Macroencapsulation Devices for Cell Therapy
Wei Liu, Yanfang Wang, Jinqiang Wang*, Olivia L. Lanier, Marissa E. Wehsler,Niholas A. Peppas,e,*, Zhen Guf,g,*
a Key Laboratory of Advanced Drug Delivery Systems of Zhejiang Province, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China
b Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
c Department of Biomedical Engineering, The University of Texas at Austin, Austin, TX 78712, USA
d Department of Biomedical Engineering and Chemical Engineering, The University of Texas at San Antonio, San Antonio, TX 78249, USA
e Division of Molecular Pharmaceutics and Drug Delivery, College of Pharmacy, The University of Texas at Austin, Austin, TX 78712, USA
f Zhejiang Laboratory of Systems and Precision Medicine, Zhejiang University Medical Center, Hangzhou 311121, China
g MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
Keywords:Drug delivery Cell encapsulation Cell therapy Cell transplantation Biomedical devices
ABSTRACT Macroencapsulation has been widely used in cell therapy due to its capability to provide immuneprivileged sites for implanted allogeneic or xenogeneic cells. Macroencapsulation also serves to provide mechanical and physiochemical support for maintaining cell expansion and promoting therapeutic functions. Macroencapsulation devices such as membrane-controlled release systems, hydrogels, microneedle (MN) array patches, and three-dimensional (3D) stents have shown promising in-lab and preclinical results in the maintenance of long-term cell survival and the strengthening of treatment efficacy.Recent studies focus on expanding the applications of these devices to new cell-based areas such as chimeric antigen receptor (CAR)-T cell delivery, cardiovascular disease therapy, and the exploration of new materials,construction methods,and working principles to augment treatment efficacy and prolong therapy duration.Here,we survey innovative platforms and approaches,as well as translation outcomes,for advancing the performance and applications of macrodevices for cell-based therapies. A discussion and critique regarding future opportunities and challenges is also provided.
1. Introduction
Cell therapies based on either native cells or engineered cells have been widely developed to treat a variety of diseases,including endocrine disorders[1–4], cardiovascular diseases [5], cancers [6–9], and neurological pathologies [10–16]. The treatments either directly utilize cells as the functional mediator or use cells to secrete active biomolecules to exert a therapeutic function [17–21].Cell therapy holds the potential to revolutionize the treatment methodology for a wide range of chronic pathologies and disorders[22]. However, the systemic or local injection of therapeutic cells can result in unsatisfactory treatment efficacy due to poor retention[23]and may induce severe safety issues[22].Distinct implantable device-based strategies for delivering cells have been engineered to overcome these obstacles and meet the vastly diverse requirements of disease treatment[24–27]. To be successful, encapsulation devices must permit the permeability of essential nutrients, such as oxygen and amino acids, in order to allow them to reach the cells, while also protecting the cells from the immune system in some cases. In addition, the encapsulation device should have ideal mechanical properties and biocompatibility to avoid inducing additional immune responses[28].In general,such approaches are classified as microencapsulation [29–31] and macroencapsulation [32,33], simply based on the scale of the device [34]. This review will focus on macroencapsulation-based devices (> 1000 μm in one dimension) because they can be efficiently functionalized and manufactured to meet diverse requirements for treating various diseases (Fig. 1). For instance, as one of the most widely-studied macroencapsulation devide, chamberbased device allows for precise control over porosity, pore size,and membrane thickness. Also, microneedle (MN) array patches can be prepared with MNs of varying lengths,geometries,density,and hardness.
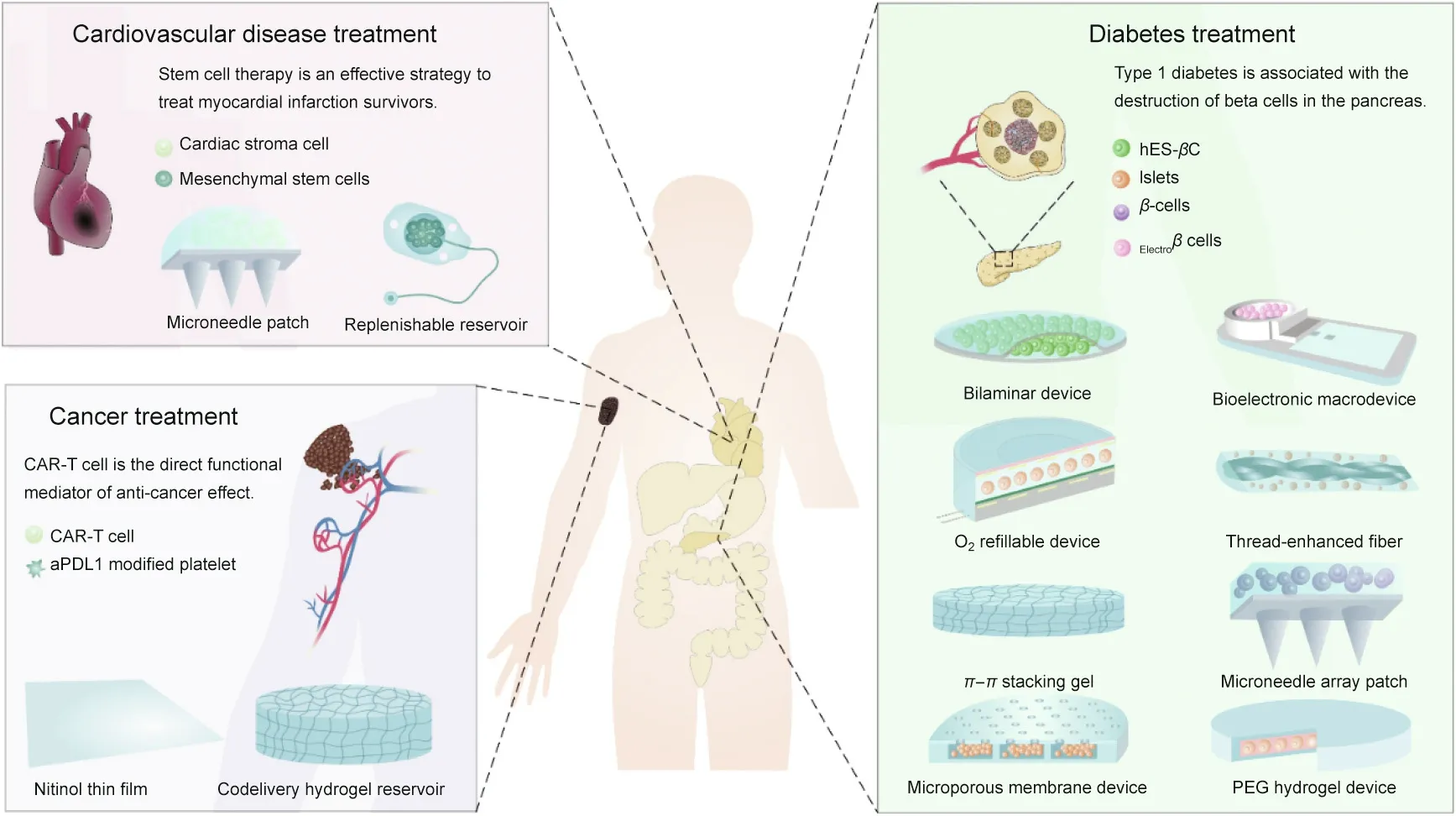
Fig. 1. The design and application of macrodevices for type 1 diabetes (T1D) treatment, myocardial infarction (MI) recovery, and cancer immunotherapy. CAR: chimeric antigen receptor; hES-βC: β-cells prepared from human embryonic stem cells; Electroβ cells: electrosensitive human β-cells; aPDL1: anti-programmed cell death ligand 1 antibody; PEG: poly(ethylene glycol).
Macrodevices can constrain transplanted cells inside devices,making the in vivo tracking of cells feasible.Furthermore,macrodevices allow for easy device retrieval from the body in case the device fails or causes an inflammatory response[32,35].Therefore,macrodevices have been used for transplanting islets or insulinsecreting cells for diabetes treatment[35–38].They have also been used to deliver antibody-secreting cells to treat neurodegenerative disorders by passive immunization [39,40]; cardiac stromal cells(CSCs) for treating cardiac disease [41]; chimeric antigen receptor(CAR)-T cells for promoting cancer immunotherapy [42]; and exosome-producing cells for treating Parkinson’s disease [43].
Here,we provide an overview of macrodevices for disease treatment, including preparation methods, materials selection, device properties, and their impact on in vitro and in vivo treatment outcomes. Because the requirements for using macrodevices to treat various diseases differ, the following sections are categorized by the disease focus of the developed device. Insights into the design strategy and function of the device are provided, along with a discussion of the opportunities and challenges in this field.
2. Macrodevices for diabetes treatment
Islet allotransplantation treatment currently remains limited to patients with severe insulin-deficient diabetes,due to the low supply of allogenic islets and the side effects that arise from the lifelong administration of immunosuppressive medication associated with transplants [44–46], which include an increased risk of inflammation and cancer [46]. Using macroencapsulation devices to isolate the implanted islets in artificial immune-privileged sites to protect the islets from the host immune system could reduce or eliminate the administration of immunosuppressive medications[18,32,47–49]. In addition, this method provides opportunities for the use of xenogeneic islets [50] and β-cells prepared from human embryonic stem cells (hES-βC) [22,51–55]. Macrodevices for these applications are categorized as membrane-controlled release systems, gel scaffold-based devices, or MN-based devices,all of which possess shared advantages such as easy retrieval and preparation[56–61].The critical issues associated with the clinical translation of macrodevices—such as fibroblastic overgrowth,poor vascularization [62], and host immune response [63]—are addressed below.
2.1. Membrane-controlled release devices for cell encapsulation
Membrane-controlled reservoir systems are generally containers sealed with a thin membrane that is distributed with suitable pores to facilitate nutrient and bioactive molecule exchange [64].The device shape and size, membrane thickness, and pore size can all be readily manipulated[35,65].To date,significant progress has been achieved in membrane-controlled release device-based cell therapies. For example, a cell-encapsulated device candidate is under development for the purpose of treating different types of diabetes[66].Meanwhile,strategies to reduce immune response[67]and provide amino acid[68]and oxygen gas diffusion through the membrane [69–71] are being pursued to improve the survival of implanted cells and augment the function of implanted devices.
2.1.1. Biocompatible devices with suitable membrane pores
Membrane-based devices consisting of two membranes and a depot in the middle have been studied recently with great success.After implantation,the foreign body response can cause the formation of fibrosis surrounding the device, especially the membrane,leading to the failure of matter transportation. Therefore, careful selection of membrane materials to reduce the foreign body response is critical for successful implantation and easy device removal. Moreover, maximizing the matter exchange rate, which is vital for cell survival and device function, is mainly determined by the pore size. However, the optimal size for membrane pores—which ranges from about 2 nm for some cytokines to 10 μm for cells—remains debatable regarding how best to achieve fluent matter exchange while minimizing the attack of harmful immune components toward implanted cells.
