Microfluidic Generation of Multicomponent Soft Biomaterials
Yuetong Wang, Luoran Shang*, Yuanjin Zhao*, Lingyun Sun*
a Department of Rheumatology and Immunology,Nanjing Drum Tower Hospital&School of Biological Science and Medical Engineering,Southeast University,Nanjing 210096,China
b School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Nanjing 210046, China
c Shanghai Xuhui Central Hospital & Zhongshan-Xuhui Hospital & Shanghai Key Laboratory of Medical Epigenetics & International Co-Laboratory of Medical Epigenetics and Metabolism of Ministry of Science and Technology, Institutes of Biomedical Sciences, Fudan University, Shanghai 200032, China
d Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health) & Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou 325001, China
Keywords:Soft biomaterial Microfluidics Multicomponent Microparticle Microfiber
ABSTRACT Soft biomaterials hold great potential for a plethora of biomedical applications because of their deformability,biodegradability,biocompatibility,high bioactivity,and low antigenicity.Multicomponent soft biomaterials are particularly attractive as a way of accommodating components made of different materials and generating combinative functions. Microfluidic technology has emerged as an outstanding tool in generating multicomponent materials with elaborate structures and constituents,in that it can manipulate multiphasic flows precisely on the micron scale.In recent decades,much progress has been achieved in the microfluidic fabrication of multicomponent soft biomaterials with finely defined physicochemical properties capable of controllable therapeutics delivery, three-dimensional (3D) cell culture, flexible devices and wearable electronics,and biosensing for molecules.In the paper,we summarize current progress in multicomponent soft biomaterials derived from microfluidics and emphasize their applications in biomedical fields. We also provide an outlook of the remaining challenges and future trends in this field.
1. Introduction
Soft biomaterials capable of readily deforming in threedimensional (3D) spaces under outside stimuli have been extensively studied in diverse areas [1,2]. Soft biomaterials, which are generally biopolymers, can be processed into different shapes and configurations and are highly attractive due to their versatile features [3–5]. Unlike hard-matter materials, soft materials typically exhibit an elastic modulus lower than 10 MPa, and their features are determined by two pivotal aspects: the properties of the structural units and their weak yet diversiform mutual effects[6,7]. Soft biomaterials possess unique properties such as shape memory, self-oscillation, stretchability, and biologically related characteristics including biocompatibility, biodegradability, low antigenicity,and high bioactivity[8–13].Therefore,soft biomaterials are broadly utilized in tissue engineering,biomedicine,biodiagnostics, electronic skins, and soft robotics [14,15].
The development of soft biomaterials has shown a trend from simple composition to multicomponent construction.Multicomponent soft biomaterials with varying composition, size, and configuration allow for the integration of different building blocks and ingredients, and can thus accommodate multiple functions [16–19]. Moreover, such composite biomaterials can be well-tailored to adapt to the requirements of more complicated application scenarios.Over the years,numerous methods have been employed for generating multicomponent soft biomaterials. These methods can be sorted into two major classes: those involving a ‘‘top-down”approach and those using a ‘‘bottom-up” approach [20,21]. A‘‘bottom-up” strategy involves the construction of building blocks including atoms,monomers,and nanoparticles through controlled self-assembly,oriented growth or reaction,and templated synthesis.Compared with‘‘top-down”methods(i.e.,traditional microfabrication methods such as photolithography), ‘‘bottom-up”strategies possess great potential to address intrinsic size limitations and processing restrictions,and are versatile in creating arbitrarily designed microstructures at relatively low cost [22].However, conventional bulk synthesis platforms are prone to exhibiting a lack of accuracy in the blending, nucleation, growth,assembly, and reaction processes, making it difficult to support the batch synthesis of multicomponent biomaterials[23,24].Thus,advanced fabrication methodologies with exquisite control over environmental parameters are desired to address these limitations.
Microfluidic technology is an excellent candidate for the generation of multicomponent soft biomaterials. It offers precise and controllable manipulation of flows at the sub-microliter scale,with liquids being confined in microchannels below a few hundred microns [25–27]. Compared with macroscopic systems,microfluidics provides a confined reaction system with a uniform physicochemical environment that is convenient to scale up[28,29]. In addition, multiphase fluids can be finely integrated via reasonable design of the microfluidic channels, thus achieving morphological and compositional diversity of the products[30–32]. This ability is highly advantageous for the preparation of multicomponent soft biomaterials. Therefore, microfluidics has been emerging as an advanced tool in the adjustable synthesis of diversified materials with sophisticated microstructures and multiple functions. A number of multicomponent soft microparticles,microfibers, and composite materials have been synthesized, and are finding extensive applications in the biomedical field [33–35].
Herein,we outline recent developments in the microfluidic synthesis of multicomponent soft biomaterials and their biomedical applications. We first introduce typical soft biomaterials and their unique physicochemical properties. Next, we describe the microfluidic fabrication platforms for the preparation of multicomponent soft biomaterials with diverse forms,particularly microparticles,microfibers,and other composites.We highlight the distinct compositions,morphologies,and salient virtues of these materials.Then, we present the applications of these multicomponent soft biomaterials in biomedical fields including drug delivery, tissue engineering, biodiagnostics, wearable devices, and soft robotics,with an emphasis on the unparalleled advantages of the microfluidic synthesis strategy. Finally, we discuss in depth the current challenges and future perspectives of microfluidic techniques for the creation of multicomponent soft biomaterials.
2. Typical soft polymers
Polymers and polymer composites account for the majority of soft biomaterials, including natural and synthetic ones [36,37].Typical soft polymers include hydrogels, elastomers, and so forth[38–40]. Hydrogels have become one of the most widely investigated soft materials, as they possess hydrophilic polymeric networks with high water volume and favorable biosecurity. They are also soft and possess Young’s modulus comparable to those of human tissues;thus,they have great value for biomedical applications, including drug release, tissue engineering, and biodiagnosis [41,42]. Elastomers—especially those with considerable toughness—can generally deform and restore their original shape under outside stimuli. Both hydrogels and elastomers are crosslinked networks of mobile polymer chains [43,44]. Several crosslinking approaches have been exploited for the manufacturing of polymer materials from monomer precursors,without which the prepolymers suffer from insufficient mechanical properties,posing a huge obstacle for practical applications [45,46]. After crosslinking,the polymeric materials obtain reinforced mechanical strength and enhanced stability due to the interconnected polymer chains. Common soft polymers and their representative crosslinking methods are listed in Table 1 [47–65].
2.1. Hydrogels
A hydrogel is a 3D network of hydrophilic polymers crosslinked via physical or chemical interactions. One of the most intriguing features of hydrogels is that they can hold water and maintain their shape, but can also deform and release water in response to a variety of external stimulating factors, such as pH, temperature,an electromagnetic field,and biological molecules[36].Hydrogels’moist properties and stimulus responsiveness are similar to those of the tissues of living systems.Through the process of responding to external stimuli,hydrogels undergo tremendous changes,which in turn modulate other properties.Abundant sources for hydrogels exist, including natural products, synthetic polymers, copolymers,and blends of natural and synthetic polymers.The chemical structures of some typical hydrogels are depicted in Fig. 1(a).
Polysaccharides, proteins, and bacterial biopolymers are common natural polymers that can be used to construct hydrogels.Polysaccharides are typical carbohydrates consisting of polymers linked by glycosidic bonds. They can be sorted into cationic, anionic, and nonionic categories depending on their electric charge[33]. Polysaccharides commonly possess a single kind of ionizable pendant group,such as the carboxylates of pectin or the amines of chitosan, which determines their charge density under certain pH conditions. Their physicochemical characteristics can easily be adjusted via surface modification. Polysaccharides exhibit favorable water solubility,biocompatibility,and biodegradability.These features, together with polysaccharides’ rich abundance and low cost, render them promising candidates for application in diversified biomedical scenarios, including efficient drug release, cell encapsulation, and biosensors [54,55].
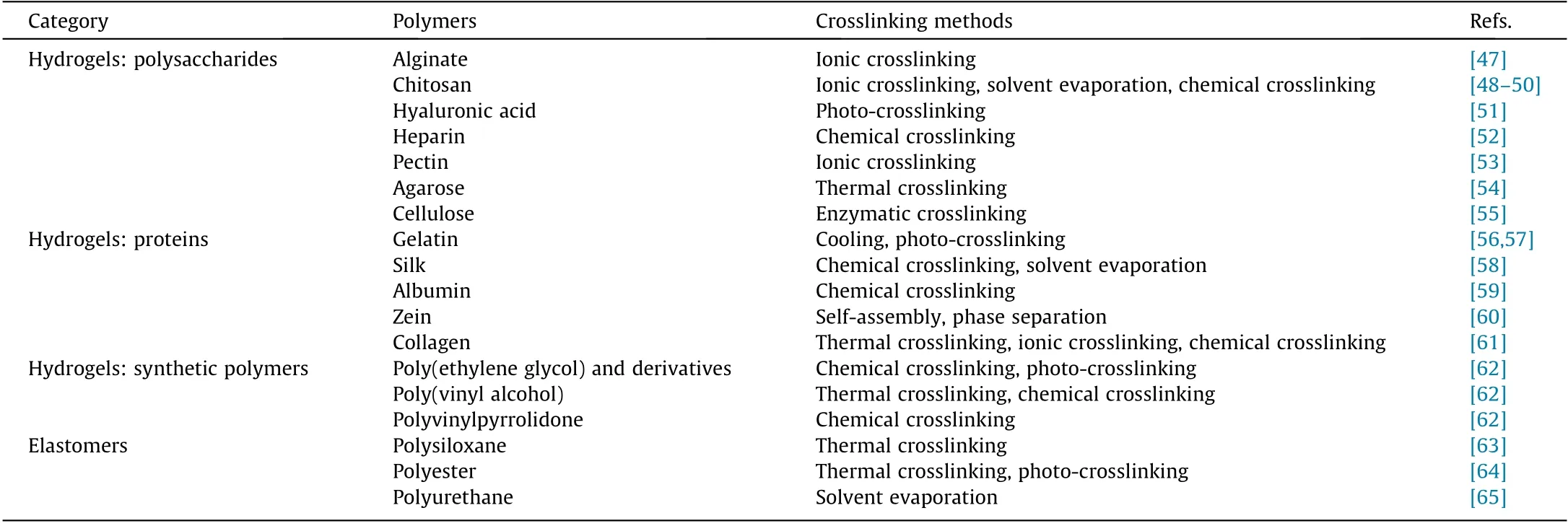
Table 1 Common soft polymers and their crosslinking methods.
