLa can significantly increase the growth of microorganisms and avoidthe limitation of hypoxia. In the initial stage of fermentation, the substrate concentration is much higher than the saturation coefficient, which indicates that microbial growth is not limited by the substrate concentration.When the sugar concentration is less than 50 g∙L-1, microbial growth can be restricted by the low sugar concentration. At this time, feeding is required to promote microbial growth. As shown in Fig. 6, we found that when the fermentation time was 50 h (the starting time of continuous fermentation in the RDMAB,the sugar concentration in the RDMAB was<50 g∙L-1,and the fermentation time in the CCAB was 70 h(the starting time of continuous fermentation in the CCAB).
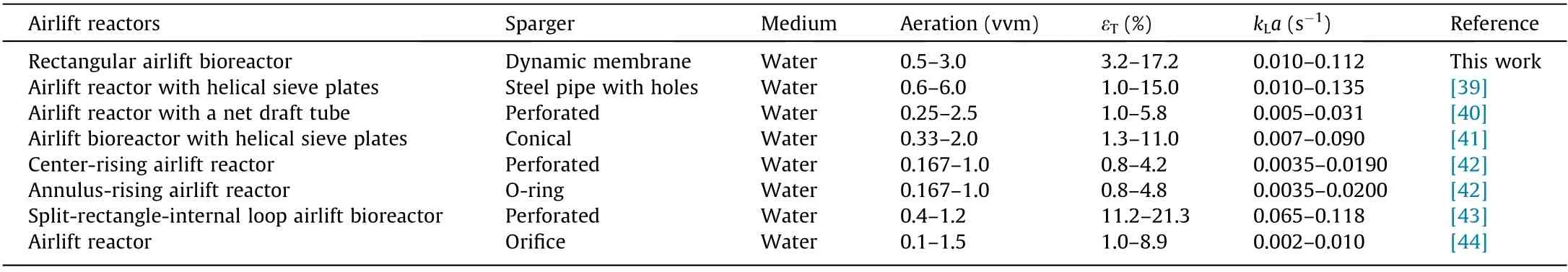
Table 2 Comparison of present data with published results.
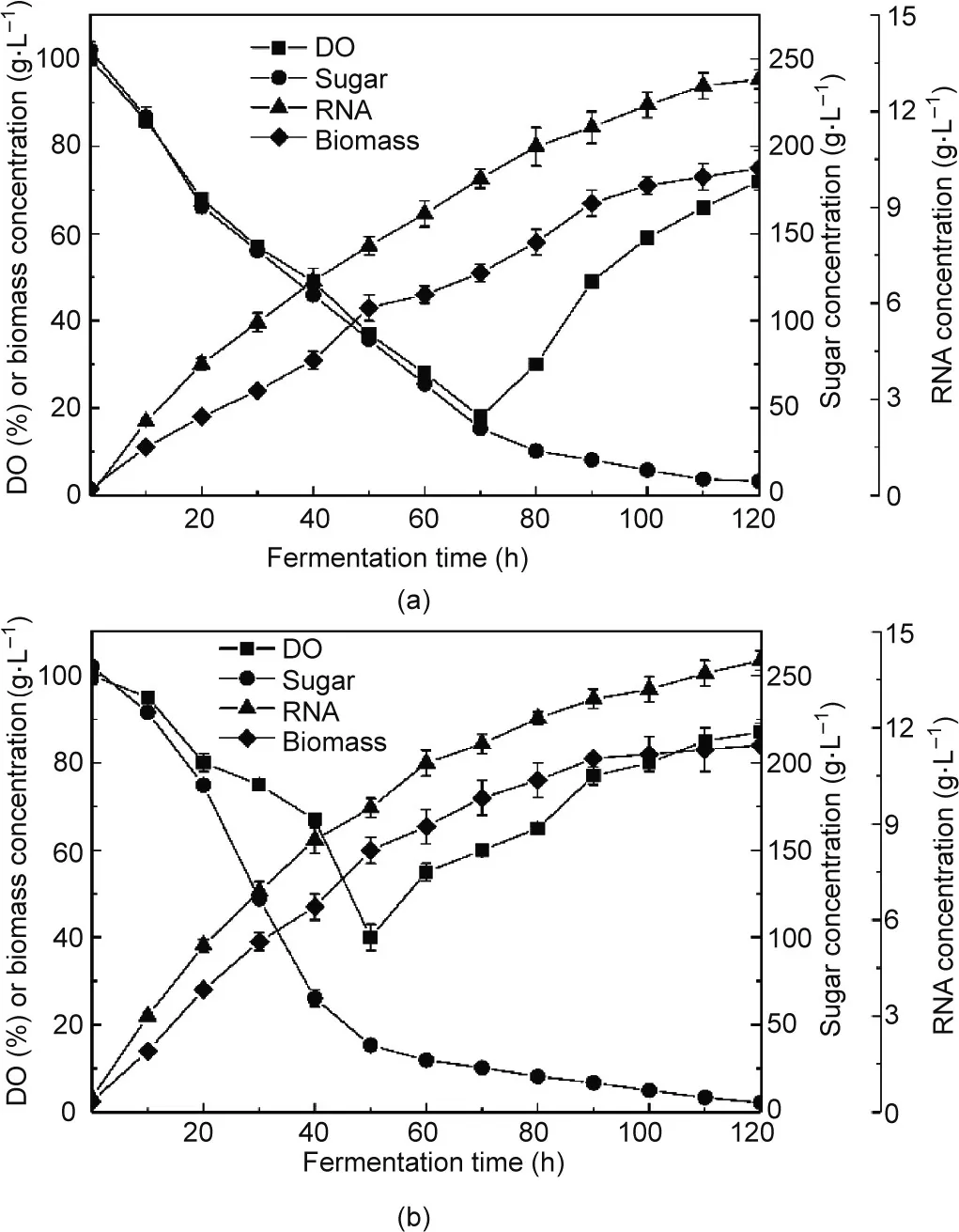
Fig.6. Batch fermentation for RNA production in (a) the CCAB and (b) the RDMAB.
The Luedeking–Piret model fitted to the RNA production is shown in Fig.7(b).The correlation coefficients(R2)for RNA concentration in the CCAB and RDMAB were 0.9553 and 0.9679, respectively. Our results show that the experimental data agreed well with the prediction model. The Luedeking–Piret parameters α and β are classified into three categories: ①α ≠0 and β = 0, product formation is growth-associated; ②α = 0 and β ≠0, product formation is non-growth-associated; and ③α ≠0 and β ≠0, product formation is mixed-growth-associated [34]. In this study,the growth correlation coefficients (α) in the CCAB and RDMAB were 0.1206 and 0.1035 g∙g-1,respectively,and the non-growth correlation coefficients (β) were 0.0004057 and 0.0005286 g∙L-1∙h-1,respectively, which showed that the Luedeking–Piret model of RNA production belonged to a mixed-growth-associated pattern(category #3). The RNA concentration increased almost proportionally with the increase in biomass during fermentation. The value of α in the RDMAB was lower than that in the CCAB, which indicates that the conversion efficiency of substrate sugars into the product was higher in the RDMAB.