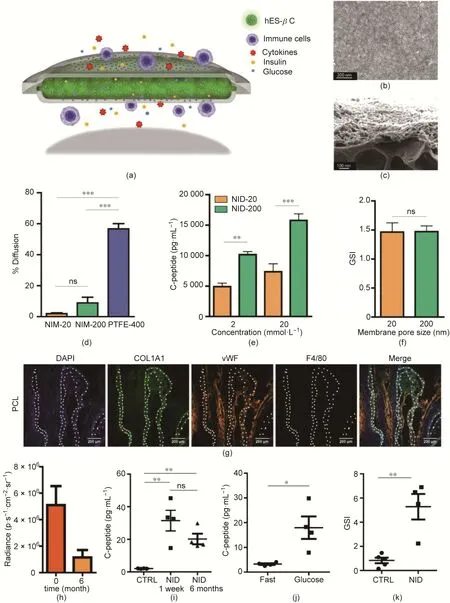
Fig.2. A PCL-bilaminar device for hES-βC encapsulation.(a)Schematic of the bilaminar device.(b)Typical scanning electron microscope(SEM)image of the PCL membrane.(c) Representative SEM image of a cross-section of the PCL membrane with the nanoporous layer and microporous layer. (d) Diffusion rate of immunoglobulin across the membranes within seven days.(e)C-peptide secretion by the encapsulated hES-βC after exposure to glucose solution(2 and 20 mmol∙L-1)(n=4).(f)GSI for hES-βC loaded in NID-20 and NID-200. The device was sequentially exposed to 2 and 20 mmol∙L-1 of glucose solution for 30 min. The ratio of the C-peptide concentration between 20 and 2 mmol∙L-1 was calculated and defined as the GSI (n = 4). (g) Representative images of the immunofluorescence staining of cell nucleus (DAPI), collagen (COL1A1),neovasculature(vWF),and macrophage(F4/80)markers of PCL thin film after it was subcutaneously implanted for four months in C57BL/6J mice.White dotted lines indicate the location of the PCL membrane. (h) Loss of implanted cells over six months, the bioluminescence intensity is expressed by radiance in photons per second per square centimeter per steradian (p∙s-1∙cm-2∙sr-1). (i) Detection of serum human C-peptide 60 min after intraperitoneal glucose challenge. Mice were implanted with NIDencapsulated hES-βC for one week and six months and fasted before the glucose challenge.Untransplanted mice were used as the control(CTRL)(n=4).(j)Serum human Cpeptide level before and 60 min after glucose challenge in mice implanted with NID-encapsulated hES-βC for six months and fasted overnight (n = 4). (k) GSI of implanted encapsulated or naked hES-βC(CTRL)for six months in NSG mice(n=4).*p<0.05,**p<0.01,***p<0.001.ns:not significant.Reproduced from Ref.[72]with permission of American Chemical Society, © 2017.
In one study,Chang et al.[72]developed a flexible bilaminar Ushaped device sealed with two layers of nanoporous polycaprolactone(PCL)membranes(Figs.2(a)–(c)).These nanoporous immunoprotective membranes (NIMs) were scattered with 20 nm (NIM-20)and 200 nm(NIM-200)pores and outperformed polytetrafluoroethylene (PTFE) membranes with 400 nm pores (PTFE-400) by inhibiting the diffusion of immunoglobulin through the PTFE-400(Fig. 2(d)). hES-βC sealed into the inner space of the device survived for longer than five weeks in the culture medium.Compared with membranes with 20 nm pores, membranes with 200 nm pores showed minor hindrance to insulin or C-peptide transportation across the membranes (Fig. 2(e)), even though a similar glucose stimulation index(GSI)of approximately 1.5 was observed for both devices(Fig.2(f)).When the membranes were implanted subcutaneously in immunocompetent C57BL/6J mice,no deposition of fibrotic tissues around the membranes was observed for one month. Meanwhile, increased blood vessel density and reduced macrophage recruitment were detected within four months (Fig. 2(g)). Significantly, nanoporous immunoprotective device with membranes with 200 nm pores (NID-200)robustly constrained the cells inside the device without leaking for six months, although approximately 75% of the cells were lost(Fig. 2(h)). After six months of implantation in immune-deficient non-obese diabetic (NOD)-severe combined immune-deficient(SCID)gamma(NSG)mice,glucose-stimulated C-peptide secretion was observed after intraperitoneal glucose injection(Figs. 2(i)and(j)), with a GSI ranging from 2 to 7 (Fig. 2(k)).
In another study, researchers tested a device that showed a reduced foreign body response and high permeability for antibodies orcytokines whileonlyblockingcells[50].Thedevicewascomprised of two parts:a polydimethylsiloxane(PDMS)reservoir with a depth of 150 μm and a porous polycarbonate track-etched (PCTE) membrane with various pore sizes (Figs. 3(a) and (b)). Engineered HEK293T cells(HEKepo cells)secreting mouse erythropoietin were encapsulated into the device.It was found that pores of 1 μm allowed macrophage infiltration into the device and that lowering the pore size to less than 0.8 μm prevented the infiltration of immune cells;although antibodies against HEKepo cells were generated in all groups,they did not cause notable graft damage.In further studies,the device was sealed with membranes with a pore size of 0.4 μm,which effectively prevented immune cell infiltrationwhile maximizing the exchange of oxygen(O2),nutrients,and bioactive molecules.
The membranes were further modified with three zwitterionic polymers and tetrahydropyran phenyl triazole (THPT)-derived polymer to reduce the foreign body response (Fig. 3(c)). The THPT-derived polymer outperformed the zwitterionic coatings in preventing fibrosis and maintaining cell survival after implantation in C57BL/6J mice. Then,islets mixed in alginate gel were encapsulated into the device, which was subsequently intraperitoneally implanted in streptozotocin(STZ)-induced diabetic C57BL/6J mice.The coated device helped maintain normoglycemia for an average of 75 d, while the uncoated device failed within 21 d (Fig. 3(d)).Moreover, only the THPT-coated cell device was able to return the blood glucose to the normal range in 120 min, similar to the blood glucose in healthy mice after the glucose challenge (Figs.3(e) and (f)).
2.1.2. O2refillable devices
Another consideration when designing membranes for cell encapsulation is an adequate O2supply, which is essential for the survival of implanted cells. One feasible method for providing O2is blowing air directly into the device.Ludwig et al.[73]developed a device called β-Air,which was integrated with an oxygen-rich air refueled tank.The O2could slowly penetrate through a PTFE membrane between the two compartments to reach the islets. Islets obtained from German Landrace sows were encapsulated inside the β-Air device [73], and then subcutaneously implanted in Göttingen minipigs for 13 d. The implanted and explanted devices exhibited GSIs of 3.5 and 6.7, respectively, when sequentially exposed to 3.3 and 16.7 mmol∙L–1of glucose solution. Later, this device was studied in combination with a growth hormonereleasing hormone (GHRH) agonist to enhance β-cell survival and cell proliferation after implantation [74]. It was further found that the surface area of the islet-containing slab, the ventilation method, and the gas composition could all affect cell survival[75]. For example, a density of 1000 islet equivalents (IEQ)∙cm-2and a thickness of 500 μm,in combination with frequent air perfusion or with the air chamber open to the air, could maintain cell viability. However, the implanted device with islets seeded at a density higher than 2000 IEQ∙cm-2failed to maintain normoglycemia in two days if no air or air with 20% or 30% O2was used.This device was then tested clinically at a dose of 2100 IEQ∙kg-1body weight[76].The air was refreshed daily to ensure continuous graft function for over ten months. The basal C-peptide level was only(0.04±0.03)mmol∙L-1,which is much lower than the average level of a healthy individual. After removing the chamber, the implanted site had a thin fibrous capsule and exhibited good vascularization and no signs of inflammation. It was notable that the islets retained their normal morphology after explantation with normal α- and β-cells.
2.1.3. Pre-vascularization devices
Pre-vascularization is another strategy used to enhance the O2and nutrient supply to promote cell survival. Pepper et al. [77]found that pre-vascularization of the implant site could greatly improve islet survival. They also designed a contractmanufactured biocompatible cell pouch (CP) device to be an immune-isolating and pre-vascularized site for islet implantation[78]. In this strategy, the CP was implanted for 4–5 weeks under the skin of mice to induce vascularization before it was filled with syngeneic islets. The blood glucose of the mice receiving islets loaded in CPs became normal progressively. One hundred days post-transplantation, 95% of the CP-treated mice reached normoglycemia.After the CPs were removed, the blood glucose returned to hyperglycemic levels within one week. It was notable that the islet grafts within the explanted CPs were viable and functioned normally. Cells transplanted without the CP failed to reverse diabetes, while a CP without pre-vascularization was not evaluated.
Mesenchymal stem cells (MSCs)and platelet-rich plasma (PRP)have also been investigated to promote angiogenesis [79,80].Inspired by this,researchers explored the use of three-dimensional(3D)-printed chambers consisting of biocompatible polylactic acid(PLA)[81,82].Microchannels were evenly distributed on the surface of the device,ensuring newly formed blood vessel penetration into the cell reservoir.PRP and MSCs were preloaded into the cell reservoir,which was further transplanted into the subcutaneous space in rats and nonhuman primates.Devices preloaded with PRP and MSCs resulted in a dense vascular network,providing a sufficiently rapid O2-transferring microenvironment to enhance the survival of the subsequently loaded islets and testosterone-secreting Leydig cells. Furthermore, a local immune-tolerant microenvironment induced by immune-suppressive molecules worked with prevascularization to further enhance the survival of grafted allogenic cells[83–85].Paez-Mayorga et al.[86]engineered a subcutaneously implantable dual-reservoir device with a central cell reservoir and bilateral drug reservoirs filled with MSCs and immunosuppressant(CTLA4Ig) to enhance angiogenesis and establish an immunosuppressive microenvironment to protect the allogeneic Leydig cells encapsulated inside the device.
Song et al. [87] developed microvascular meshes suitable for cell attachment to device surfaces in order to promote O2and nutrient supply by promoting neoangiogenesis and anastomoses.Human umbilical vein endothelial cells (HUVECs) incubated in a fibrin matrix could self-assemble into a vascular mesh on a micropillar substrate.This mesh stimulated the formation of dense functional vascularization on a diffusion chamber after two weeks.In SCID-Beige mice,the subcutaneous transplantation of a chamber with islets and microvascular meshes was able to maintain glucose in the normal range for three months.