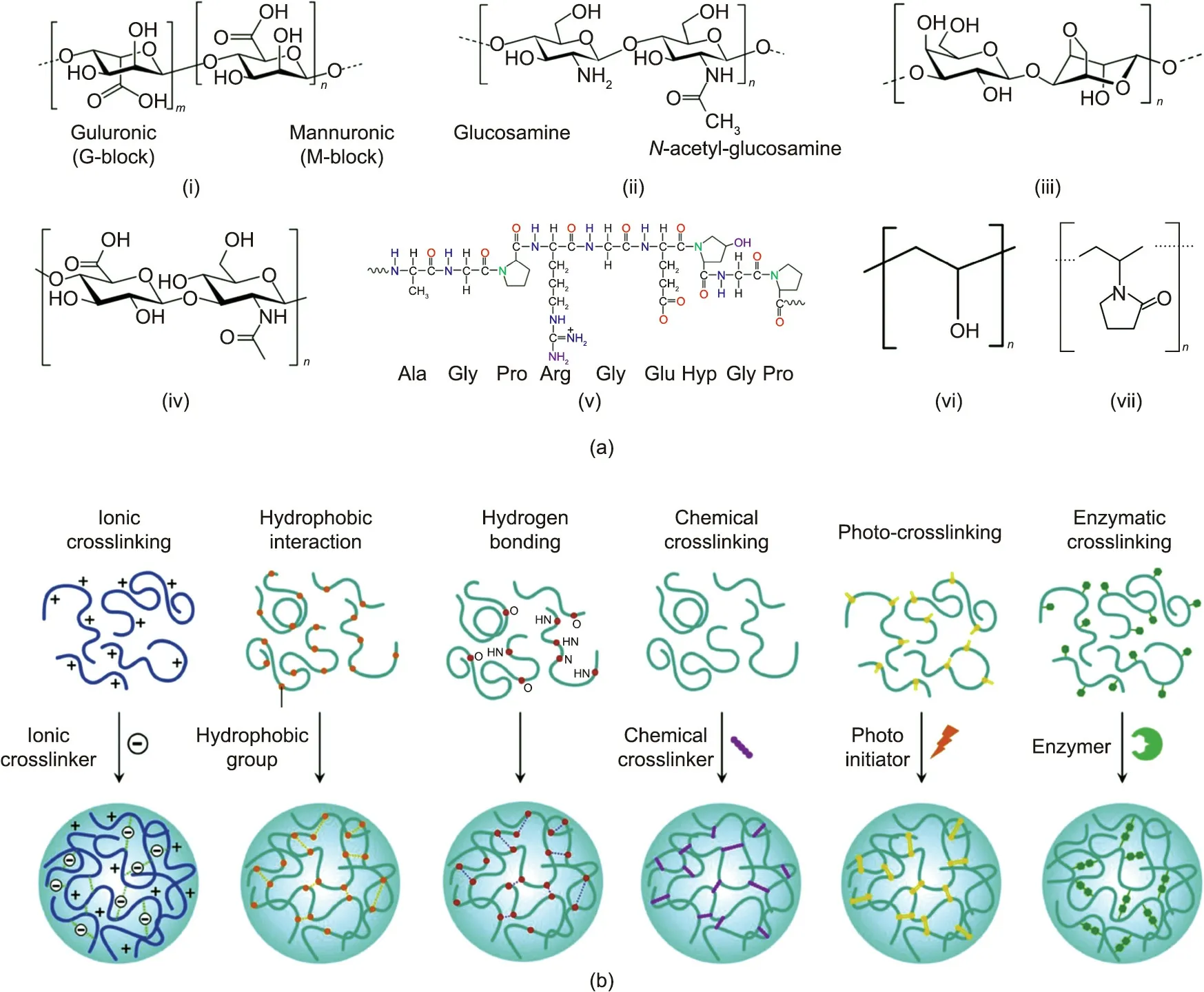
Fig.1. Chemical structures and common crosslinking strategies of the typical soft polymers.(a)Chemical structures of(i)alginate,(ii)chitosan,(iii)agarose,(iv)hyaluronic acid, (v) gelatin, (vi) poly(vinyl alcohol), and (vii) polyvinylpyrrolidone [33,62]. (b) Crosslinking strategies for generating soft biomaterials from monomer precursors,including ionic crosslinking, hydrophobic interaction, hydrogen bonding, chemical crosslinking, photo-crosslinking, and enzymatic crosslinking. (b) Reproduced from Ref.[33] with permission.
Proteins are generally made up of multiple amino acid residues linked by peptide bonds [66,67]. Globular and fibrous proteins are two major categories of proteins. Proteins play vital roles in maintaining life activities, including supporting and regulating the functions of cells, tissues, and organs. A protein’s sequence of amino acids determines its molecular weight,conformation, and other characteristics [68,69]. Proteins have been widely exploited to create soft biomaterials for various biomedical applications.
In addition to natural polymers, synthetic polymers are important sources of hydrogel materials. Synthetic polymer hydrogels differ from natural polymers in their properties because of the varied chemical structures of their building blocks. One of the most remarkable features of synthetic polymer hydrogels is that specific functions can be achieved to meet certain requirements. Tailoring the chemical ingredients or parameters involved in the synthesis(i.e., temperature, precursor concentration, crosslinking strategy,crosslinking elements, etc.) may give rise to novel properties.Poly(ethylene glycol) (PEG) is one of the most extensively utilized hydrogels. PEG-derived hydrogels are characterized by favorable biocompatibility, low toxic impact, and good swelling capacity,making them excellent candidates for drug delivery [70,71].Polyvinyl alcohol(PVA)-based hydrogels provide a humid environment that is beneficial for wound dressing and reconstruction and regeneration [72]. Polyvinylpyrrolidone (PVP)-based hydrogels,which can be obtained through a simple and clean radiation technique, are also widely used in biomedical fields [73]. As for novel soft biomaterials,previous works have shown that hydrogels with reversible crosslinking and dynamic networks can possess unique viscoelastic properties, which are important for applications in drug delivery and cell regulation[74–76].These viscoelastic materials can be incorporated into microfluidic soft biomaterials systems. For example, nanogels or nano-composites can be encapsulated into microspheres or microfibers, thereby facilitating control over the macro- and micro-properties of these materials.
2.2. Elastomers
Elastomers are polymers that possess an elasticity similar to that of rubber;they can repeatedly be deformed upon the application of an outside load and recover their primary shapes when the load is removed. Polyurethane (PU), polyolefin, and polyester are typical elastomers.Their elasticity mostly depends on the polymer dispersity and the molecular weight uniformity at the crosslinking positions [77]. Robust mechanical strength is derived from homogeneous polymeric networks. Elastomers are key substrates for smart textiles, wearable devices, and soft robotics. Polysiloxanes are the mostly commonly applied polymers in this field due to their favorable dielectric properties, convenient modification, and ready processing[63,78].The flexible material PU has high permittivity and outstanding abrasive resistance, while thermoplastic polyurethane (TPU) has superior shape-memory function and is capable of holding and recovering predetermined deformations under external stimuli.
2.3. Crosslinking of soft polymers
Typical crosslinking approaches for the fabrication of polymer materials from monomer precursors are shown in Fig. 1(b) [33].Supramolecular assemblies can be formed from chemical species held together by reversible and weak intermolecular mutual effects, such as ionic interactions, hydrophobic forces, and hydrogen bonding [79]. For example, adding divalent cations (e.g.,Ca2+) to polymer solutions (e.g., alginate and pectin) can result in fast solidification through ionic crosslinking [80]. Heatresponsive hydrogels based on protein, such as milk protein, can be made via thermal denaturation through hydrophobic interactions.For some polysaccharides(e.g.,agarose),cooling the solution after heating results in gelation,and the 3D structure can be stabilized via hydrogen bonding [81].
Chemical crosslinking is another method for crosslinking in which covalent bonds form between reactive functional groups.Gel networks linked by covalent bonds are relatively robust and durable, in contrast to those held together through physical interactions, and commonly display strengthened mechanical characteristics and prolonged stability within in vivo environments[82].Among the available chemical crosslinking strategies,Schiff’s base reaction is one of the most widely used ways of generating an imine linkage within amino and aldehyde groups under physiological conditions [83]. Photo-crosslinking and enzymatic crosslinking strategies are also appealing for curing in situ due to their fast reactions, which only require about 10 min [84].
