Mapping a stable adult-plant stripe rust resistance QTL on chromosome 6AL in Chinese wheat landrace Yibinzhuermai
Mei Deng,Li Long,Yukun Cheng,Fangjie Yao,Fangnian Guan,Yuqi Wang,Hao Li,Zhien Pu,Wei Li, Qiantao Jianga,, Yuming Wei, Jian Ma, Houyang Kang, Pengfei Qi, Jirui Wang,,Youliang Zhenga,, Yunfeng Jiang,, Guoyue Chena,,
a State Key Laboratory of Crop Gene Exploitation and Utilization in Southwest China, Wenjiang, Chengdu 611130, Sichuan, China
b Triticeae Research Institute, Sichuan Agricultural University, Wenjiang, Chengdu 611130, Sichuan, China
c College of Agronomy, Sichuan Agricultural University, Wenjiang, Chengdu 611130, Sichuan, China
Keywords:Puccinia striiformis QTL mapping Single nucleotide polymorphism Yellow rust
A B S T R A C T Stripe rust,caused by Puccinia striiformis f.sp.tritici(Pst),is one of the most important diseases threatening the yield and stability of wheat production in China and many other countries.Identification and utilization of new genes for durable stripe rust resistance are important for ongoing control of this disease.The objectives of this study were to identify quantitative trait loci (QTL) associated with adult-plant stripe rust resistance in the Chinese wheat landrace Yibinzhuermai(YBZR)and to provide wheat breeders with new sources of potentially durable resistance. A total of 117 recombinant inbred lines (RILs) (F5:8)derived from a cross between YBZR and highly susceptible cultivar Taichung 29 (TC29) were assessed for stripe rust severity in field experiments at Wenjiang in 2016 and 2017 and Chongzhou in 2016,2017,2018,and 2019 in Sichuan following inoculation with a mixture of current Pst races.The RILs were genotyped using the Wheat55K single nucleotide polymorphism (SNP) array. Three QTL were identified on chromosome arms 6AL, 5BL and 7DS. QYr.YBZR-6AL and QYr.YBZR-7DS conferred major effects in all field environments, explaining 10.6% to 14.7% and 11.5% to 21.2% of phenotypic variation, respectively.The QTL on 5BL and 7DS likely correspond to previously known QTL, whereas QYr.YBZR-6AL is probably novel. Haplotype analysis revealed that the resistance allele at QYr.YBZR-6AL was present in 2.8% of 324 Chinese wheat landraces. SNP markers closely linked with QYr.YBZR-6AL were converted to kompetitive allele-specific PCR markers and validated in the RIL population and a subset of 92 wheat cultivars. QYr.YBZR-6AL and its markers should be useful in breeding programs to improve the level and durability of stripe rust resistance.
1. Introduction
Stripe rust,caused byPuccinia striiformisf. sp.tritici(Pst),is the most common and widespread disease of wheat(Triticum aestivumL.)worldwide[1,2].If the weather is favorable for development the disease can cause complete yield loss in susceptible cultivars [3].Severe epidemics of wheat stripe rust in China occurred in 1950,1964, 1990, and 2002, causing losses of approximately 6.0, 3.2,1.8, and 1.4 million metric tons, respectively [4,5]. Breeding for disease resistance can contribute to a reduced frequency of disease outbreaks with less severity and thereby lower yield loss. The use of wheat cultivars resistant to stripe rust is the most practical and sustainable method to control this disease [6]. Long-term production trials have shown,however,that disease management relying on single major resistance genes is short-term as selection for virulent races will occur. Breeding cultivars with durable stripe rust resistance has thus been emphasized in modern wheat breeding programs.
More than 300 genes/quantitative trait loci(QTL)for stripe rust resistance have been reported in common wheat and its relatives[7,8]. These genes can be broadly categorized into two groups:all-stage resistance (ASR) and adult-plant resistance (APR). ASR is usually controlled by single genes with large effect and is effective against only a subset of races,whereas APR is mostly quantitatively inherited and is manifested as non-race specific resistance at post-seedling stages. Compared with ASR, APR often confers durable resistance but the individual genes generally do not provide a high level of resistance. For example, resistance conferred by the best-known APR gene,Yr18[9], has remained durable with no observed evolution of increased virulence for more than 50 years [9-11], but this gene alone does not provide adequate resistance under high disease pressure. As an effective strategy for durable resistance, the pyramiding of multiple APR genes,which often show positive additive effects, has been successfully applied to achieve highly effective resistance to stripe rust[1,12,13]. Although, a large number of QTL that confer various degrees of APR to stripe rust have been identified, major APR QTL that significantly reduce stripe rust severity are scarce resources in wheat breeding program. The identification of additional major APR QTL that are useful in breeding programs is thus required.