2.1.4. Wireless bioelectronic device
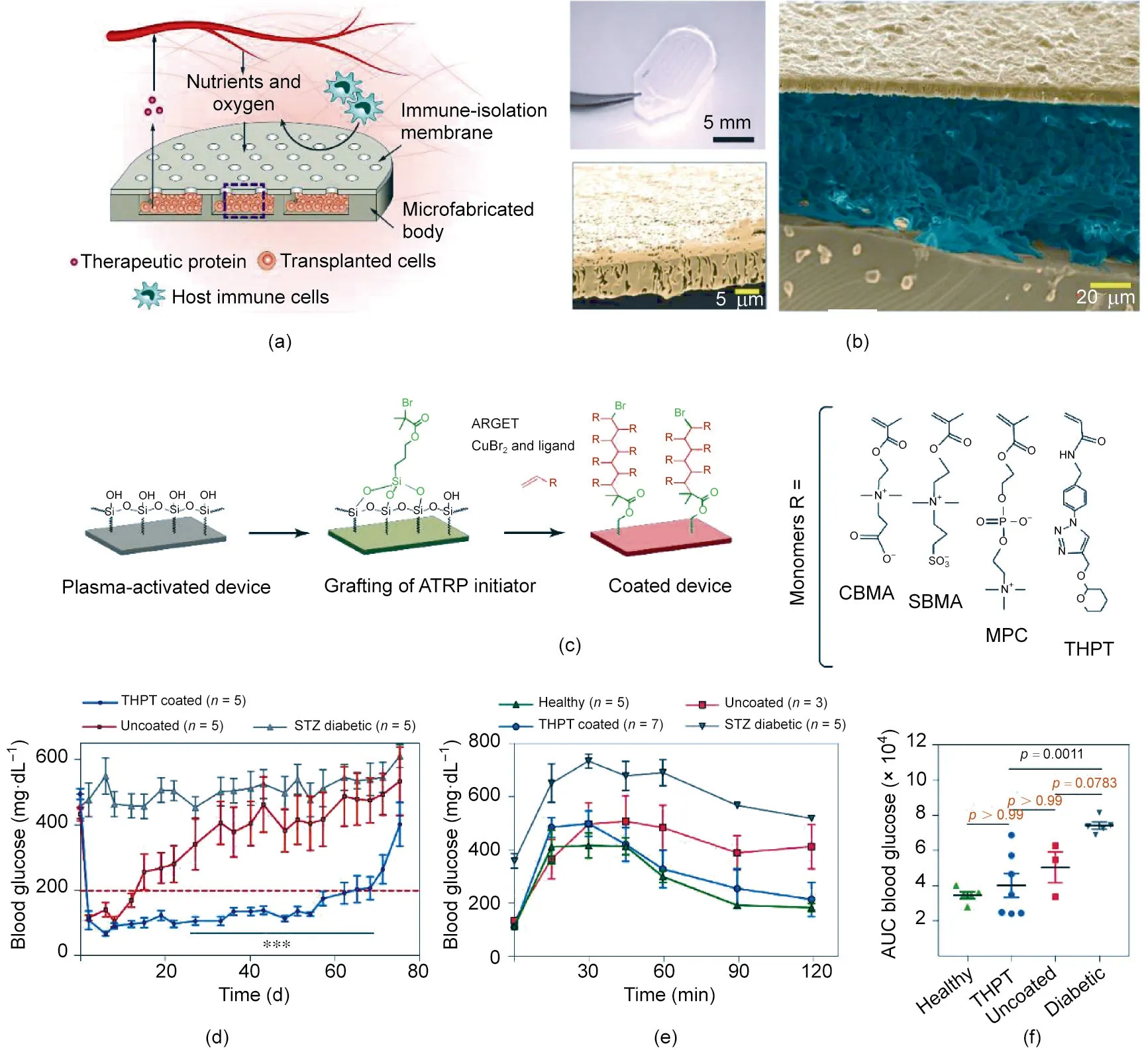
Fig.3. Microporous device for cell and islet delivery.(a)Schematic of the microporous device.(b)Representative images of the device:(top left)picture of a typical device for the mice experiment; (right) typical SEM image of the cross-section, where the image is false-colored to show the membrane, cells, and silicone body; (bottom left) falsecolored image of the membrane.(c)Chemical modification of the membrane.(d)Blood glucose levels of type 1 diabetic mice treated with the device loaded with islets(200 to 300 islet equivalents(IEQs)per device,n=5).(e)Blood glucose levels of healthy mice and diabetic mice after glucose challenge.The diabetic mice were treated with a device for 15 d. (f) Analysis of the data in part (e). ATRP: atom transfer radical polymerization; ARGET: activators regenerated by electron transfer; CBMA: carboxybetaine methacrylate;SBMA:sulfobetaine methacrylate;MPC:2-methacryloyloxyethyl phosphorylcholine;AUC:area under the curve.Reproduced from Ref.[50]with permission of Springer Nature Limited, © 2020.
The devices mentioned above all exerted their glucoseresponsive insulin secretion through the inherent glucoseresponsive circuit of β-cells. However, how to manipulate this circuit to control insulin release via an external stimulus remains elusive. β-cells in the islets of Langerhans secrete insulin in response to blood fluctuation. When the blood glucose elevates,the uptake of glucose through the glucose transporters of β-cells can generate adenosine triphosphate (ATP)intracellularly,causing an increase in the ATP/adenosine diphosphate (ADP) ratio. Then,ATP-sensitive K+channels close, leading to membrane depolarization and the subsequent opening of voltage-gated calcium channels (Cav), which results in an expedited inflow of calcium and the promotion of insulin secretion [88].
Building on this principle, Krawczyk et al. [89] designed an implant that contained electrosensitive human β-cells (Electroβ cells) and a customized bioelectronic interface, aiming to control the insulin release remotely for the treatment of type 1 diabetes(T1D)mellitus(Fig.4(a)).HEK293T cells were selected to be transfected with plasmids to express one of the L-type voltage-gated calcium channels on the membranes and secrete human placental-secreted alkaline phosphatase (SEAP) as the reporter.HEK293T cells expressing both the voltage-gated calcium channel Cav1.2 and the inwardly rectifier potassium channel Kir2.1 exhibited a reduced basal SEAP level and promoted a depolarizationtriggered SEAP release profile, as compared with the cells that expressed only voltage-gated calcium channels (Figs. 4(b) and(c)). A β-cell line variant named INSVescwith normal insulin secretion machinery but without glucose-sensing ability was then engineered to express both Cav1.2 and Kir2.1 and proinsulin-NanoLuc;this allowed the insulin secretion ofElectroβ cells to be easily controlled and monitored (Figs. 4(d)–(f)).
A wireless electrostimulated implant was designed and prepared for in vivo implantation(Fig.4(a)).In this macrodevice,platinum electrodes were installed on each side of the semipermeable membrane enveloping theElectroβ cells to apply electrical pulse stimulation to the cells. Furthermore, a switchboard was connected to the device to generate square unipolar pulses for electrostimulation. In a glucose tolerance test, the diabetic mice treated with the bioelectronic implant presented a blood glucose response profile similar to those treated with human islets, which quickly recovered to normoglycemia after 60 min with electric stimulation (Fig. 4(g)). In real-time blood glucose monitoring, the implant restored normoglycemia within 1 h after 30 min of electrostimulation (Fig. 4(h)). During the three weeks of transplantation, the implant containingElectroβ cells showed good biocompatibility, with negligible immune cell infiltration or device-related cytotoxicity.This system is advantageous,as it comprises real-time monitoring and response to glucose levels in the blood; however, it may be challenging to scale up production for these devices.
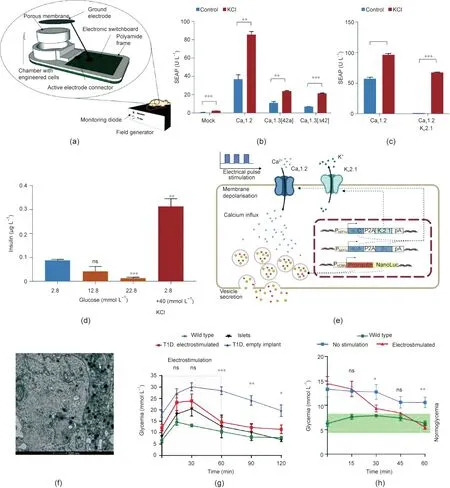
Fig.4. A wireless bioelectronic macrodevice loaded with Electroβ cells.(a)3D model of the durable microporous membrane device.(b)Comparative effect of calcium channels.U∙L-1:unit per liter.(c)Comparative performance of cells with Cav1.2 or cells co-expressing Cav1.2 and Kir2.1.Cav1.2,Cav1.3[42a],and Cav1.3[Δ42]are three L-type voltagegated calcium channels. Data are means ± standard error of the mean, n = 3; **p < 0.01, ***p < 0.001. (d) Glucose-induced release of insulin of Electroβ cells after exposure to varied glucose solution. Data are means±standard error of the mean,n= 3;**p<0.01, ***p<0.001. (e)Schematic representation of the insulin secretion pathway of Electroβ cells under electrical stimulation. PhEF1α-α2/β1-P2A-β3-pA and PhEF1α-α1C-P2A-Kir2.1-pA are domains of transgenic for constitutive expression of Cav1.2 and Kir2.1.(f) Representative transmission electron microscope (TEM) image of Electroβ cells. The white arrow points to a vesicle with insulin. (g) Glucose tolerance tests. After 48 h of implantation, the Electroβ cells in the device were electrostimulated for 60 min (red zone); then, the blood glucose levels of mice were monitored after the intraperitoneal injection of glucose.Wild type,n=8;T1D,implant electrostimulated,n=6;T1D,empty implant,n=10;islets,n=3.(h)Real-time monitoring and recording of blood glucose.Fasted type 1 diabetic mice implanted with devices were electrostimulated for 30 min,and blood glucose levels were monitored.The green zone indicates the normoglycemic range. Wild type, n = 6; no stimulation, n = 6; electrostimulated, n = 7. Reproduced from Ref. [89] with permission of AAAS, © 2020.
2.2. An MN array patch for cell delivery
MN array patches are another type of encapsulation device that has been investigated for the treatment of diabetes.In past studies,these devices have been widely investigated as a painless method for delivering insulin [90–92], vaccines [93,94], anticancer drugs[95–97], living cells [98], and other medications [99–102] for the treatment of various diseases [103–106]. The patches contain arrays of MNs on the surface,which can deliver the drug transdermally.In addition,MN array patches can function as a communication channel between cells located on the back of the MN patch and the physiological environment with which the MNs are in contact [41,107].