3. Multicomponent soft microparticles from microfluidics
A diversity of multicomponent soft microparticles have been designed by applying microfluidics, which are generally derived from an emulsion template.Microfluidic platforms obtain uniform emulsion droplets by means of a multiphase fluid platform in which one fluid traverses through an immiscible flow of another liquid, leaving behind flexible interfaces. Microfluidics offers several intriguing advantages in the preparation of microparticles,such as accurate control over size, morphology, surface topography,compositions,and so forth [85]. A variety of multicomponent soft microparticles have been synthesized via emulsion droplets templates, including microspheres, non-spherical microparticles,hollow/porous microparticles,Janus microparticles,microcapsules,and complex multicore or higher order compartmental soft microparticles.
3.1. Droplet microfluidic technique
The most pivotal characteristic of the microfluidic-emulsion technique is its outstanding ability to continuously generate monodispersed emulsion droplets. Droplet generation can be accomplished by adjusting two immiscible solutions at the intersection position, where viscous stress and interfacial tension induce the pinching off of a dispersed fluid and thereby generate droplets. Most importantly, tuning the intersection geometry and the fluid parameters of the two phases allows the droplet size and monodispersity to be well controlled. Thanks to these advantages, droplet microfluidics has been broadly utilized to generate microparticles comprising diversified material components.
The most broadly adopted geometries are the co-flow(or coaxial junction), flow-focusing, and cross-flow (or T-junction) geometries, as shown in Figs. 2(a)–(c) [33]. In the co-flow channel, the inner and outer phases flow via two pipelines in a concentric manner (Fig. 2(a)). In the flow-focusing geometry, the dispersed phase is infused through an orifice and embraced by two streams of the continuous phase (Fig. 2(b)). In the T-junction geometry,two-phase fluids flow in a vertical configuration (Fig. 2(c))[33,85].The droplet size can be controlled by adjusting the viscosity of the dispersed and continuous phases,the flow rate ratio,the capillary tip size, and the surfactant concentration. In addition to passive droplet generation, active methods have been developed that rely on external forces,such as electromagnetic fields,thermocapillary actuation, acoustic-driven systems, and so forth. For example, Wang et al. [86] designed an electrodynamic fluidic microfluidic system that implemented an electrode-driven droplet generation setup. Firstly, the liquid was extruded out of the container under the actuation of an electric field.Then,when the electrode was switched off, the flow was turned off to form a narrow neck and ultimately pinched off, forming a droplet, as shown in Fig. 2(d) [86]. Low-volume requirements are actually a barrier in fabricating microparticles. In-air microfluidics produces droplets using jet ejection into the air and induces jet breakup and coalescence. The droplet generation frequency is twice that of chipbased microfluidic methods. In this method, as shown in Fig. 2(e)[87], a droplet train is extruded out of nozzle 1 and collides with a jet ejected out of nozzle 2.
3.2. Janus soft microparticles and multicompartmental soft microparticles
Janus microparticles are particles with a strictly biphasic geometry and two hemispheres of distinct compositions—with characteristics that endow two totally different sets of physicochemical properties and directionality to a single microparticle.When two dispersed phases come into contact with each other and are simultaneously emulsified by the continuous phase,a droplet with binary or multicompartmental phases can be generated[88,89].Janus or multicompartmental particles can then be synthesized by solidifying the corresponding droplets through phase separation, polymerization, solvent evaporation, and so forth.When the two internal components are soluble with each other,symmetry of the channel structure is required to ensure that the internal phases maintain an intact Janus structure inside the microfluidic channel.Otherwise,the two internal components tend to blend when forming droplets. However, when the two internal components cannot dissolve in each other, the extent of mixing between the two phases can be neglected [90]. Hence, apart from a symmetrical chip architecture,asymmetrical geometric channels(e.g., a T-junction) can also be used to generate Janus droplets,which greatly simplifies the microfluidic platform setup. Based on these principles,Zhang et al.[91]fabricated multicompartmental microgels by injecting multiple hydrogel precursor solutions through distinct channels and applied rapid gelation to each solution in formed droplets.As shown in Fig.3(a)[91],a flow-focusing polydimethylsiloxane (PDMS) chip was constructed to fabricate monodisperse alginate droplets. When alginate and calcium (Ca)ions came into contact, microgels with sharp interfaces between distinct compartments were formed. This approach is an example of the continuous production of multicompartmental soft microparticles with precise control over their structure. Maeda et al.[92]demonstrated a novel fabrication strategy for multicompartmental Ca–alginate hydrogel microparticles with uniform configurations by integrating a droplet generation platform based on centrifuge and a multi-barreled capillary (Fig. 3(b)). They utilized a capillary-based microfluidic chip and directed two independent inner fluids into the same outer phase.The fluids were then broken up into Janus and multicompartmental droplets.
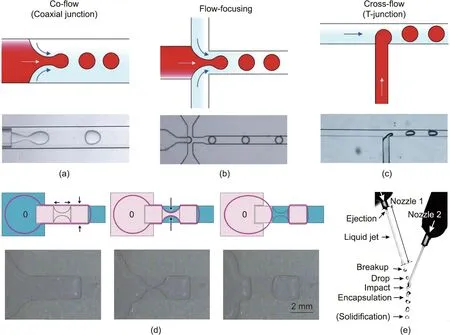
Fig. 2. Drop generation based on microfluidics. Microchannel geometries for producing droplets, including the (a) coaxial junction, (b) flow-focusing, and (c) T-junction regimes. (d) Schematics and images of an electrodynamic fluidic microfluidic system with an electrode design for the pinching off of droplets. (e) Photograph of in-air microfluidics in the ‘‘drop-jet” mode. (a–c) Reproduced from Ref. [33] with permission; (d) reproduced from Ref. [86] with permission; (e) reproduced from Ref. [87] with permission.
Aside from simultaneous emulsifying streams of multiple polymeric solutions,other approaches have been developed to generate Janus or multicompartmental polymer microparticles possessing clearly distinguishable anisotropic features. Min et al. [93]reported the generation of multicompartmental microparticles by taking advantage of phase separation,as shown in Fig.3(c).Homogeneous droplets composed of two immiscible polymers were fabricated from a capillary-based microfluidic chip. As the solvent evaporated,the two polymers’phase separation was triggered following the solidification process, resulting in the corresponding multicompartmental microparticles. Poly(N-isopropylacrylamide)(PNIPAM)is a polymer that is broadly utilized for synthesizing soft materials with biocompatible properties. In recent years, a supersaturated N-isopropylacrylamide (NIPAM) solution was reported to undergo a liquid–liquid phase separation process. Ghosh and Schurtenberger [94] prepared snowman-shaped Janus hydrogel particles with just one monomer aqueous solution flowing through the microchannel and breaking into droplets(Fig.3(d)).The partial evaporation of water triggered the phase separation of the NIPAM precursor solutions, resulting in the corresponding Janus microdroplets. Thermo-responsive PNIPAM microparticles were then obtained through the photo-crosslinking of the monomers via ultraviolet (UV) irradiation.
3.3. Core–shell soft microparticles
Core–shell microparticles are composed of a core in different forms encapsulated within a shell layer. Due to the exquisite core–shell structures, these microparticles provide diverse functionalities, such as efficient encapsulation and sustained or stimuli-responsive release. In conventional strategies such as bulk emulsification, the resultant core–shell soft microparticles suffer from nonuniformity in configurations, which makes them inadequate for practical requirements [95,96]. Droplet microfluidics addresses this dilemma with its capability to fabricate homogeneous droplets with highly controllable size and shape.Core–shell microparticles can be created by means of phase separation,assembly methods, solubility variation, and surface reactions. Yu et al.[97] employed the solvent evaporation and spontaneous miniemulsion polymerization principle to fabricate novel core–shell particles, as shown in Fig. 3(e). Alternatively, a multiple emulsion system can serve as the template for the preparation of core–shell microparticles via different solidification processes of the shell.For example, Li et al. [98] fabricated monodisperse particles comprising gelatin methacrylate (GelMa) cores and poly(lactic-coglycolic acid) (PLGA) shells derived from double-emulsion templates, with two distinct biopolymer solutions serving as the dispersed and continuous phases. The droplets then solidified after photopolymerization and solvent evaporation,as shown in Fig.3(f).
3.4. Porous soft microparticles
Porous particles are of great significance in biomedicine due to their intriguing morphology and application values. When generating porous particles using a droplet microfluidic platform,the pore formation mechanism can be categorized into five methods:triggered polymerization,a sacrificial template,a flow reaction,self-assembly, and flow lithography [99]. Inverse-opals are typical porous structures prepared by means of the sacrificial template method. Chen et al. [100] sacrificed photonic crystal particle templates and further obtained macroporous chitosan inverse-opal spheres with an interconnected porous structure.The silica colloidal crystal beads(SCCBs)were first derived from a droplet-templated,confined assembly of silica nanoparticles. Pregel solution was infiltrated into the spaces between the nanoparticles and was polymerized through UV exposure.After removing the template SCCBs in hydrofluoric acid, macroporous gel scaffolds were formed(Fig. 4(a)) [100]. The chitosan inverse-opal microparticles demonstrated outstanding performance in facilitating cell distribution and migration,as well as temperature-responsive drug release.Microparticles with a golf-ball shape exhibit dimple surface patterns and thus possess larger surface areas than smooth ones.Hwangbo et al. [101] designed microparticles with a golf-ball-like dimpled morphology and a porous interior through a solvent evaporation-induced phase separation process. Building on Hwangbo et al.’s achievements, Lee et al. [102] further increased the extent of the surface dimples by increasing the ratio of the polymers,as depicted in Fig.4(b).
Aside from applying single droplets as templates,double emulsions with several internal droplets can also generate porous microparticles. Costantini et al. [103] realized the fabrication of extremely adjustable porous microparticles by coupling droplet chips with secondary breakup units controlled via a pulsed electric field,as depicted in Fig.4(c).Firstly,monodisperse oil/water(O/W)emulsions were generated.Then,the emulsions were broken down into double microdroplets under the influence of the electric field,acting as templates to obtain porous microparticles.