Landraces were the main wheat cultivars in China until the 1950s. During long-term cultivation and natural and artificial selection, many landraces were adapted to disease stress in stripe rust epidemiological regions and demonstrated to be reservoirs of uncharacterized stripe rust resistance genes [1]. A few major genes/QTL have been identified thus far in Chinese wheat landraces using bi-parental mapping populations, includingYr1[14],Yr18[9],Yr81[15],YrYL[16],YrBai[17],QYr.caas-5AL[18],QYr.cau-6DL[19],QYr.cau-2AL[20],QYr.GTM-5DL[21], andQYr.AYH-5BL[22]. In addition, our recent genome-wide association studies(GWAS) have uncovered dozens of potentially novel genes that can be used to enrich the current gene pool [23-28].
The Chinese wheat landrace Yibinzhuermai (YBZR) collected from Sichuan, where stripe rust epidemics are a frequent occurrence, has displayed high levels of APR to ChinesePstraces in the field across many years. In this study, we constructed a highdensity genetic map using the Wheat 55K SNP array in a recombinant inbred line (RIL) population from a cross between YBZR and highly susceptible cultivar Taichung 29 (TC29) to identify QTL for stripe rust resistance in YBZR. We then converted markers closely linked to a potential novel QTL to kompetitive allele-specific PCR(KASP) markers to enable marker-assisted selection in breeding programs.
2. Materials and methods
2.1. Pst races
The resistant parent YBZR(AS1591,AS refers to germplasm held by the Triticeae Research Institute, Sichuan Agricultural University) was crossed as female parent with the highly stripe rust susceptible parent TC29. A mapping population of 117 F5:8RILs was developed by single-seed descent. Three lines, Mingxian 169,SY95-71,and Avocet S(AvS),were planted as susceptible controls.A collection of 324 Chinese wheat landrace accessions was used for SNP-based haplotype analysis ofQYr.YBZR-6AL[27,29]. A panel of 92 wheat cultivars was used to determine the polymorphism of markers tightly linked withQYr.YBZR-6AL. ThePstraces(including CYR32, CYR33, CYR34, G22-14, Su11-4, Su11-5, and Su11-7) were provided by the Plant Protection Research Institute, Gansu Academy of Agricultural Sciences, Lanzhou, Gansu, China.
2.2. Phenotyping of stripe rust resistance
Seedling evaluations for stripe rust were conducted under controlled greenhouse conditions at the Plant Protection Institute of the Gansu Academy of Agricultural Sciences. The current predominantPstrace CYR34 was used to test the seedling resistance of YBZR and TC29.Ten seeds were planted in a plastic pot filled with soil mixture, and seedlings were inoculated with CYR34 when plants were at two-leaf stage. Inoculated plants were incubated at 10 °C in a dew chamber for 24 h in darkness, and then moved to a greenhouse maintained at 16 °C [22]. The susceptible control was Mingxian 169, and infection types (IT) were recordeded 18-21 days after inoculation using a 0-9 scale [30].
To evaluate adult-plant stripe rust responses, the 117 RILs and parents were grown in the field at Wenjiang (30°43′N, 103°52′E)during two growing seasons (2015-2017 referred as 2016WJ and 2017WJ)and at Chongzhou(30°33′N,103°39′E) during four growing seasons (2015-2019 referred as 2016CZ, 2017CZ, 2018CZ, and 2019CZ). The field trials were conducted in randomized complete blocks with three replications. Plots consisted of 2.0 m long rows spaced 30 cm apart, with 10 cm between plants in each row.Approximately 20 seeds were sown in each row. The highly susceptible line SY95-71 was planted in every 20th row to aid disease spread. Both susceptible and spreader rows were inoculated approximately 1 month after planting using a mixture of the current predominantPstraces (including CYR32, CYR33, CYR34,G22-14, Su11-4, Su11-5, and Su11-7). Disease severity was scored as the percentage of infected leaf area (0, 5%, 10%, 20%, 40%, 60%,80%, or 100%) in accordance with the Chinese National Standard,GB/T 15797-2011. When the susceptible check SY95-71 and AvS were fully covered with rust pustules, the final disease severity(FDS) of each line in each trial was recorded and used for phenotypic analysis.