Ye et al. [107] exploited the usage of MN array patches for the delivery of insulin-producing cells to treat T1D (Fig. 5(a)). In this strategy, an MN array patch was prepared from hyaluronic acid(HA)(Fig.5(b)),which was further crosslinked using methacrylate groups and exposure to ultraviolet(UV)light.In order to maintain the survival of β-cells, the cells were encapsulated in alginate microgels applied to the back of the MN array patch (Fig. 5(c)). A synthetic glucose-signal amplifier(GSA)was incorporated because the diffusion of glucose from the interstitial fluid to the alginate microgel was slow.This patch could be directly applied to the skin,with the MNs inserted into the dermal tissue to sense the blood glucose fluctuation and subsequently release insulin in response to hyperglycemia.The insulin-secreting capability was maintained for at least three days. In type 1 diabetic mice, this cellularsynthetic hybrid patch could sense hyperglycemia and release insulin accordingly to maintain blood glucose in the normal range for more than 10 h (Fig. 5(d)). Moreover, sequential treatments of two MN array patches did not cause hypoglycemia (Fig. 5(e)). In this design,both the MN array patch and the alginate gel provided immune isolation for β-cells, protecting these cells from the immune rejection reaction. Meanwhile, the microscopic porous structure of the MNs offered a communication channel for glucose from the interstitial fluid and insulin-secreting β-cells. More work is needed to scale this device up for clinical applications.
2.3. Hollow porous fibers for cell encapsulation
An ideal macrodevice would be accompanied by dense vascularization on the device surface,which would promote matter transfer between the device and its surrounding physiological environment[70]. Meanwhile, increasing the relative surface area of the device could also facilitate matter exchange. In this pursuit, Tan et al.[108] fabricated a macrodevice using hollow microporous poly(ether sulfone)fibers with pore sizes of(0.27±0.02)μm.The surface of the membrane was further modified with the anti-inflammatory cytokine interleukin-4(IL-4)to enhance the polarization of macrophages toward the anti-inflammatory M2-type.Furthermore,fibronectin (FN) was used to modify the internal surface of the fiber to establish a biomimetic environment,thus promoting the adherence of a monolayer of β-cells to the internal surface of the fiber membrane.No significant difference in pore size before and after membrane modification was identified. The FN-modified device achieved a 10-fold increase in the number of β-cells attached to fibers,which confirmed the feasibility of this strategy.
Similarly, a polymeric nanofibrous device for syngeneic, allogeneic, or xenogeneic rodent islet transplantation to treat T1D has been investigated [109]. The device consisted of an immunoprotective hydrogel core with a nanofibrous skin made from electrospun medical-grade thermoplastic silicone–polycarbonate–urethane. The nanofiber with diameter less than 500 nm can prevent immune cell infiltration.When the device was implanted into the peritoneal cavity, diabetic behavior was reversed in immunodeficient and immunocompetent mice. When the device was scaled up for dog studies, xenogeneic immune responses were observed, although viable human β-cells were detected.
2.4. Hydrogels for cell encapsulation
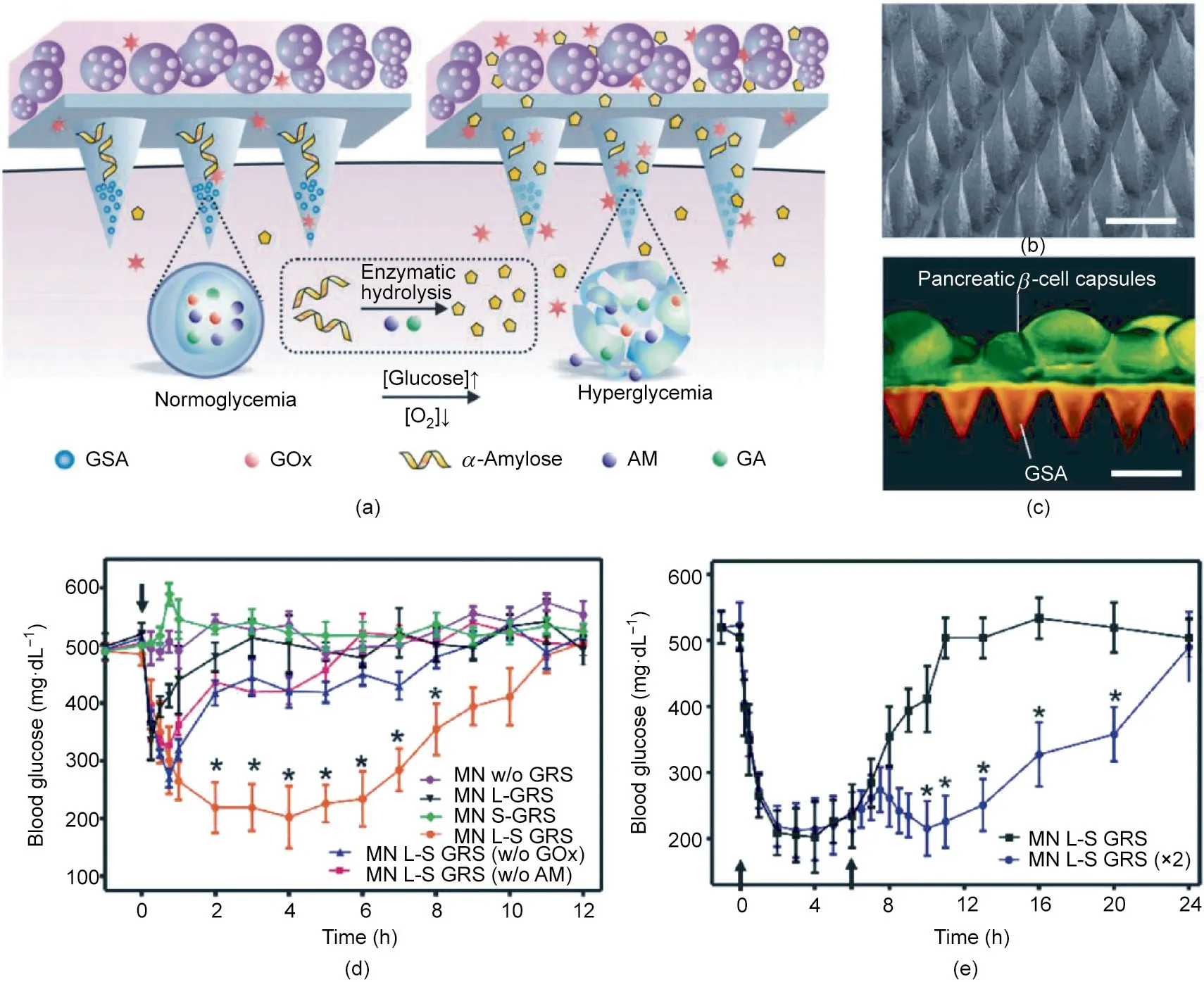
Fig. 5. MN array patch for the delivery of insulin-producing cells. (a) Schematic of the glucose-responsive mechanism. [Glucose] and [O2] represent the concentrations of glucose and O2,respectively.GOx:glucose oxidase;AM:amylase;GA:glucoamylase.(b)Typical SEM image.(c)Fluorescence image of cell-integrated MN array patch.GSAs were labeled with rhodamine,while cells were labeled with calcium-α-AM.(d)In vivo blood glucose regulation ability of the cell MN patches in type 1 diabetic mice.MN w/o GRS: empty MN;MN L-GRS:MN integrated with cell-encapsulated gel;MN S-GRS:MN with the amplifier but without cells; MN L-S GRS:MN was loaded with gel and was composed of amplifier system;MN L-S GRS (w/o GOx): MN L-S GRS without the usage of GOx; MN L-S GRS (w/o AM): MN L-S GRS without the usage of amylose.(e) Blood glucose of type 1 diabetic mice after treatment with two sequential MN patches. Reproduced from Ref. [107] with permission of John Wiley & Sons, Inc., © 2016.
Hydrogels are crosslinked macromolecular networks that swell in water or biological fluids.As their hydrophilic chains can hold a large quantity of water,hydrogels can serve as reservoirs for drugs,proteins,and cells for specific therapeutic purposes.Hydrogels can be prepared from either natural macromolecules such as alginate and gelatin or synthetic polymers such as poly(ethylene glycol)(PEG) and poly(2-hydroxyethyl methacrylate) (PHEMA) [110–116].Currently,researchers are aiming to enhance the mechanical strength of hydrogels, reduce the foreign body response to them,and improve their nutrient and O2supply in order to enhance cell survival and device function.
2.4.1. Polymer-thread-reinforced alginate hydrogel
Alginates are currently the most widely used materials for preparing hydrogels due to their satisfactory biocompatibility and easy hydrogel preparation[117–119].However,alginates present several limitations that need to be addressed. For example,alginates inevitably contain lipopolysaccharide, peptidoglycan,and lipoteichoic acid,all of which may be recognized as xenobiotic by the host immune system, leading to foreign body response and fibrosis. Moreover, alginate hydrogels cannot be used or removed easily due to their low mechanical strength. Thus, a handful of strategies have been developed to enhance their mechanical strength [120,121]. New biocompatible materials are also being developed to overcome the limitations of alginate [122–124].
An et al. [120] developed alginate gels with Ca2+-releasing poly(methyl methacrylate) derived nanoporous-coating threads for crosslinking alginate solution containing suspended islets to reinforce the strength of the hydrogel (Fig. 6(a)). The prepared alginate gel adhered to the thread tightly (Fig. 6(b)), with most of the islets remaining viable (Fig. 6(c)). The encapsulated islets secreted insulin in a similar dynamic to that of naked islets when exposed to glucose solution. This fiber gel loaded with rat islets was implanted into the peritoneal cavity of diabetic C57BL/6J mice, whose blood glucose decreased to the normal range two days after implantation and remained normal for four weeks(Fig. 6(d)). An intraperitoneal glucose tolerance test (IPGTT)(2 g∙kg-1) was performed on day 28 after transplantation. The blood glucose levels of either treated diabetic or healthy mice were normalized in 2 h (Fig. 6(e)). Furthermore, human islets loaded in this device were able to reverse hyperglycemia in diabetic immunodeficient SCID-Beige mice (Fig. 6(f)) and maintained normoglycemia for longer than four months. In addition, when a 10 in (1 in = 2.54 cm) long alginate gel was prepared and placed between the liver and diaphragm of dogs, the explanted islets remained alive and were able to secrete insulin in response to glucose changes (Fig. 6(g)).