3.5. Multicomponent soft microparticles with complex structures
Multicomponent soft microparticles with other configurations also exhibit salient characteristics and are useful in certain applications. By confining photocurable droplets inside microchannels with various sizes and configurations, anisotropic microparticles can be obtained either by means of UV-induced photopolymerization or through a thermal process. For example, Xu et al. [104]designed a comprehensive approach for producing tripropylene glycol diacrylate(TPGDA)microparticles with manifold shapes via tuning the droplet size and the cross-sectional construction of the microfluidic channels. When the droplet size was larger than the channel diameter, the droplets were remodeled to form nonspherical shapes (e.g., rods, cuboids, and disks). Wang et al. [105]encapsulated uniform microdroplets into polymeric networks through surface crosslinking within microchannels to generate fibrous matrices containing droplets. By stretching and squeezing the fiber-like matrices,inner microdroplets with diversified shapes could be formed. From these deformed droplets, diversified microparticles could be created, including tablet-, rod-, and needle-like microparticles. These particles were capable of magnetic-actuated rotational and translational behavior(Fig. 4(d)) [105]. Miyama et al. [106] prepared gel microparticles with a special yarn-ball shape using microfluidics, as shown in Fig. 4(e). These yarn-ball-shaped microparticles displayed the virtues of both spheres and fibers, and possessed an surface-area-tovolume ratio (SA:V) significantly higher than that of spherical microparticles.
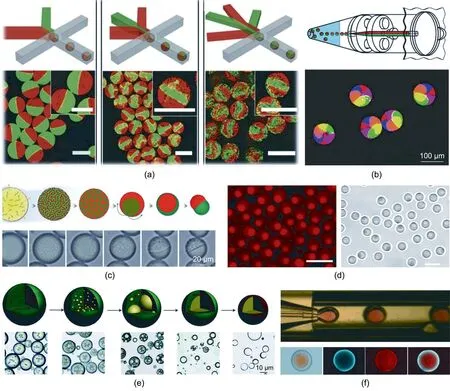
Fig.3. Microfluidic fabrication of Janus,multicompartmental,and core–shell soft microparticles.(a)Fluorescent microscopic images showing multicompartmental hydrogel microparticles made using microfluidic chips with several channels for dispersed phases (scale bar: 100 μm). (b) Centrifuge-based droplet formation from multi-barreled capillaries. (c) Series of schematic and optical microscope images showing the formation of acorn-shaped microparticles. (d) Fluorescent and optical microscopic images of snowman-shaped Janus poly(N-isopropylacrylamide)(PNIPAM)microparticles(scale bar:5 μm).(e)Poly(lactic-co-glycolic acid)(PLGA)core–shell microparticles originating from solvent evaporation and spontaneous mini-emulsion polymerization. (f) Core–shell microparticles possessing gelatin methacrylate (GelMa) cores and PLGA shells derived from double-emulsion templates.(a)Reproduced from Ref.[91]with permission;(b)reproduced from Ref.[92]with permission;(c)reproduced from Ref.[93]with permission; (d) reproduced from Ref. [94] with permission; (e) reproduced from Ref. [97] with permission; (f) reproduced from Ref. [98] with permission.
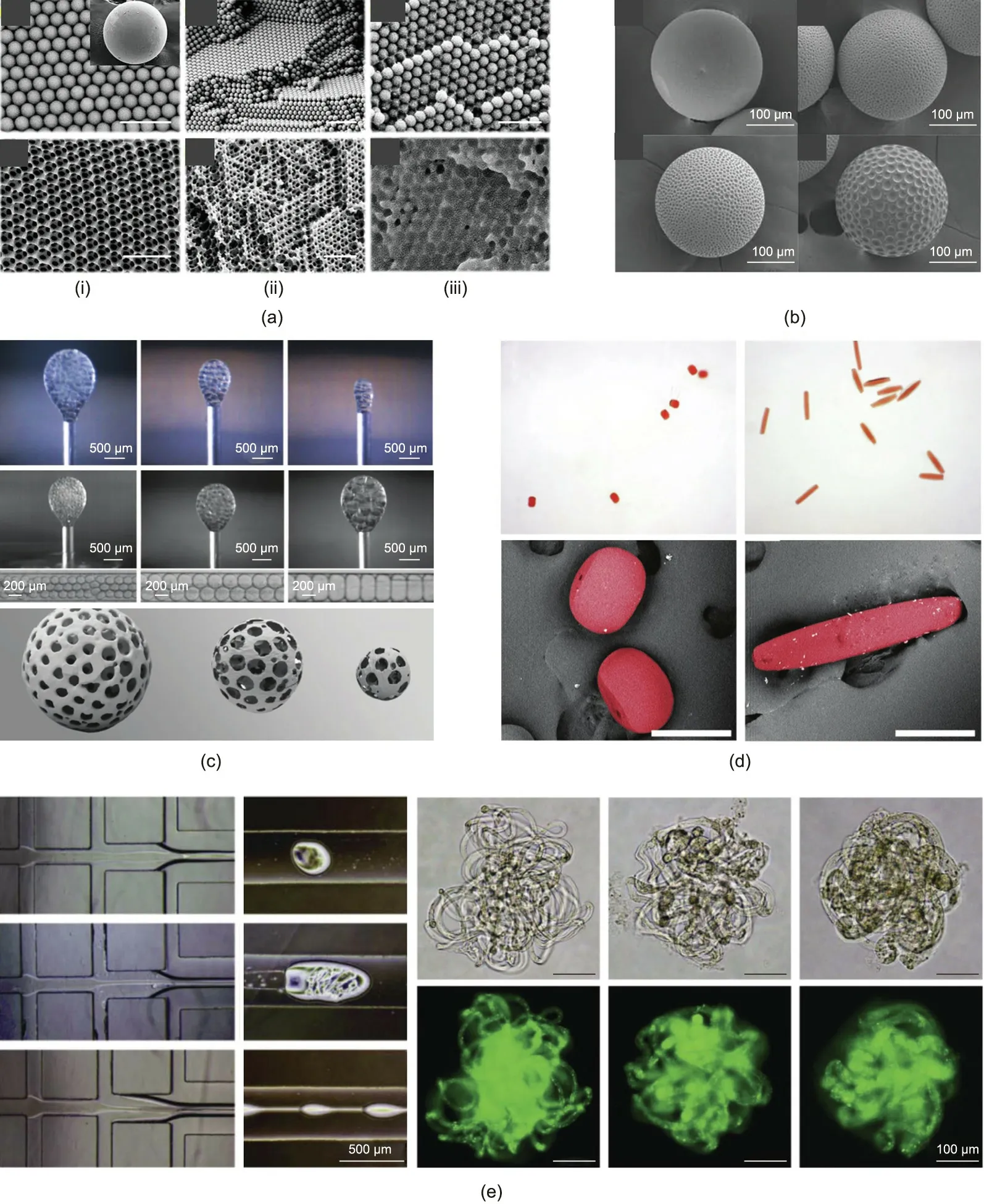
Fig.4. Microfluidic fabrication of porous soft microparticles and particles with other types of complex shapes.(a)Scanning electron microscope(SEM)images of the surface and inner microstructure of (i) SCCBs, (ii) inverse-opal particles with interconnected pores, and (iii) SCCBs with chitosan hydrogel filling the pores (scale bar: 500 nm).(b) SEM images of golf-ball-shaped microparticles. (c) Microscopy images of the production of monodisperse oil in water droplets and porous microbeads at three voltages with different values.(d)Images of tablet-like and rod-like poly(ethylene glycol dimethacrylate)(poly(EGDMA))microparticles solidified from deformed droplets in stretched fiber-like matrices(scale bar:200 μm).(e)Graphs demonstrating the multiphase fluids in the channels and the resultant yarn-ball-like microparticles.(a)Reproduced from Ref.[100]with permission;(b)reproduced from Ref.[102]with permission;(c)reproduced from Ref.[103]with permission;(d)reproduced from Ref.[105]with permission;(e) reproduced from Ref. [106] with permission.
4. Microfluidic spinning of multicomponent soft microfibers
Microfibers include filaments with anisotropic properties and the materials composed of these filaments. Similar to the structures and functions of natural fibers, the shape of the fiber or the arrangement of the filament units contributes largely to the tensile strength or rigidity in a specific direction [107]. These fibrous materials play an important role in biomedical applications such as sensors, wearable devices, and biomedicine. Thus far,microfluidic spinning has been employed in the continuous fabrication of fiber materials with rich morphology and structures.The basic principle of microfluidic spinning is the convergence and solidification of a central jet within a sheath flow.To form a robust fiber prototype, the instability of the two-phase fluid interface must be attenuated, regardless of the channel geometry[108,109]. The methods used to solidify the central fluid can be roughly divided into three main types according to the properties of the central fluid:UV-induced photopolymerization,phase inversion, and ion crosslinking [110].