Substrate utilization is related to microbial growth, RNA production, and substrate uptake, which are required for cell maintenance. In the fermentation process, sugars are used as carbon sources, and a kinetic model of sugar utilization was established using the modified Luedeking–Piret model, as shown in Fig. 7(c).The correlation coefficients (R2) of the model for the CCAB and RDMAB were 0.9834 and 0.9732,respectively,which indicates that the model fitted to the experimental data satisfactorily. The yield coefficients of biomass on the substrate (YX/S) in the CCAB and RDMAB were 0.8178 and 0.4686 g∙g-1, respectively, and the yield coefficients of RNA on the substrate (YP/S) were 0.04127 and 0.09196 g∙g-1,respectively,indicating that sufficient oxygen levels are more favorable for the conversion of substrate sugars into RNA production. kLa is a key fermentation parameter for producing highly aerobic products. Compared with those in the CCAB, the dynamic membrane in the RDMAB can produce higher kLa values[9].Chang et al.[48]found that a high kLa could effectively increase the docosahexaenoic acid concentration, docosahexaenoic acid productivity, and conversion yield, which is consistent with the results of our study.
The models were validated by changing the initial sugar concentration (200 g∙L-1) of the medium. The results of the Logistic–Monod model fitted to microbial growth, Luedeking–Piret model fitted to RNA production,and modified Luedeking–Piret model fitted to substrate utilization were more than 90% accurate, which indicates that the models are reliable and robust.