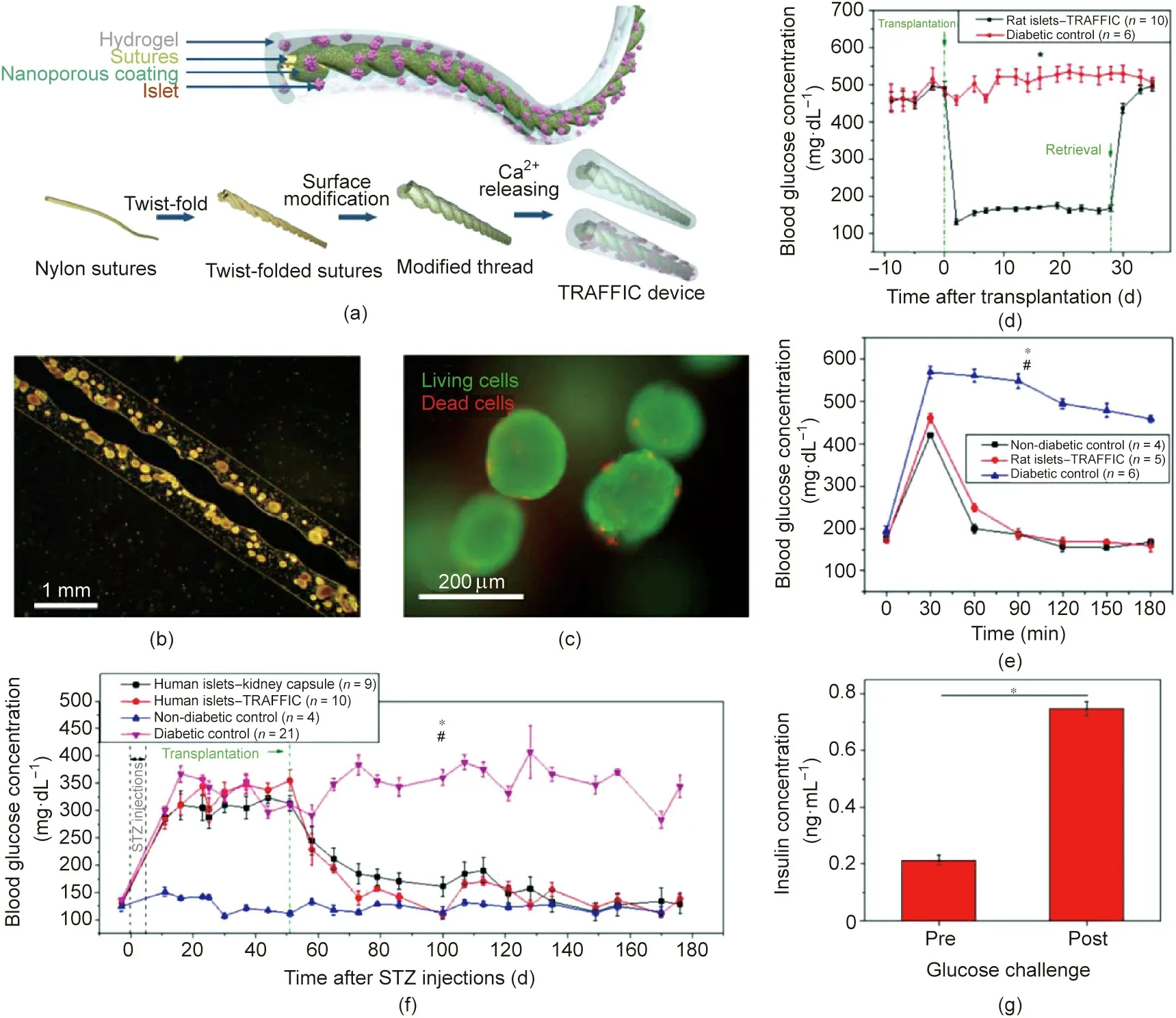
Fig.6. Thread-enhanced alginate fiber for islet delivery.(a)Schematic of the device.TRAFFIC:thread-reinforced alginate fiber for islets encapsulation.(b)Typical islet-loaded device image.(c)Live and dead staining of islets encapsulated in fiber.(d)Blood glucose of diabetic C57BL/6J mice transplanted with the device loaded with rat islets,n=6–10, *p < 0.05. (e) Blood glucose levels of treated diabetic mice after glucose challenge before retrieval of device, n = 4–5, *p < 0.05 (diabetic control vs rat islets-device),#p>0.05(nondiabetic control vs rat treated with device).(f)Blood glucose levels of diabetic SCID-Beige mice after transplantation with various types of human islets,n=4–21,*p<0.05(human islets-device vs diabetic control), #p>0.05(human islet-kidney capsule vs human islets-device).(g)In vitro glucose-stimulated insulin secretion of islets encapsulated in fiber gel before and after implantation(n=3),*p<0.05.All data presented as mean±standard error of the mean.Reproduced from Ref.[120]with permission of National Academy of Sciences, © 2018.
2.4.2. Disk-like triazole-zwitterionic (TR-ZW) hydrogel
Another method for enhancing the mechanical strength of a hydrogel device is to develop and utilize bulk hydrogels. Among hydrogels, the TR-ZW hydrogel has some key advantages, including high tensile property and significant mechanical stability. Liu et al.[123]recently developed several disk-like hydrogel prepared from TR-ZW hydrogel from polymers, including poly(quaternized triazole carboxybetaine acrylamide) or P(qTR-CB), poly(triazole carboxybetaine acrylamide)or P(TR-CB),and poly(triazole sulfobetaine acrylamide) or P(TR-SB), for islets transplantation. The mechanical strength of this type of hydrogel could be enhanced by increasing the monomer concentration during synthesis, as increased concentration promoting strong ionic bond formation[125]. P(TR-SB)that was prepared from triazole sulfobetaine acrylamide (TR-SB) exhibited a 20-fold higher breaking strain than polycarboxybetaine (PCB) hydrogels and showed minor foreign body response after being implanted subcutaneously in healthy C57BL/6J mice. Rat islets encapsulated in P(TR-SB) hydrogel disks and implanted in STZ-induced diabetic C57BL/6J mice for one month mostly maintained a relatively normal blood glucose level, while the islets encapsulated in the alginate gels failed to regulate blood glucose progressively. After retrieval, the device showed a 1.5-fold increase in insulin secretion when the islets were transferred from 2.8 to 16.7 mmol∙L-1glucose buffer and incubated for 60 min. Enhanced surrounding vascularization around the P(TR-SB) device was also identified.
2.4.3. Non-degradable PEG hydrogel
In order to improve the vascularization around a different encapsulation device, Weaver et al. [126] prepared a proteasedegradable PEG hydrogel, which was further functionalized by Arg–Gly–Asp(RGD)and vascular endothelial growth factor(VEGF).RGD was used to provide adhesive sites for cells, while VEGF was used to stimulate the growth of blood vessels.The researchers used these materials to create a multi-layer encapsulation device with the vascularized degradable PEG coating a smaller nondegradable PEG disk encapsulating the islets [127]. The islets in PEG hydrogels grew better than those loaded in alginate hydrogel,which was studied as a control representing a traditionally used encapsulation material.The device loaded with islets of male Lewis rats was implanted at the omentum of female Lewis rats. A significantly increased vascular density was observed when VEGF was incorporated into the outer layer of the PEG hydrogel.
2.4.4. Porous tubular hydrogel
As mentioned earlier, an increase in the relative surface of a device can enhance matter exchange. Recently, Naficy et al. [128]prepared a retrievable porous device for β-cell or islets encapsulation and implantation.The device had a tubular shape,with a porous shell prepared from polypropylene glycol (PPG) and hydrophilic polyurethane (HPU). The HPU–PPG hydrogel mainly offered mechanical strength,and the stiffness of the hydrogels ranged from 100 to 400 kPa.When preparing the device,NaCl particles were used to fill the pores and were later removed by washing the gel in water. The pores were then filled with alginate gel loaded with human insulin-producing pancreatic β-cell lines or porcine neonatal islet cell clusters. The gel material was compatible with the cells enveloped inside the gels. The device loaded with the βcell line could release insulin slightly faster when exposed to 33 mmol∙L-1glucose solution in Krebs–Ringer Bicarbonate N-(2-hydroxyethyl)piperazine-N′-2-ethane sulfonic acid (HEPES) buffer than when incubated in general RPMI 1640 medium. When the device was loaded with islets,the insulin release in 24 h was doubled when the device was exposed to a 33 mmol∙L-1glucose culture medium compared with a standard RPMI 1640 culture medium. These results indicate that islet cell clusters may be better than β-cell lines for controlling insulin release. Moreover, the gel was quite durable, which allowed for easy removal after implantation.
3. Macrodevices for cardiovascular disease treatment
Myocardial infarction (MI) can cause damage to heart muscle cells, and about 36% of MI survivors are exposed to a high risk of heart failure [129]. Stem cell-based therapies are emerging as effective strategies to treat MI survivors using a paracrine mechanism via secreted regenerative factors [130,131]. Thus, prolonging the retention of stem cells at the site of MI could potentially improve the treatment efficacy. Injectable scaffolds and cardiac patches have been developed to improve cell delivery efficacy.Injectable scaffolds can provide physiological support for implanted cells, induce vascularization, and relieve fibrosis [132].In comparison,cardiac patches can provide direct mechanical support to enhance heart function,as well as a suitable microenvironment for cell growth.
3.1. Cardiac patches for MI treatment
Kim et al. [133]stamped decellularized cell-derived extracellular matrices (CDM) onto a poly(vinyl alcohol) (PVA) hydrogel to obtain a stretchable extracellular matrix membrane for stem cell delivery. NIH-3 T3 fibroblast cells proliferated robustly in this device, due to increased cell attachment onto the CDM. In a rat MI model,MSCs delivered in this membrane exhibited higher therapeutic efficacy than MSCs injected directly or used together with FN/PVA hydrogels, indicating the superiority of this device. Bejleri et al. [134] also engineered a bioprinted cardiac patch using the extracellular matrix of the myocardium and gelatin methacrylate(GelMA) to deliver pediatric human progenitor cells (hCPCs). This cardiac patch presented a suitable mechanical strength, improved angiogenic potential, and was retained in vivo for over 14 d.
Song et al.[135]developed a polyacrylic acid-incorporated oxidized alginate/gelatin hydrogel, which is advantageous for selfhealing, microscopic homogeneous conductivity, and biocompatibility,as well as in matching the mechanical strength of the cardiac tissue.Neonatal cardiomyocytes cultured inside the gels exhibited improved differentiated sarcomeres and cell-to-cell contacts,along with well-organized sarcomeric α-actinin and directionally distributed sarcomeres. The hydrogel patch significantly inhibited cardiac fibrosis,increased the thickness of the left ventricular wall,and promoted angiogenesis.