4.1. Microfluidic spinning technologies
Microfluidic spinning benefits from laminar fluid flow due to the micro-dimension of the channel. When multiple streams flow in contact with one another,their mixing is dominated by diffusion through the interface. Therefore, a flow of precursor solution fluid can be readily converted into solid fibers in situ via rapid solidification. Typically, a sample fluid containing precursor solution is pumped into a separate inlet channel. By adjusting the channels’construction, flows can be morphed into various patterns. Fluids with a desired configuration can be solidified to generate fibers and preserve the designed shapes. In this process, a rapid solidification process is crucial for maintaining the fibrous shape. In contrast to the conventional wet spinning process, massive solidification approaches can be integrated with microfluidic spinning technologies, including photopolymerization, chemical/ionic crosslinking, phase separation, solvent evaporation/exchange, and interfacial assembly [111–114]. These strategies make it possible to employ a wider range of materials for the fibers.Many soft biomaterials have been used in the preparation of fibers, including alginate, chitosan, GelMa, PLGA, and poly(ethylene glycol) diacrylate(PEGDA).Additives such as carbon nanotubes,metal nanoparticles, graphene oxide (GO), and cellulose have also been introduced into the spinning process to endow fibers with specific functions. In addition, responsive polymer components such as NIPAM can impart favorable dynamic responsiveness to the prepared microfibers [115–119]. Devices used for microfluidic spinning include glass micropipettes, PDMS microchannels, metal needles,and tubes[107].Meng et al.[120]reported a design rationale for tough and highly viscoelastic hydrogel microfibers by applying a microfluidic strategy. The steps combined the fabrication of a double network—which involved the rapid gelation of an agar phase through a thermal transition—and the subsequent UV-induced radical polymerization of acrylamide (AAm)-based monomer solutions. Ice water in an external square capillary efficiently cooled the inner tubular capillary, resulting in the formation of the first network.
Functional ingredients can also be synthesized in situ within the microfibers and can achieve well-defined functions. Because microfluidic spinning technology realizes single-step fabrication in a continuous manner, it is suited for the inclusion of functional ingredients within microfibers. By tuning the dimensions and geometries of the microchannels, diversified microfibers with different diameters and configurations can be fabricated, including hollow, core–shell, helical, and other complex shapes, some of which are difficult to achieve by means of traditional wet or dry spinning methods. Therefore, microfluidic spinning technology,with its advantages of cost efficiency, rapid mass transfer, high controllability, and continuous production ability, is excellent for producingmicrofiberswithstructuralandfunctional controllability.
4.2. Core–shell soft microfibers
Core–shell fibers can be fabricated using a coaxial microfluidic chip. For example, Liu et al. [121] developed a new type of fastresponse and highly elastic hydrogel material and subsequently applied it to a coaxial microfluidic chip to produce thermally responsive fibers with a core–shell structure, as shown in Fig. 5(a). Due to their remarkable temperature responsiveness,the fibers shrank in an environment with increased temperature;the shrinkage from the neatly arranged fiber array drove the hydrogel to deform, which simulated the muscle-mimetic fiber’s movement.
4.3. Tubular soft microfibers
Tubular fibers are advantageous for physiological simulation,tissue engineering, and drug delivery. In general, hollow fibers are spun through a microfluidic device with a three-phase coaxial construction. The inner and outer diameters of the tubular microfibers can be tuned through the flow rate. Moreover, the high-throughput generation of such tubular fibers can be realized by means of parallelization of the fluid channels. Fujimoto et al.[122]designed a 12-microchannel microfluidic platform to support the high-speed, mass production of alginate microtubes. Daniele et al. [123] prepared poly(ethylene glycol dimethacrylate)(poly(EGDMA)) microtubes with a square cross-section using hydrodynamic shaping in square microchannels through in situ photopolymerization.Hu et al.[119]fabricated tubular microfibers by applying a series of materials including gelatin–hydroxyphenylpropionic acid (Gtn–HPA) and polysulfone. Live cells and biologically active ingredients, including growth factors, extracellular matrix (ECM), and nutrients, were immobilized into the hydrogel composite. Huynh et al. [124] designed a stretchable liquid handling device and an electrochemical impedimetric immunebiosensor made of tubular and conductive microfibers (Fig. 5(b)).These tubular microfibers,which were composited with a reduced graphene oxide (rGO) and PU matrix, were fabricated by means of a microfluidic wet spinning process and constituted the microchannels of the immune-biosensor device. Their highly conductive and stretchable properties enabled them to act as outstanding electrochemical working electrodes for detecting human sweat. Similar strategies for simultaneously generating solidified microfibers can be realized with micro-hole arrays.
4.4. Helical soft microfibers
Helical microfibers can be fabricated in a microfluidic device under certain conditions in terms of the viscosity and flow rates of the fluids,such that the fibers undergo a coiling instability.Furthermore, by integrating a variety of fluids, helical fiber materials with two or even multiple compartments can be prepared. Yu et al. [25] employed a coaxial capillary microfluidic system for the continuous preparation of helical microfibers, as shown in Fig. 5(c). Spinning was initiated when an inner sodium (Na)-alginate fluid flow was introduced into a CaCl2fluid flow, and the diffusion of Ca2+ions resulted in rapid gelation. Interestingly, the inner liquid jet started to change the flow patterns from a straight to helical state when the ratio of the inner to the outer flow rates increased.Accordingly,the diameter and pitch of the helical microfibers could be precisely controlled.
4.5. Janus, triple, and heterogeneous soft microfibers
Heterogeneous fibers with diverse cross-section morphologies,including Janus, multicompartmental, and other types, have been fabricated through microfluidic spinning.To generate Janus microfibers,a tri-phase microfluidic device equipping two separate input channels and one external channel is commonly utilized. Jeong et al. [125] fabricated Janus hydrogel fibers using colored and uncolored 4-hydroxybutyl acrylate (4-HBA) solutions as the core streams. As an alternative, Jung et al. [126] applied a microfluidic device with a simple diphase design to synthesize Janus PU fibers.During the spinning, PU isocyanate groups reacted with water in the sheath fluid and generated carbon dioxide bubbles. The bubbles then migrated upward and created a porous upper interface,thereby creating a Janus fiber with both porous and non-porous characteristics. Aside from Janus fibers, Kang et al. [127] also realized triple alginate microfibers concurrently encoded with different colored dyes; the device they used was composed of six individually controlled inlets equipped with pneumatic valves. It was notable that the fibers’ composition and morphology could be comprehensively regulated. Similarly, Bell et al. [128] realized the microfluidic spinning of triple‘‘toothpaste”asymmetric supracolloidal microfibers. An assembly of Laponite clay disc- and branched copolymer-stabilized emulsion droplets resulted in the generation of tough but light composite microfibers.
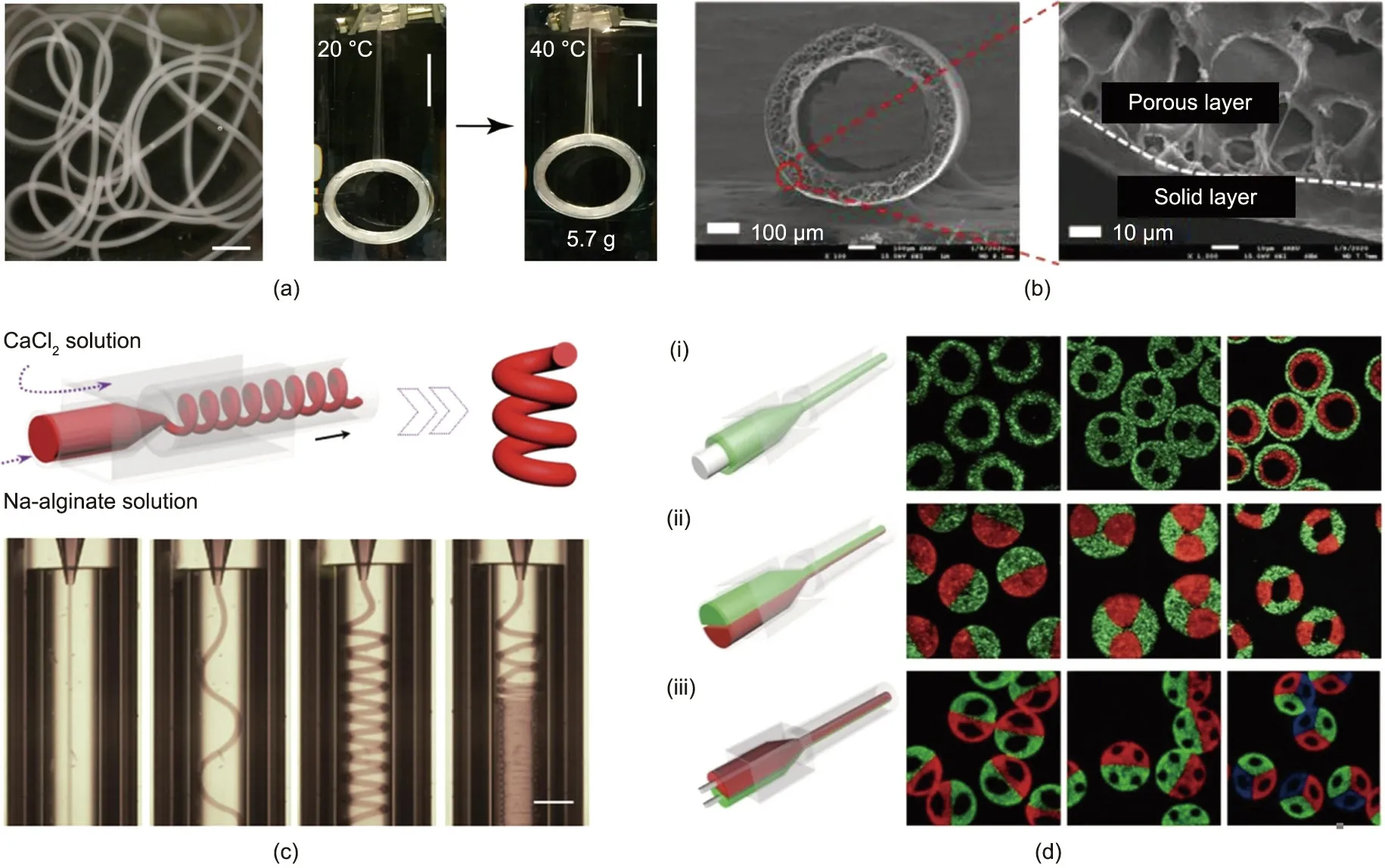
Fig.5. Diverse shapes of microfluidic-spun soft fibers.(a)Core–shell temperature-responsive hydrogel microfibers(scale bar in left:1 mm;scale bars in middle and right:20 mm). (b) Field-emission SEM images of an rGO/PU tubular microfiber and its wall including the solid and porous layer. (c) Helical alginate microfibers (scale bar: 350 μm).(d) Schematic illustrations and cross-sectional microscopy images of Janus, triple, and heterogeneous soft microfibers. (a) Reproduced from Ref. [121] with permission;(b)reproduced from Ref.[124]with permission;(c)reproduced from Ref.[25]with permission;(d-i,d-ii)reproduced from Ref.[129]with permission;(d-iii)reproduced from Ref. [130] with permission.