4.7. Continuous fermentation in the CCAB and RDMAB
Continuous fermentation, based on batch fermentation, needs to meet the following assumptions: ①The bioreactors are in the chemostat culture state in the continuous fermentation process;②the physiological state of each stage in the bioreactor is maintained in the continuous fermentation process in the chemostat;and ③the parameters of continuous fermentation,such as microbial growth,product formation,and substrate consumption,are in good agreement with those of batch fermentation [33,49]. Therefore, the initial dilution rate for continuous fermentation was obtained using the batch fermentation kinetic model.
The dilution rate was the feed flow rate divided by the culture volume.In the continuous fermentation mode,the specific growth rate (μ) is related to the dilution rate (Dr) in the mass balance model [50]:
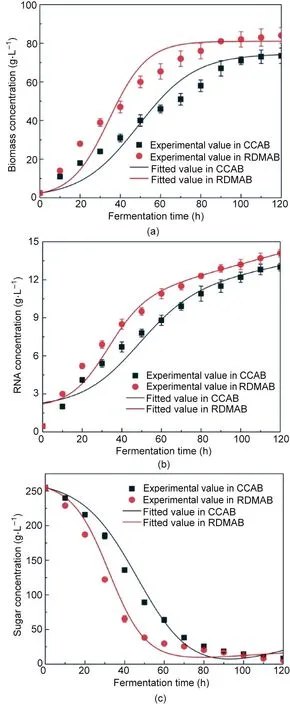
Fig.7. Model fitting curves of(a)biomass in the CCAB(R2=0.9496)and the RDMAB(R2 = 0.9527); (b) RNA production in the CCAB (R2 = 0.9553) and the RDMAB(R2=0.9679);and(c)sugar concentration in the CCAB(R2=0.9834)and the RDMAB(R2 = 0.9732).

where Dris the dilution rate(h-1) and μ is the specific growth rate(h-1).
At the steady state,μ = Dr; thus, the initial dilution rate can be determined by the specific growth rate. In addition, Drshould not exceed μmaxto prevent washout.
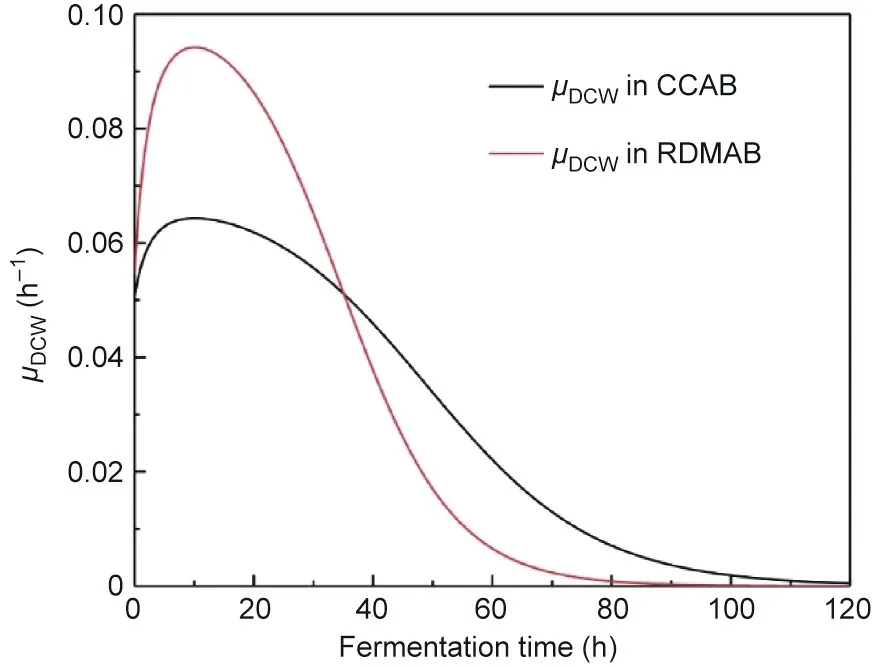
Fig. 8. Specific growth rates (μDCW) of C. tropicalis in the CCAB and the RDMAB.
The specific growth rates of microbial biomass in the batch fermentation mode in the CCAB and RDMAB are shown in Fig. 8. In the initial stage of fermentation, the specific growth rate in the RDMAB was significantly higher than that in the CCAB because of sufficient oxygen and substrate levels.With the rapid consumption of the substrate, the specific growth rates in both the CCAB and RDMAB decreased significantly.By comparing the maximal growth rate reported,the maximal growth rates in the RDMAB and CCAB in this study were lower than those previously reported due to high substrate concentrations. However, the average growth rate was consistent with previously reported results[19].The starting times for continuous fermentation in the CCAB and RDMAB were determined to be 70 and 50 h, respectively, based on batch fermentation. The initial dilution rates during continuous fermentation in the CCAB and RDMAB were 0.01297 and 0.01684 h-1,respectively.The initial dilution rate during continuous fermentation in the RDMAB was higher than that in the CCAB,which shows that rapid substrate consumption promotes biomass increase and substrate accumulation in the RDMAB.