In addition, Song et al. [136] prepared a pre-vascularized cardiac patch using reprogrammed fibroblasts. Moreover, to avoid the reduction of cell viability in the device and to improve the preservation and application of the patch, an artificial cardiac patch bearing secreted cell factors was developed by Huang et al.[137] for cardiac repair after MI.
3.2. An MN array patch for MI treatment
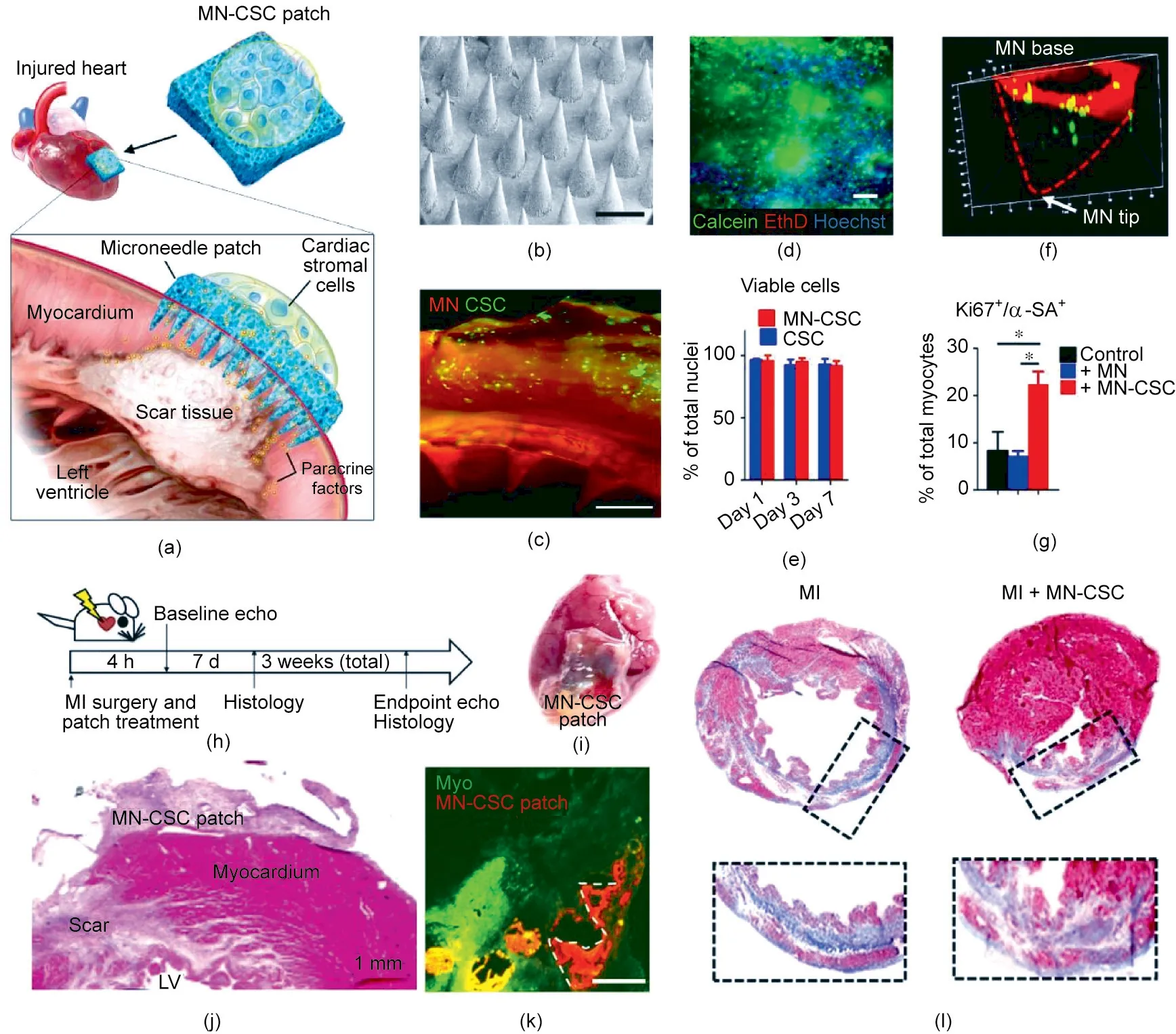
Fig. 7. MN array patch for delivering cardiac cells to treat MI. (a) Schematic of the design of the cell-integrated MN patch. (b) Representative image (scale bar: 500 μm).(c)Fluorescence image of cell-integrated MN patch.(d)Representative image of cells stained with calcein(live)/EthD(dead)(scale bar:200 μm).(e)Quantitative analysis of cell viability. Data are means ± standard deviation (SD), n = 3. (f) Representative confocal image of cell (green) integrated with MN patch for three days. (g) Quantitative analysis of neonatal rat cardiomyocytes(NRCMs)stained with proliferation marker Ki67 and α-sarcomeric actin(α-SA).NRCMs were cultured alone or in the presence of MN or an MN-CSC patch (n = 3). Data are means ± SD. (h) Schematic of the overall design of the animal study. (i) Representative image of the heart treated with a cell patch;hematoxylin and eosin (H&E) staining is shown in (j). LV: left ventricular. (k) Fluorescence image of heart treated with an MN patch seven days after treatment (scale bar:400 μm). (l) Representative images of myocardial sections using Masson’s trichrome staining protocol. The hearts were obtained three weeks after MI. Blue indicates scar tissue; red indicates viable myocardium. Reproduced from Ref. [41] with permission of AAAS, © 2018.
The problem of how to enhance matter exchange between patches and the site of MI remains a major challenge. In order to resolve this issue, Tang et al. [41] created a CSC patch to enhance the transportation of regenerative factors into the injured myocardium (Fig. 7(a)). The MN array patch was prepared from PVA that was highly pronged to form crystallized structures [138] (Fig. 7(b)).These MNs could be inserted into the heart to connect directly to the host myocardium. After absorbing some interstitial liquid,the PVA MNs swelled slightly, and micrometer-sized channels formed inside the gels. CSCs were encapsulated into a biocompatible fibrin gel located on the back of the MN patch (Fig. 7(c)) and survived well (Fig. 7(d)). This method prolonged the retention of CSCs at the site of injury, in comparison with intramuscular injection.When cultured in a medium,the cells integrated on top of the patch survived after seven days (Fig. 7(e)). In addition, CSCs migrated from the fibrin gel into the cavity of the MNs (Fig. 7(f))and secreted regenerative factors into the culture medium,thereby enhancing the viability of the cardiomyocytes(Fig.7(g)).This integrated patch was tested on a rat MI model(Fig.7(h)).An MN patch containing 1×106stromal cells was applied onto the newly formed MI area(Figs.7(i)and(j)),where it remained for more than seven days(Fig.7(k)).Reduced myocardial apoptosis and increased angiomyogenesis were observed after treatment in comparison with the control group (Fig. 7(j)), and a thicker infarct wall and viable tissue were observed(Fig.7(j)).Three weeks after treatment,the hearts that had received MN-CSC transplantation exhibited reduced fibrosis (Fig. 7(l)).
3.3. Refillable cell reservoir for MI treatment
In another study,Whyte et al.[139]developed a therapeutic cell reservoir to treat infarcted hearts via paracrine factors secreted by stem cells. The device was mainly composed of three parts: a cell reservoir attached to the heart, a subcutaneous port, and a refillable tube connecting them.The permeable membrane of the reservoir faced the surface of the heart, while the impermeable membrane faced outward. Mouse MSCs constrained inside the device remained alive in the culture medium for 28 days and secreted G-luciferase into the surrounding medium. Furthermore,the implementation of refilling cells into the reservoir through the refill tube was confirmed to be feasible. Because of the short survival time of the MSCs, this multiple bolus-based cell administration strategy could provide the delivery of cellular factors,including cytokines, chemokines, and paracrine factors, over multiple timescales.
4. Macrodevices for CAR-T cell therapy
CAR-T cell therapy has shown tremendous effects in treating pre-B cell acute lymphoblastic leukemia(ALL)or B cell lymphomas[140,141], but remains challenging in treating solid tumors[142,143]. Since the CAR-T cell is the direct functional mediator exerting anticancer activity, designs for devices for CAR-T cell delivery in treating solid tumors aim to concentrate the CAR-T cells at the tumor site and provide physiochemical support to enhance the survival and anticancer activity of the CAR-T cells.
In pursuit of this end, Coon et al. [42] developed thin-film nitinol(TFN) for CAR-T cell delivery.The film had a micropattern that enabled a uniaxial stretch of over 100%when an external force was applied. A thin layer of fibrin gel was coated onto the device to facilitate CAR-T cell adhesion and migration. In addition, antibodies against CD3, CD28, and CD137 were conjugated to the fibrin gel to stimulate the expansion of CAR-T cells. An in vitro culture on the TFN retained CAR-T cell function after incubation for 6 d.In an unresectable ovarian carcinoma model,CAR-T cells delivered by the TFN were found to be able to eradicate tumors in seven out of ten mice and achieved an average survival time of 80 days—much longer than the survival time of mice that had been intravenously or intratumorally injected with CAR-T cells. This method provides an alternative for enhancing CAR-T cell antitumor activity toward solid tumors.
A hydrogel implant made from GFOGER-modified alginate hydrogel has also shown the ability to enhance T cell proliferation and retention at the tumor site, achieving significantly promoted anticancer activity against 4T1 resected tumors and a multifocal ovarian cancer model [144]. Moreover, an injectable, biocompatible, and functionally modified polyisocyanopeptides hydrogel was developed by Weiden et al. [145], allowing the injection of T cells into tumor sites to enhance their persistence and cellrelease sustainability. In addition, the injection of IL-15-secreting CAR-T cells in a PEG–chitosan hydrogel to retinoblastoma tumors significantly improved the CAR-T cells’ anticancer activity, subsequently preserving the vision of mice [146]. These strategies have achieved encouraging treatment outcomes;however,the immunesuppressive and hypoxic tumor microenvironment could hamper the anticancer activity of CAR-T cells against solid tumors. Therefore, some strategies have recently been developed to address these issues.