Cheng et al. [129,130] improved a simple microfiber spinning capillary device with multiple inlet channels and hierarchical injection channels in order to obtain microfibers with more complicated structures,as shown in Fig.5(d).Hollow alginate microfibers were fabricated by inserting one more calcium chloride (CaCl2) inlet channel in the previous alginate core channel (Fig. 5(d-i)) [129].The alginate was crosslinked immediately at the inner and outer interfaces once the three streams came into contact with each other.As a result, a hollow microfiber was continuously achieved in the channel.Similarly,microfibers with multiple holes were fabricated by inserting several CaCl2inlet channels inside of the alginate channel, while hollow microfibers with multiple shell layers were achieved by increasing the number of hierarchical injection channels in the capillary device.To achieve multicompartmental microfiber spinning, multi-barrel capillaries were used as the injection channel, instead of the single-channel injection capillary used in the original microfiber spinning device(Fig.5(d-ii))[129].Multiple fluids of alginate solution were pumped into the injection capillaries simultaneously and solidified in situ to form multicompartmental microfibers.Laminar flow was formed in the channels,and no obvious diffusive mixing occurred between different alginate streams,resulting in sharp and obvious interfaces between the different compartments of the microfibers. Microfibers with multicompartmental core–shell structures were also achieved by combining the features of the hollow fiber device and the multicompartmental fiber device,as presented in Fig.5(d-iii)[130].
4.6. Other composite soft microfibers
In addition to the abovementioned microfiber configurations, a great deal of research has been focused on constructing composite microfibers with the integration of microparticles. Xie et al. [131]presented necklace-like knotted fibers using a novel oil-free microfluidic spinning method. They also controllably fabricated hemisphere-and petal-knotted fibers,as shown in Fig.6(a).Shang et al. [132]integrated microfluidic spinning with emulsification in a single microfluidic chip comprising a coaxial double-layer injection tube.Through control over the flow rates,the outer layer fluid could be pinched off into spindle-shaped droplets strung on fibers that were generated through the fast gelation of the innermostlayer fluid. The resultant fibers possessed periodic spindle knots,and joints were generated,as shown in Fig.6(b)[132].In contrast,Yu et al.[109]presented a type of fiber with droplets encapsulated inside, which shrank into a bamboo-like morphology after dehydration, as shown in Fig. 6(c).
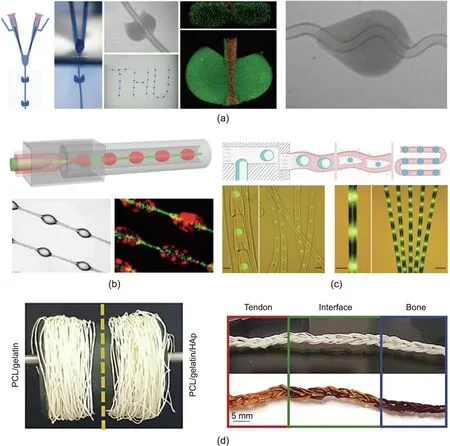
Fig.6. Examples of other composite soft microfibers.(a)Hollow alginate microfibers with hemisphere-and petal-shaped knots.(b)Spindle-knotted microfibers derived from a microfluidic device with a coaxial double-layer injection tube.(c)Bioinspired bamboo-like microfibers after dehydration.(d)Wet-spun microfibers collected in rollers,and their 3D scaffolds generated through crochet to mimic tendon, interface, and bone (PCL: polycaprolactone; HAp: hydroxyapatite). (a) Reproduced from Ref. [131] with permission; (b) reproduced from Ref. [132] with permission; (c) reproduced from Ref. [109] with permission; (d) reproduced from Ref. [133] with permission.
In addition,various strategies have been developed to assemble microfibers into higher order structures to create various 3D macroscopic fibrous constructs as tissue mimics. Calejo et al.[133] designed a 3D fibrous scaffold by assembling microfibers with a certain topography and composition that were tailored to mimic tendon and bone, as shown in Fig. 6(d). To realize this end,wet-spun microfibers were first generated from polycaprolactone (PCL)/gelatin and PCL/gelatin/hydroxyapatite (HAp) nano-tomicroparticles. Different flow rates in microfluidics were adjusted to tune the microfibers’diameter and mechanical properties.Then,crochet technique was applied to construct a 3D fibrous scaffold,and the scaffold’s biological performance was evaluated by culturing stem cells onto it. Besides weaving, a 3D microstructure was achieved through the layer-by-layer stacking of microfluidic-spun fibers—a method that could hold great significance for constructing tissue-mimetic fibrous networks in biological research [130]. Bioprinting is a promising technology for building arbitrarily shaped 3D fibrous architectures.By combining multiple microfluidic channels with bioprinting,a multicompartmental fiber assembly can be generated.Typically,a microfluidic bioprinting head with a multiinlet Y-junction channel feeding into a coaxial needle can be employed for this purpose [117]. More complex engineered tissue-like constructs can be fabricated by the further deposition of materials and cells through this type of printing head. These fiber materials have a wide range of applications in the coculture of different cells, multiplexed detection, and multiple sensing. It is worth mentioning that the slender and soft characteristics of the fibers make them flexible for further assembly into more complex fabrics and give rise to specific functions.
5. Applications of multicomponent soft biomaterials
Microfluidic technology can create diverse multicomponent soft materials in a uniform and highly controllable manner. Through the integration of specific components, novel materials can be imparted with certain physicochemical and biological characteristics, such as stimulus-responsive capacity, mechanical strength,biocompatibility, and flexibility. Hence, they have been widely applied in various fields, especially in biomedicine.
5.1. Drug delivery
The encapsulation and controlled release of active agents is essential in exploiting delivery systems for drugs, nutrients, fragrances, and cosmetics. For efficient drug delivery in particular,agents can be encapsulated within carriers in appropriate doses and released at a specific target location [134–136]. In drug delivery applications, the host’s immune response to the engineered soft biomaterials is a vital factor in their functionalization. The interactions of drug-loaded soft biomaterials with cells, tissues,and organs can assist the delivery process and the subsequent immune responses. These interactions are governed by the particle’s properties, including chemical moieties, rigidity, size, shape,mechanical properties, and surface charge. In particular, the surface morphology of drug-loaded soft biomaterials plays a role in efficient controlled drug release and local immunomodulation.However, drug carriers prepared through conventional batch methods suffer from a low encapsulation rate and poorly controlled release kinetics.Materials synthesized by microfluidics possess several advantages to overcome this dilemma. Hussain et al.[137] produced PLGA-b-PEG microparticles with uniform size and controllable surface textures by taking advantage of interfacial instability and the binary blending approach using the microfluidic flow-focusing technique,as shown in Fig. 7(a).The resultant polymer particles exhibited varied roughness and were employed for drug encapsulation. Interestingly, by varying the surface textures of the microparticles,the drug-loading efficiency and release kinetics could be tuned. It was demonstrated that these microfluidic polymer particles, which had a size of about 3 μm, could trigger the desired cellular uptake and exhibited superior biocompatibility.Based on the research by Hussain et al.,Wang et al.[138]developed anisotropic pollen-like wrinkled microparticles with tunable roughness through evaporation-induced interfacial instability, as shown in Fig. 7(b). The researchers found that these highly textured drug carriers can easily adhere to the forepart of the colon mucosa. Thus, drugs could be loaded into the polymer matrix for further interaction with human colon cancer cells and intestinal inflammation sites.
Hydrogel microparticles can also be applied in applications where physical isolation of transplanted cells from the immune system is needed to facilitate a therapeutic effect following delivery in vivo. For example, the delivery of insulin-producing islets has been widely studied for the treatment of diabetes. However,overcoming premature immune rejection following transplantation has been a major challenge. Hydrogel microparticles are an ideal platform for islet transplantation because they can be used to provide an immunoprotective barrier.The shorter diffusion distances (compared with those of bulk hydrogels) ensure the efficient exchange of nutrients and oxygen with the surrounding tissue to maintain islet survival and enhance the transfer of insulin from the islets to the surrounding vasculature [139]. Islets encapsulated in alginate microparticles prepared from microfluidic spraying can be used as a platform to achieve immunoisolation and enhance the therapeutic effect, as shown in Fig. 7(c) [139].Interestingly, one study examined the influence of material size by comparing host responses to alginate hydrogels prepared as microspheres of 100–1000 μm in diameter.Tuning the microchannels’ intersection geometry or the fluid parameters of the two phases made it possible to control the resultant microspheres’size,which subsequently helped in achieving the size-dependent immune response [140].