Due to the high oxygen demand for the growth, maintenance,and metabolism of C. tropicalis, a sufficient oxygen supply is required in the bioreactor. The dynamic membrane in the airlift bioreactor provides fine bubbles by combining membrane micropores and the dissipation energy produced by dynamic membrane rotation.The bubble size in dynamic membrane aeration can reach the micron level to increase the specific surface area of the bubbles,which significantly increases the kLa and provides sufficient DO for microbial culture. Fig. 9 shows the biomass concentration, aeration, RNA concentration, and sugar concentration at 20% DO in the bioreactor for the CCAB and RDMAB while maintaining a constant dilution rate.Biomass accumulation in the RDMAB was 9.71%higher than that in the CCAB, and the RNA concentration in the RDMAB was 11.15% higher than that in the CCAB. The yield of RNA (RNA production/sugar consumed) in the RDMAB was 0.069 g RNA per gram of sugar,which was 11.29%higher than that in the CCAB(0.062 g RNA per gram of sugar).The average productivity of RNA in the RDMAB was 0.117 g∙L-1∙h-1, which was 8.3%higher than that in the CCAB (0.108 g L-1∙h-1). However, the air aeration of the CCAB was 59.4% higher than that of the RDMAB.If the CCAB and RDMAB need to provide a constant DO level, theCCAB must increase air aeration.However,with the increase in air aeration, bubbles break and coalesce in the solution, which may increase the shear stress and affect cell growth in the CCAB. The speed of the RDMAB was relatively similar to the reported speed of the stirred reactor [9]. The shear stress induced by stirring in the RDMAB had little adverse effect on the microorganism, which was confirmed by the RNA yield and biomass concentration. For aerobic fermentation, DO is not only a nutritional factor, but also an environmental factor. Compared with the perforated pipe on the CCAB, the rotation of the dynamic membrane in the RDMAB can increase the liquid velocity to promote gas–liquid mixing and further increase the kLa.The CO2level of the gas after fermentation in the RDMAB was 23.1% higher than that in the CCAB.

Table 3 Parameters of kinetic models.
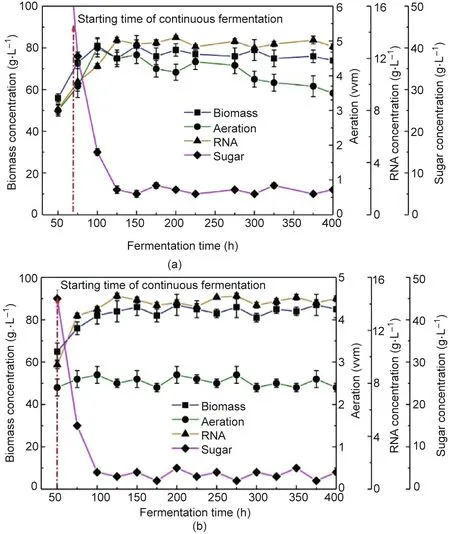
Fig. 9. Biomass, aeration, RNA concentration, and sugar concentration at 20% DO in (a) the CCAB and (b) the RDMAB.
4.8. Dilution rate during continuous fermentation
In aerobic fermentation bioprocesses,the oxygen demands vary between different microbial strains and fermentation stages. The DO level in the fermentation broth can directly affect enzyme activity,metabolic pathways,and product yield in microorganisms.During the fermentation process,kLa is mainly affected by the concentration of DO in the fermentation broth and the transfer resistance [45,51]. Dynamic membranes enhance the gas–liquid mass transfer coefficient by producing fine bubbles and promoting gas–liquid mixing[9].Therefore,it is important to study the effects of DO levels on fermentation to improve bioprocesses.The dilution rate is the key index for continuous fermentation.The dilution rate is directly proportional to the maximum specific growth rate,indicating that the higher the specific growth rate,the higher the dilution rate. As shown in Fig. 10, under the same air aeration (3.0 vvm),the DO in the CCAB was 76.9%lower than that in the RDMAB,which led to different dilution rates in the two airlift bioreactors in the continuous fermentation mode.The average dilution rate in the RDMAB was 39.6%higher than that in the CCAB.Therefore,the production capacity of the RDMAB in continuous fermentation mode may be greatly improved. Under the same air aeration (3.0 vvm),the RDMAB had a higher level of DO,which promoted the synthesis of the product.