To reverse the tumor-suppressive microenvironment, Hu et al.[147] designed a biodegradable HA hydrogel reservoir for codelivering chondroitin sulfate proteoglycan 4 (CSPG4)-targeting CAR-T cells,anti-programmed cell death ligand 1 antibody(aPDL1)modified platelets (P-aPDL1), and the cytokine IL-15-encapsulated poly(lactic-co-glycolic acid) (PLGA) nanoparticles (Figs. 8(a) and(b)). The hydrogel was embedded into the tumor cavity of the resected melanoma to inhibit both local tumor recurrence and the growth of distant tumors. The hydrogel reservoir sustainably and slowly released CAR-T cells to the surrounding tumor tissues,avoiding the massive inactivation of CAR-T cells caused by burst release. Meanwhile, the cytokine IL-15 loaded in the hydrogels maintained the activity of the CAR-T cells (Fig. 8(a)). In addition,the postoperative inflammatory environment triggered the activation of platelets and the subsequent release of anti-PD-L1 antibodies, thereby inhibiting the immune checkpoint inhibition pathway and enhancing the killing effect of the CAR-T cells.In vitro,platelets were released entirely from the hydrogel within 96 h; in comparison, only 50% of the CAR-T cells were released within the same period (Fig. 8(c)). Also, hydrogels loaded with P-aPDL1 and CAR-T cells were shown to exhibit anticancer activity via a coculture assay (Fig. 8(d)). In vivo, the hydrogel reservoir loaded with CART cells and P-aPDL1 showed significantly higher tumor-inhibition activity than other groups (Fig. 8(e)). Notably, the systemic anticancer activity of this hydrogel confirmed that unilateral administration of the hydrogel could inhibit the growth of the contralateral tumor (Fig. 8(f)).
To improve the hypoxic tumor microenvironment, Luo et al.[148] prepared a porous hydrogel-coated immune-microchip system for co-delivering CAR-T cells, IL-15, and O2carriers into solid tumors. The gel layer degraded gradually to release O2into the tumor microenvironment, thus providing O2to the CAR-T cells,which migrated into the tumors from the immune microchip.This method enhanced the survival and infiltration of T cells into the tumor stroma, leading to improved inhibition of tumor growth,as indicated by the decreased HIF-1α and Ki67 expression of the tumor cells.
5. 3D-printed hydrogels for establishing vascular networks
Solid organs contain interpenetrating vascular networks for fluid transportation. Printing 3D entangled vascular networks in biocompatible hydrogels is critical for cell survival in artificial organs, including vascularized bone, skeletal muscle, cardiac tissues,and liver[149].For example,Cui et al.[150]combined regional bioactive factor immobilization with 3D printing technology to establish complex vascularized bone, in which hard PLA and cellladen soft GelMA were used to build bone scaffolds and vessels,respectively. In addition, Zhang et al. [151] combined vasculogenesis and angiogenesis by building on the self-promotion of endothelial cells and using a direct 3D printing technique to prepare hydrogel-based vascular myocardial tissues.
Recently, Grigoryan et al. [152] developed a 3D printing strategy for creating biocompatible hydrogels with internal entangled vascular networks, which could be further functionalized with intravascular 3D fluid mixers and bicuspid valves. In this study,tartrazine, a non-toxic food additive, was used as the photoabsorber to facilitate the projection stereolithographic production of hydrogels with high z-axis resolution, which enabled the fabrication of soft hydrogels with patent vessels perpendicular to the direction of projected light [152]. Functional intravascular static mixers and bicuspid venous valves have also been printed (Fig.9(a)), demonstrating the ability of this method to produce functional intravascular topologies. The researchers also printed a discontinuous cubic lattice and a knot-entangled torus(Figs.9(b)and(c)),and confirmed intervascular O2transportation(Fig.9(d)).Furthermore, the researchers established a hydrogel with an alveolar model topology for studying oxygen exchange during breathing(Fig. 9(e)). The branching vascular network, hydrogel distention,and blood redirection all led to faster oxygen exchange.Hydrogels seeded with mammalian cells were also prepared,which validated the compatibility of this printing strategy with living mammalian cells.The researchers further printed an advanced hydrogel carrier integrated with hepatic aggregates within a fibrin gel, a vascular compartment seeded with endothelial cells, and hydrogel anchors for retaining the fibrin gel (Figs. 9(f) and (g)). In a chronic rodent liver injury model, the hepatic cells were able to survive in vivo for more than 14 days. The presence of host blood cells in the explanted device was identified in microvessels adjacent to the hepatic aggregates.This 3D printing technology for printing vascular networks could potentially enhance the O2and nutrient exchange in a large 3D device.
6. Discussion and conclusions
In the last few years, macrodevices with various architectures have been prepared from either biocompatible inert or degradable materials for cell delivery to treat many diseases (Table 1)[41,42,50,72–74,77,78,81,87,89,107,108,120,123,134–136,139,144,146–148,150–152]. Such macrodevices are generally designed to support the survival and function of cells—either allogeneic or xenogeneic—by incorporating specific strategies to meet the cells’demand for nutrients, oxygen, cytokines, and a safe immuneprivileged space. Strategies developed in recent years for the prevascularization of implanted devices and the establishment of a local immune-suppressive environment are vital for maximizing O2and nutrient supply. These devices are also designed to maximize the loading capacity of cells to exert a practical therapeutic function and minimize the device’s size to facilitate the implantation. It must be noted that macrodevices are customized to match the specific requirements for treating each disease.
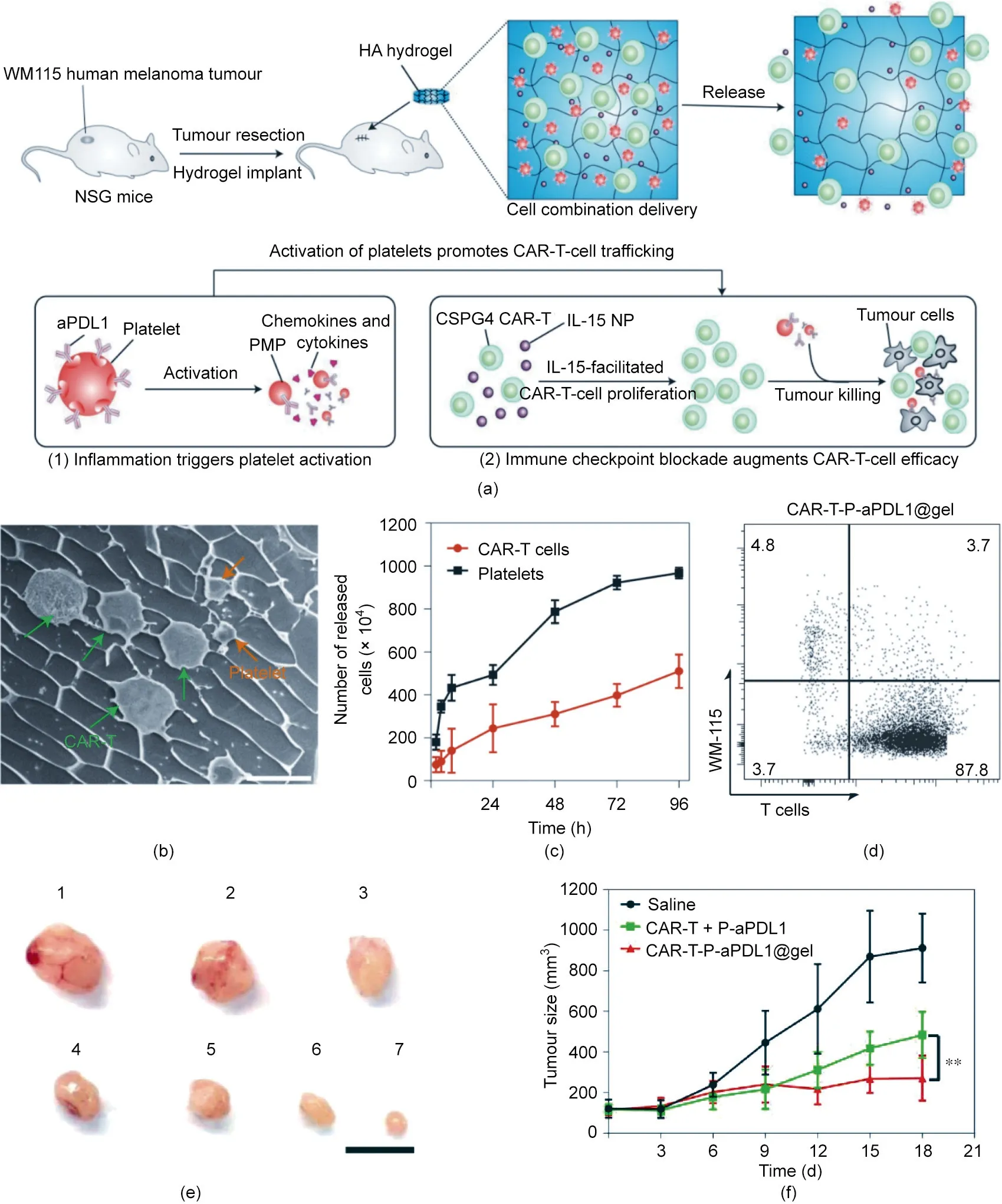
Fig. 8. A hydrogel reservoir for the co-delivery and gradual release of CAR-T cells and anti-PDL1-conjugated platelets. (a) Schematic of the mechanism of the hydrogel for post-surgery cell immunotherapy.PMP:platelet-derived microparticles;NP:nanoparticle.(b)Typical cryo-SEM image of hydrogels loaded with cells and platelets(scale bar:10 μm).(c)Release of CAR-T cells and platelets from the hydrogel in vitro;1×107 CAR-T cells and 1×107 platelets were loaded into the hydrogel(n=3).(d)Flow cytometry plots of cells after co-culturing tumor cells and T cells for 72 h.Data are means±SD(n=3).(e)Representative image of tumors from mice after 3 weeks of treatment.Mice were treated with:1:saline;2:P-aPDL1@gel;3:CAR-T cells;4:CAR-T+P-aPDL1;5:CAR-T@gel;6:CAR-T@gel+P-aPDL1;and 7:CAR-T-P-aPDL1(scale bar:1 cm).(f)The size of tumors on the left side after treatment of the primary tumor on the right side(n=6).**p=0.0023.All data are means±SD.Reproduced from Ref.[147]with permission of Springer Nature Limited, © 2021.
Macrodevices for treating T1D must constrain the functional cells inside the device during the whole treatment period, while insulin is required to be secreted from the device in order to lower blood glucose levels. It is challenging to maintain the survival and function of insulin-secreting cells for a sufficiently long duration or even life-long period.Success has been achieved using biocompatible and immune-compatible materials to reduce the foreign body response and fibrosis in order to ensure fluent mass transfer,thereby maintaining the device’s function and the entrapped cells.Decreased fibrosis also reduced the device’s adherence to the surrounding tissues and facilitated device removal after failure. The current focus of research on the device for islets or β-cell implantation remains on optimizing the characteristics of the device membranes of the chamber to reduce fibrosis and enhance neovascularization.