Like various other microparticles, microfibers can be a type of drug carrier. Compared with particulate carriers, fiber carriers typically show a lower initial burst release rate and a controlled zero-order release profile, making them ideal candidates for drug delivery applications. Moreover, by virtue of the cylindrical shape,fibers typically possess a high SA:V. Furthermore, compared with spherical vehicles, whose SA:V can only be adjusted by varying the radius, both the length and the cross-sectional radius can be varied for fibers.This adjustability is vital for drug delivery systems in practical applications. Marimuthu et al. [141] successfully utilized microfluidics to fabricate microfibrous scaffolds composed of amphiphilic triblock copolymer, with controlled porosity.Control over the porosity was obtained by combining the effects of immersion precipitation and solvent evaporation with the microfluidic spinning process.It was demonstrated that the porosity of the fiber had a notable effect on the release of the model protein, fibronectin.
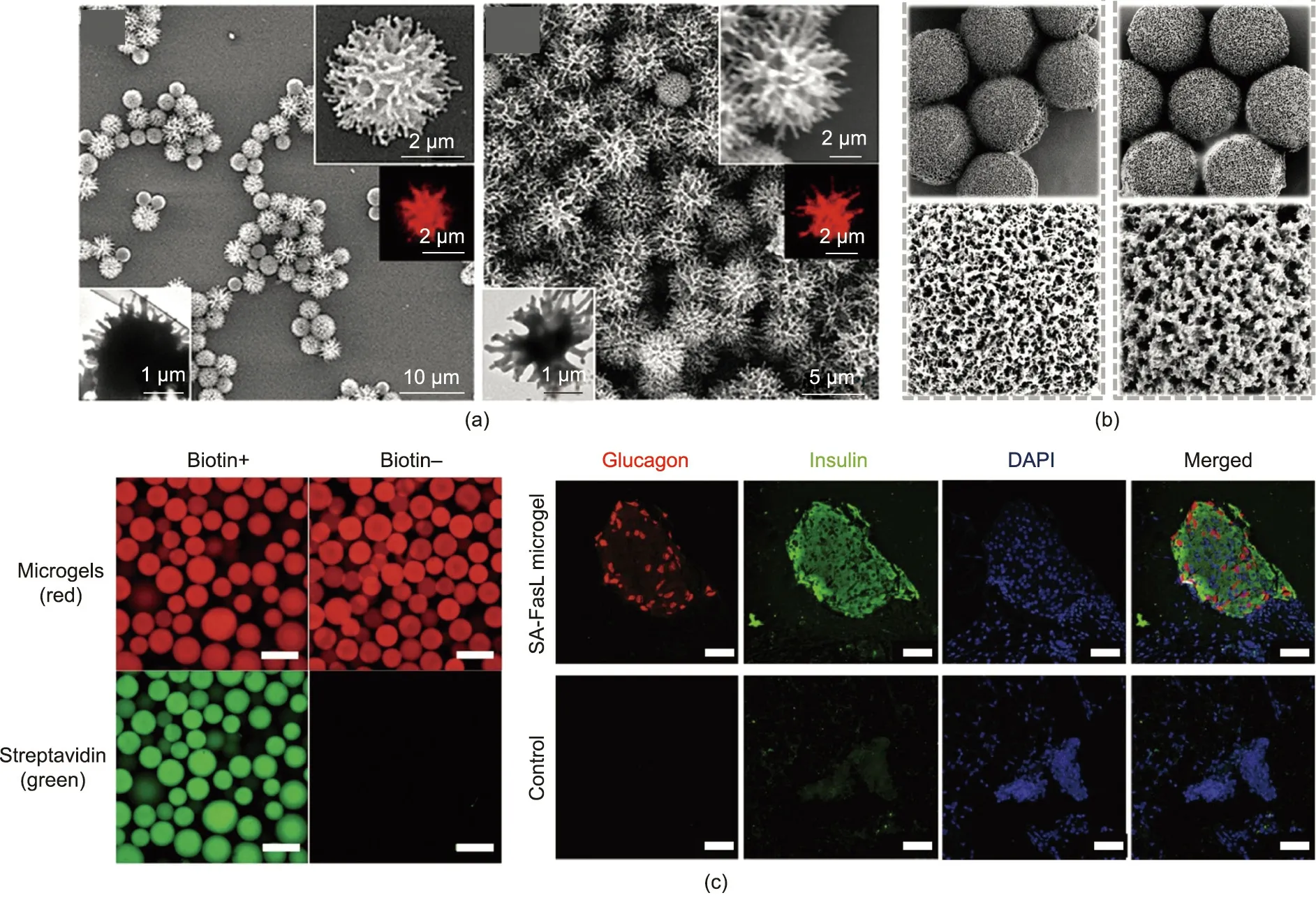
Fig. 7. Applications of multicomponent soft materials in drug delivery. (a) PLGA-b-PEG microparticles with varied roughness for drug encapsulation. (b) Highly textured microparticles that easily adhere to the colon mucosa and release drugs slowly.(c)(Left)Biotinylated microgels and their ability to capture streptavidin(scale bar:200 μm);(Right) immunostaining graphs of a long-term functioning graft from mice receiving microgels,showing glucagon-positive (red)and insulin-positive(green)structures and DNA(blue)(SA-FasL:Fas ligand with streptavidin;DAPI:4′,6-diamidino-2-phenylindole;scale bar:50 μm).(a)Reproduced from Ref.[137]with permission;(b)reproduced from Ref. [138] with permission; (c) reproduced from Ref. [139] with permission.
5.2. Tissue engineering
Tissue engineering has been demonstrated to be promising for tissue/organ regeneration and transplantation.The principle of tissue engineering involves several steps: Healthy cells are first isolated from the patient’s biopsy, then cells are expanded in vitro and seeded or encapsulated in a carrier.The engineered tissue construct is typically precultured in vitro and transferred into the patient to replace the damaged tissues. Tissues are 3D constructs consisting of multiple types of cells along with the ECM.The function of certain tissues is mediated by multiple cues, including intercellular signaling and cell–cell or cell–ECM interactions. Different types of cells can be morphed into microgel ‘‘modules”and integrated to recapitulate various types of tissues [142,143].Microgels prepared from microfluidics can serve as basic units for the construction of tissue engineering scaffolds. The applications of these tissue constructs have been extensively studied,and include bone/cartilage regeneration, stem cell culture and therapy, and more.
Gel matrices can be generated with hierarchical structures on demand to support cell culture and form 3D assemblies in which cells are organized in configurations analogous to those found in vivo, such as yarn-ball-shaped, core–shell, or tubular architectures. Fibers with core–shell structure can encapsulate cells. By applying a capillary microfluidic chip, Onoe et al. [117] fabricated meter-long core–shell hydrogel microfibers encapsulating ECM proteins and differentiated cells, including muscle cells, endothelial cells, and nerve cells. The fibers can be used to reconstitute the intrinsic morphological and functional properties of living tissues, such as blood vessels, muscle fibers, and nerve bundles, as shown in Fig. 8(a) [117].
Extensive research has been reported on the use of microfluidic spun microfibers to guide different cell types, such as myoblasts,fibroblasts,and cardiomyocytes.Kang et al. [144]fabricated cylindrical alginate fibers with microscale grooves engraved on the surfaces (diameter < 100 μm). Neuron cells were seeded onto the grooved fibers (Fig. 8(b)) [144] and aligned along the grooves.Aside from grooved fibers, patterned microfibers fabricated via other methods have also demonstrated capability for cell guidance.Yang et al. [145] constructed alginate fibers with an orientated submicron topography. The structure of the hydrogel fiber was tunable by changing the perfuse-in and spin-out rates of the fibers.Cells seeded on the surface of the shear-patterned fibers displayed an ordered spreading tendency along the fiber axis. Nguyen et al.[146] produced composite soft microfibers with a tadpole-egg shape by applying a triple-flow PDMS microfluidic device, as shown in Fig.8(c).These microfibers then acted as building blocks to create a 3D microwell template for cell cultures,thus providing tremendous differentiation capacity to chondrocytes for bone repair strategies.
5.3. Multiplexed bioassays
The increasing application of high-throughput assays in biomedical areas,including drug discovery and clinical diagnostics,demands effective strategies for multiplexing assays. One promising strategy is the use of barcode soft particles that encode information about their specific compositions to enable simple identification [147]. Microfluidics is an effective approach for the synthesis of barcode soft particles. The resultant particles have found important applications in the detection of multiple biological species, as they possess the properties of high flexibility, fast reaction times, less reagent consumption, and good repeatability.Compared with isotropic microcarriers, which commonly enclose a dye or fluorescent molecule for encoding, anisotropic multicomponent soft microparticles can effectively achieve amplified coding quantities: The extra coding information they carry, as well as the microparticles’own shape,composition,and certain patterned surface features, can become additional multiple coding elements.Therefore, the detection efficiency of anisotropic multicomponent soft barcodes is improved in comparison with that of traditional,isotropic ones [148]. Derived from microfluidic multipleemulsion templates, large amounts of exquisite multicomponent soft barcode particles can be fabricated. Kim et al. [149] utilized a sequential emulsification microfluidic method to encapsulate water-soluble red, green, and blue (RGB) coloring pigments in a transparent ethoxylated trimethylolpropane triacrylate (ETPTA)resin through a water-in-oil-in-water (W/O/W) double emulsion and constructed multicomponent encoded microcapsules. This system combined optical and patterned coding methods,achieving precise control of capsule size and composition by adjusting the flow rates. After surface modification, these microcapsules can be used for the specific recognition and detection of antigens and antibodies. Shang et al. [150] presented a photonic crystal (PhC)bubble barcode by generating microcapsules with a semipermeable membrane shell and a nanoparticle-encapsulated liquid core by means of droplet microfluidics(Fig.8(d)).The encapsulated colloidal nanoparticles self-assembled into a hollow spherical PhC shell at the inner wall of the microcapsules via solvent extraction.The overall density of the PhC microbubbles could be adjusted to match the density of a detection solution so that they remained in suspension. This strategy improved the barcode’s mobility and thus increased the detection efficiency. Bian et al. [151] decorated PhC barcodes with GO to quantify tumor-related microRNA(miRNA). The encapsulation of GO on the barcodes’ surface strengthened their robustness and resistance to incoherent light scattering, endowing the barcodes with a constant characteristic reflection peak. Moreover, GO fixed outside the barcodes could combine with miRNA probes via simple absorption,allowing these microparticles to become an effective platform for miRNA screening, as shown in Fig. 8(e) [151].