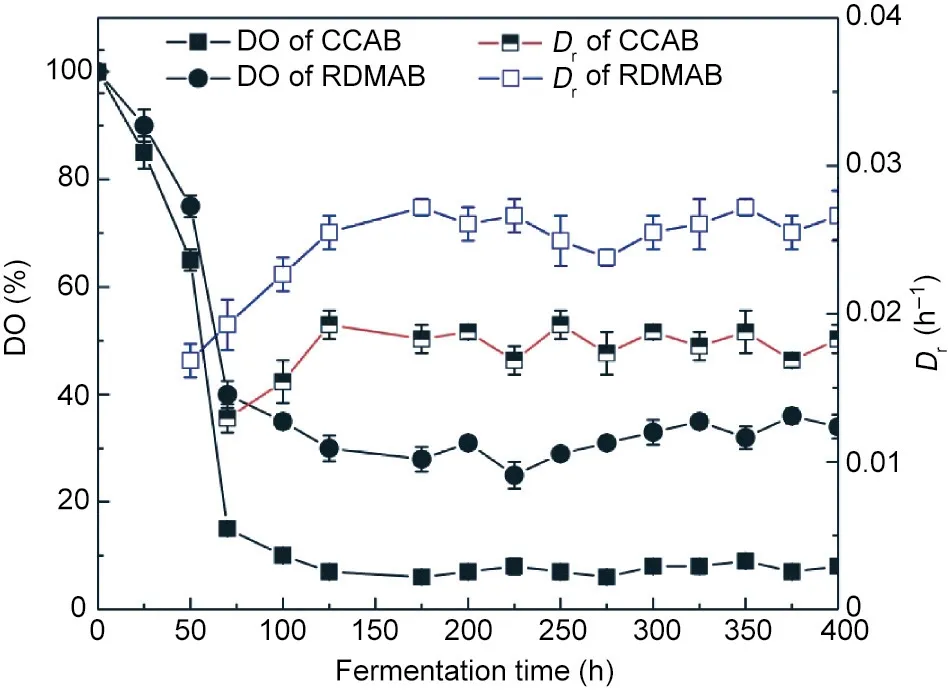
Fig.10. DO level and dilution rate at the same aeration rate (3.0 vvm) in the CCAB and the RDMAB.
5. Conclusions
In this study, we developed a novel RDMAB for producing fine bubbles to enhance the volumetric oxygen mass transfer coefficient (kLa) and gas holdup, as well as improve the biological process in the bioreactor.In addition,unstructured kinetic models for microbial growth, substrate utilization, and RNA formation by C. tropicalis were established. During batch fermentation, the biomass, RNA yield, and substrate utilization in the RDMAB were higher than those in the CCAB. We found that the starting time of continuous fermentation in the RDMAB was 20 h earlier than that in the CCAB, which greatly improved the bioprocess. The biomass accumulation in the RDMAB was 9.71% higher than that in the CCAB, and the RNA production was 11.15% higher than that in the CCAB. Finally, the dilution rate in RDMAB was 39.6% higher than that in CCAB. To summarize, the RDMAB was superior to the CCAB in both batch and continuous fermentation modes. Improving the structure of the CCAB has far-reaching implications for bioprocessing, in addition to providing a reference for the improvement of other types of bioreactors.
Acknowledgments
This work was supported by National Key Research and Development Program of China (2020YFE0100100, 2021YFC2104100,and 2018YFA0901500), Basic Science (Natural Science) Research Project of Jiangsu Province Colleges and Universities(21KJB530014), and Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture.
Compliance with ethics guidelines
Ganlu Li, Kequan Chen, Yanpeng Wei, Jinlei Zeng, Yue Yang,Feng He,Hui Li,and Pingkai Ouyang declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Electric Air Taxis Create Megadeal Buzz
- Tissue Engineering and Regulatory Science
- Factors Predicting Progression to Severe COVID-19: A Competing Risk Survival Analysis of 1753 Patients in Community Isolation in Wuhan,China
- A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses
- Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers
- Past and Future Changes in Climate and Water Resources in the Lancang–Mekong River Basin: Current Understanding and Future Research Directions