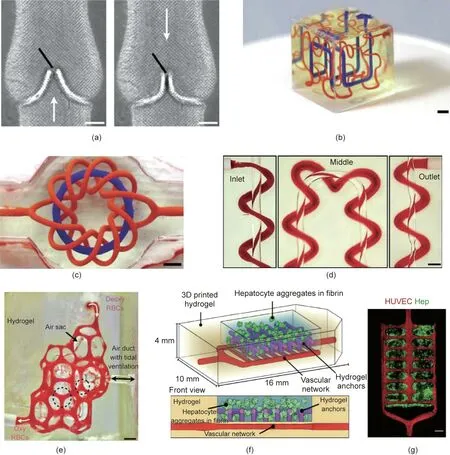
Fig. 9. 3D printing of soft hydrogels with intricate and functional vascular architectures. (a) 3D printing of entangled axial vessel and helix within a hydrogel from 20 wt%poly(ethylene glycol) diacrylate (PEGDA; 6 kg∙mol-1) (scale bar: 3 mm). (b) Hilbert curves within a hydrogel (scale bar: 3 mm). (c) Torus and torus knot (scale bar: 3 mm).(d)Axial vessel and encompassing helix.Red blood cells flowed from inlet toward outlet(scale bar:1 mm).(e)Photograph of an alveolar-like construct with flowing red blood cells.Deoxy RBCs:deoxygenated red blood cells;oxy RBCs:oxygenated red blood cell.(scale bar:1 mm).(f)Multi-compartment hydrogel construct loaded with hepatocyte aggregates.The vascular network was seeded with endothelial cells.(g)Confocal microscopy images of the hydrogels in part.Hep:hepatocyte aggregates.Reproduced from Ref. [152] with permission of AAAS, © 2019.
Thanks to the recent development in engineering pluripotent stem cells to produce insulin-secreting cells, the shortage of allogenic islet supply has been somewhat relieved. Also, an improved survival rate of cells differentiated from pluripotent stem cells to islets after implantation may further benefit patients with insulin deficiency. However, pluripotent stem cells are more dangerous than islets in causing potential systemic disease or cancer [22].The devices used for such insulin-secreting cells must be designed to be exceptionally robust and durable, and the implantation sites need to be carefully selected to reduce the likelihood of collision and the rupture of the device. In addition, it is crucial for cell survival to monitor the immune responses.However,more research is required to confirm the year-long effect of antibodies against implanted cells.Moreover,the chamber may be preferable for stem cell transplantation because of its higher strength compared with the gels.
Even if the islet shortage could be resolved by the emerging supply of insulin-releasing cells,implanting large numbers of cells into a size-acceptable device is another challenge [153]. IEQs of 300 000 are estimated to be required to achieve normoglycemia in recipients [154]. However, the slow nutrient and oxygen exchange between the membrane-controlled release system and the physiological environment requires the thickness of the container to be less than 300 μm [155]. Moreover, there is limited space available in the body for the implantation of a cell-loaded device.How to increase the density of the functional cells in a clinically relevant confined diffusion chamber while maintaining their survival and functionality remains a challenge. Transforming the two-dimensional (2D) structure into three dimensions or supplying external nutrients and oxygen (as the ‘‘β-Air” device did) [75]is an option to solve this problem. In addition, the 3D printing of vascular networks or air networks inside the implant may be helpful in solving this problem.
In the future, insulin-secreting cell-loaded macrodevices could be an ‘‘off-the-shelf” product that are available for people with deficient endogenous insulin production. The macrodevice could be implanted through minimally invasive surgery. These devices could potentially be refillable, with stem cell-derived therapeutic cells as another ‘‘off-the-shelf” product ready to replace the existing cells via injection through a port connected to the device.Moreover, the insulin-producing cells and glucagon-producing cells could be loaded into the device to improve the blood glucose control synergistically.
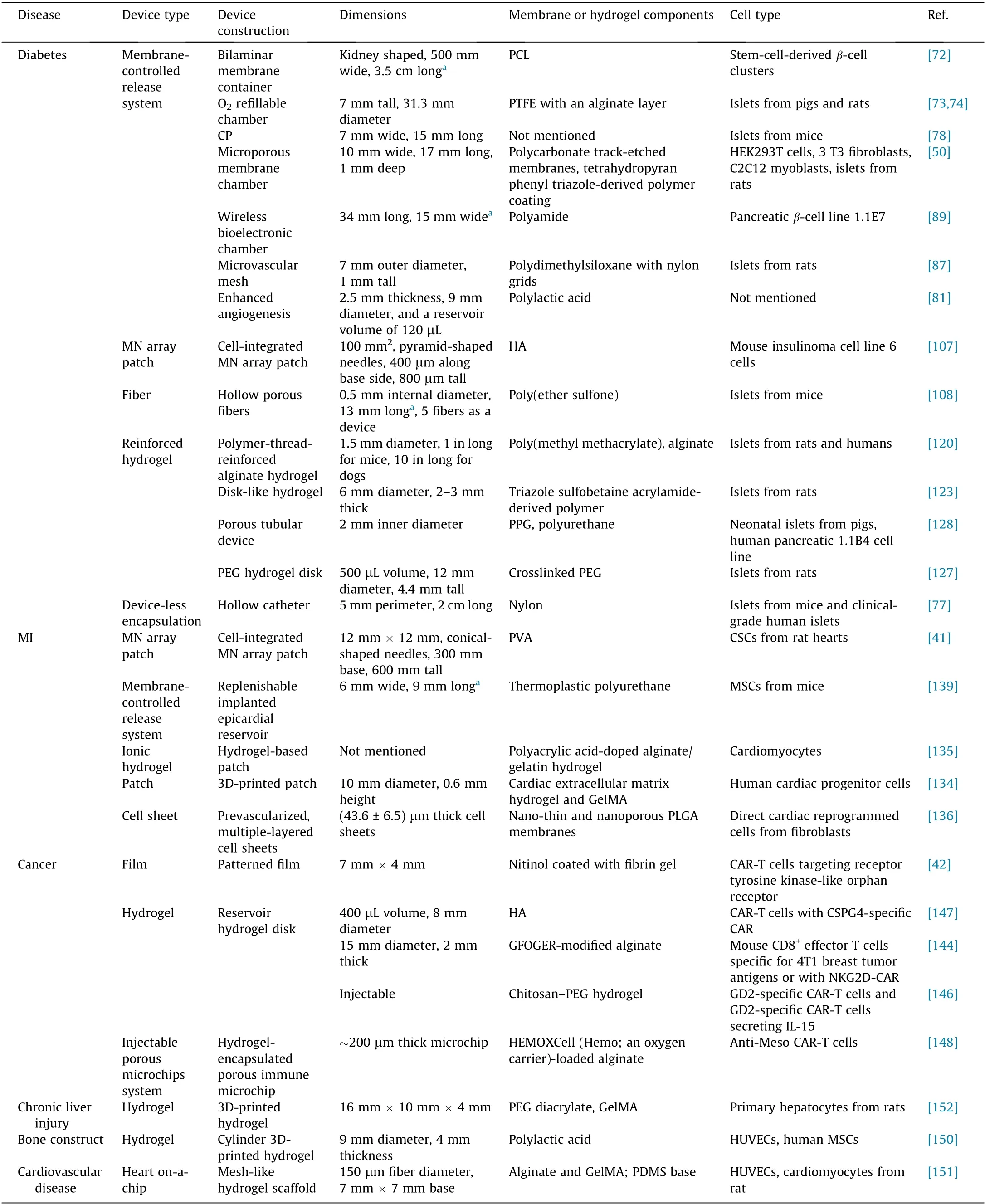
Table 1 Representative devices for cell delivery.
In MI therapy using stem cells,the cells were implanted on the heart, and a refillable device was developed to facilitate a prolonged nutrient supply [139]. Extra nutrient and oxygen supply may be an option to prolong cell survival while reducing the need for the complicated cell-refilling procedure. Also, if the device is biodegradable and it is not necessary to remove the implanted cells, retrieval of the implanted device could be avoided, and patient compliance could be enhanced. Attachment of the device onto the surface of the heart is challenging due to the heart’s constant beating. Therefore, devices used for treating MI need to be flexible, sticky, durable, and biocompatible. In the future, MItreatment devices could provide both monitoring and celldelivery functions.The cells used for treating MI could be adjusted in type and number according to the development of the disease status.
Regarding CAR-T cell immunotherapy, the focus of the device design has shifted from providing physical support to maximizing the therapeutic function of CAR-T cells or genetically engineered T cells that need to be released from the device into tumors.In addition, the device is generally implanted at the site of tumors for onsite cell delivery and to provide bioactive molecules to stimulate CAR-T cell proliferation and promote tumor cell-killing ability.For example, the sustained and gradual release of IL-15 encapsulated in PLGA nanoparticles significantly stimulated the proliferation and survival of CAR-T cells,thereby enhancing the anti-solid tumor activity. In the future, developing strategies for implanting cellreleasing devices into the tumors of internal organs—rather than those of superficial organs such as the skin and eyes—could further expand the clinical applications of such devices.
Acknowledgments
This work was supported by the grants from JDRF(2-SRA-2021-1064-M-B), and Zhejiang University’s start-up packages, the Kunpeng Program grant and Fundamental Research Funds for the Central Universities (2021FZZX001-46). The work contributed by Nicholas A. Peppas and his associates was supported in part by grants from the National Institutes of Health (EB022025 and GM043337)and the nCockrell Family Regents Chair in Engineering(UT Austin) for the Institute for Biomaterials, Drug Delivery, and Regenerative Medicine, and the UT-Portugal Collaborative Research Program. Nicholas A. Peppas is an International Member of the Chinese Academy of Engineering.
Compliance with ethics guidelines
Wei Liu,Yanfang Wang,Jinqiang Wang,Olivia L.Lanier,Marissa E. Wechsler, Nicholas A. Peppas, and Zhen Gu declare that they have no conflict of interest or financial conflicts to disclose.
Prof.Zhen Gu is the co-founder of Zenomics Inc.and Zencapsule Inc. Prof. Nicholas A. Peppas is the co-founder of Appian Laboratories.
- Engineering的其它文章
- Electric Air Taxis Create Megadeal Buzz
- Tissue Engineering and Regulatory Science
- Factors Predicting Progression to Severe COVID-19: A Competing Risk Survival Analysis of 1753 Patients in Community Isolation in Wuhan,China
- A Vaccine Based on the Receptor-Binding Domain of the Spike Protein Expressed in Glycoengineered Pichia pastoris Targeting SARS-CoV-2 Stimulates Neutralizing and Protective Antibody Responses
- Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers
- Past and Future Changes in Climate and Water Resources in the Lancang–Mekong River Basin: Current Understanding and Future Research Directions