6. Summary and outlook
Soft biomaterials have long been used in biomedical fields such as tissue engineering, regenerative medicine, soft robotics, wearable devices, and biodiagnosis. In recent years, there has been a trend of diversifying these materials in terms of their structures and components for the purpose of integrating multiple functions.This concept has been turning into a reality with the simultaneous advance of manufacturing techniques, and microfluidics in particular. In this review, we summarized the microfluidic generation of multicomponent soft biomaterials. We first briefly introduced typical soft polymers,mostly hydrogels and elastomers,along with their ways of crosslinking. We then described how these polymer building blocks are shaped into multicomponent soft biomaterials with finely tunable configurations through microfluidics, with an emphasis on multicomponent particles and microfibers. Next, we discussed in detail the applications of these multicomponent soft biomaterials in biomedical fields.
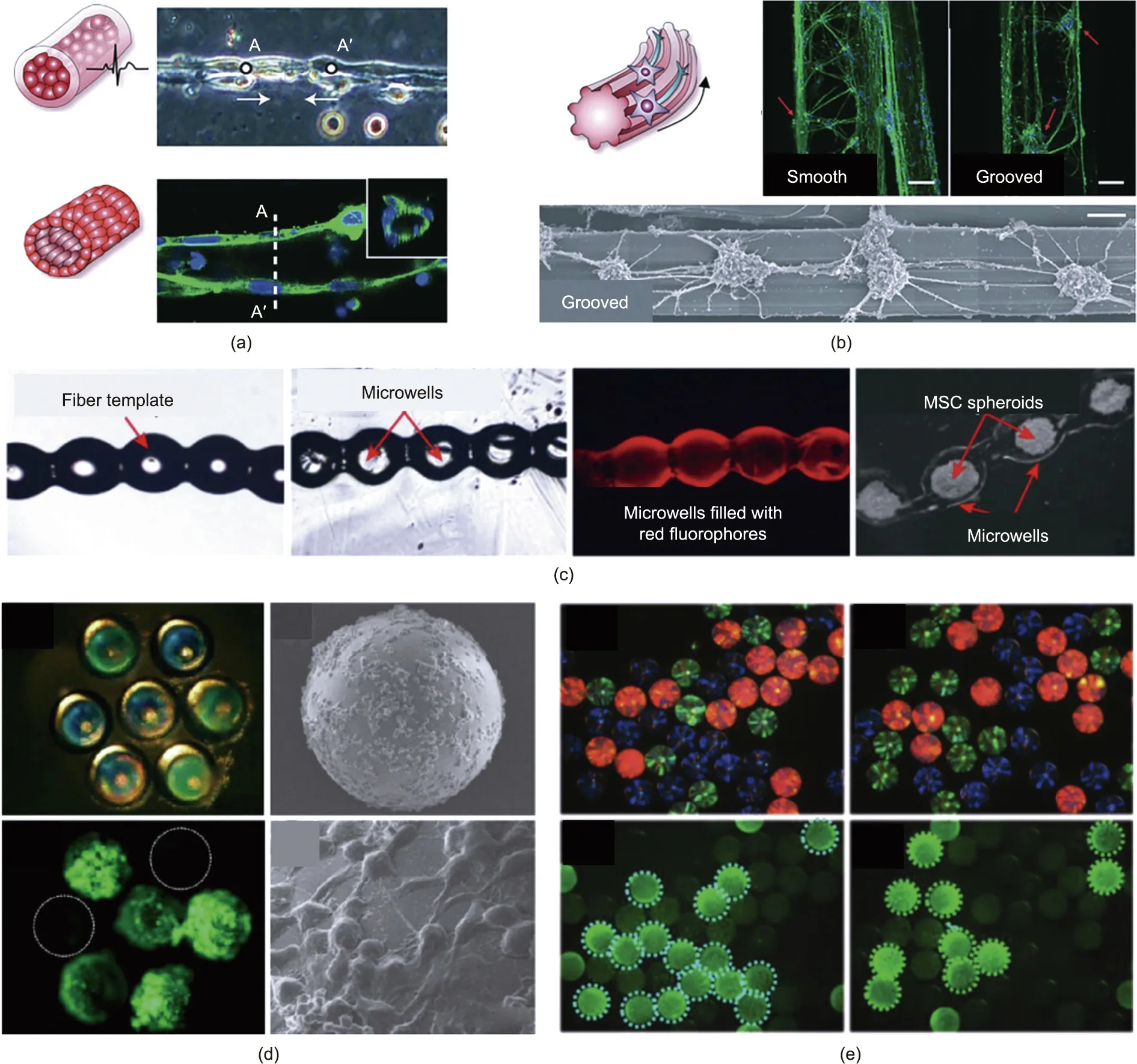
Fig.8. Applications of multicomponent soft materials in tissue engineering and multiplexed bioassays.(a)Fibrin fibers for primary cardiomyocytes encapsulation.(b)Cortical neuron alignment on flat micro-grooved alginate fibers (scale bar: 50 μm). (c) Application of tadpole-egg-shaped alginate microfibers for the culture of mesenchymal stem cell(MSC)spheroids.(d)Encoded photonic crystal(PhC)bubbles after immunoassay and HepG2 cell culture.(e)Optical microscopic images and fluorescence images of three kinds of GO-decorated PhC barcodes after incubating with target miRNA. (a) Reproduced from Ref. [117] with permission; (b) reproduced from Ref. [144] with permission;(c) reproduced from Ref. [146] with permission; (d) reproduced from Ref. [150] with permission; (e) reproduced from Ref. [151] with permission.
Despite the exciting achievements that have been made in recent decades, challenges remain in the microfluidic synthesis of multicomponent soft biomaterials and their applications in biomedical fields. To promote these techniques, diverse problems must be cautiously taken into account, and concerns need to be addressed. Firstly, new sources of soft materials are anticipated.Taking hydrogel as an example, in order to fulfill specific functions, a change in the chemical composition or synthesis factors may lead to novel biomaterials with desired functions and application values. To achieve this end, an in-depth understanding of the chemical, mechanical, and rheological behaviors of soft biomaterials is a prerequisite. Persistent endeavors to investigate various materials, including basic polymers and their composites, should be made. Secondly, for the fabrication of multicomponent soft biomaterials in microfluidics, more intricate channel designs might be anticipated to accommodate novel components. For example, some of the polymer solutions or melts used in microfluidics are non-Newtonian liquids, and their fluidic behaviors at the microscale should be taken into account. Endeavors aimed at scaling-up manufacture with uniform populations are also anticipated. Benefiting from the size limitation of the microchannels, the current maximum mass production rate has been dramatically affected. To realize batch production at an industrial level, novel and stable designs of scalable chips need to be adopted. Current achievements demonstrate that highly monodispersed droplets and the resultant microparticles can be obtained through parallelizing.However, less research has been conducted on the parallelized synthesis of microfibers and other complex composites. Finally,the utilization of multicomponent soft biomaterials is still in its infancy, especially in clinical translation. More comprehensive and profound research on characterizing the functionality and safety of multicomponent soft biomaterials in vivo is imperatively needed for more predictable biomedical applications.
To address these concerns, further studies may focus on two major directions: pursuing ingenious material components and developing novel microfluidic platforms to support the generation of novel soft biomaterials and realize mass production. Scalable production of high-quality complex composites is urgently anticipated to be achieved by exploiting systematic microfluidics with reference to integrated circuits. In addition, by further converging with other biofabrication techniques including 3D bioprinting, this technology can foster the fabrication of biomimetic lab-grown tissues. Given multidisciplinary efforts in soft matter physics, chemistry, life sciences, material science and engineering, and technical innovations, remarkable achievements can be anticipated in addressing current constraints in terms of either microfluidics or soft biomaterials. We believe that multicomponent soft biomaterials from microfluidics will inevitably provide new insights and guide substantial advancements in biomedical industries.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0908200), the National Natural Science Foundation of China (22002018, 52073060, and 61927805), and the Shenzhen Fundamental Research Program(JCYJ20190813152616459 and JCYJ20210324133214038).
Compliance with ethics guidelines
Yuetong Wang, Luoran Shang, Yuanjin Zhao, and Lingyun Sun declare that they have no conflict of interest or financial conflicts to disclose.
- Engineering的其它文章
- Electric Air Taxis Create Megadeal Buzz
- Tissue Engineering and Regulatory Science
- Factors Predicting Progression to Severe COVID-19: A Competing Risk Survival Analysis of 1753 Patients in Community Isolation in Wuhan,China
- A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses
- Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers
- Past and Future Changes in Climate and Water Resources in the Lancang–Mekong River Basin: Current Understanding and Future Research Directions