Broad sense heritabilities (H2) were estimated using the ‘‘AOV”tool in QTL IciMapping v4.2 (http://www.isbreeding.net) [31].Pearson’s correlation coefficients among different environments were calculated using SPSS Statistics v17.0 (IBM Corp., Armonk,NY, USA), and Student’st-tests (P<0.05 andP<0.01) conducted in the same program were used to evaluate significant differences between groups.
2.3. Genotyping, linkage map construction, and QTL analysis
Genomic DNA was extracted from non-infected seedling leaves of the 117 RILs and two parents using the cetyl-trimethylammonium bromide method [32]. The Affymetrix Wheat 55 K SNP Array (CapitalBio Corporation, Beijing, China) was used to genotype the complete genomes of the 117 RILs and parents.Monomorphic SNPs and SNP loci with minor allele frequencies <0.3 were excluded from further analysis [33]. Redundant SNP markers displaying identical segregation patterns were deleted using the BIN tool in IciMapping v4.2 [31], and a single marker with the minimum amount of missing data from each bin was selected and used to construct a genetic map.Linkage groups were assigned by referring to annotated chromosomal location markers.Unlinked markers were excluded from the QTL analysis. Genetic distances were calculated using the Kosambi mapping function[34] in JoinMap v4.0 [35]. Linkage maps were graphically visualized with MapDraw v2.1 [36].
Inclusive composite interval mapping using the additive tool(ICIM-ADD) in QTL IciMapping v4.2 was undertaken to detect the positions of QTL based on the mean FDS data in each environment.A logarithm of odds(LOD)threshold of 2.5 was chosen to declare a QTL as significant.Stepwise regression was performed to calculate percentages of phenotypic variation explained by individual QTL and their additive effects at LOD peaks. The identified flanking sequences of all SNPs were subjected to BLAST searches against the Chinese Spring wheat reference genome (IWGSC RefSeq v1.0,https://urgi.versailles.inra.fr/blast_iwgsc/blast.php) and Ensembl Plant database (http://plants.ensembl.org/ info/website/ftp/index.html) to determine their physical positions.
2.4. Haplotype analysis
Haplotype analysis was performed to identify haplotype variants ofQYr.YBZR-6ALin the collection of 324 Chinese wheat landrace accessions. Informative markers linked to the target QTL were screened from the Wheat55K or Wheat660K SNP array data in accordance with the method described by Wu et al. [21] and Long et al. [22]. Marker selection criteria were as follows: (1) the marker was located in the confidence interval of the target QTL;with (2) minor allelic frequency greater than 5% and missing values <10%in the 324 landrace accessions.The SNP and phenotypic data were obtained from previous studies by Cheng et al.[23],Long et al.[24],Yao et al.[25,26],Ye et al.[27],and Wang et al.[28].To analyze haplotypes,marker groups were clustered and linkage disequilibrium plots based on the alternate D’/LOD color scheme were generated in Haploview v4.2 (http://www.broad.mit.edu/mpg/haploview/).Haplotypes detected in at least 5 accessions were considered to be major haplotypes. Phenotypic differences between haplotype groups were highlighted using box plots based on the average FDS values of accessions. The geographic distribution of the superior haplotype ofQYr.YBZR-6ALamong landraces from the 10 major agroecological production zones of China.
2.5. KASP marker development and validation
SNP markers flankingQYr.YBZR-6ALwere used to develop KASP markers using PolyMarker [37] (http://www.polymarker.info/designed_primers).KASP assays were performed on a CFX96 thermal cycler (Bio-Rad Laboratories, Inc.) in 10 μL reaction volumes containing 2.0 μL genomic DNA (50-100 ng), 5 μL of HiGeno 2× Probe Mix B (JiaCheng Biotech Co., Ltd., Beijing, China),0.24 μmol L-1of each forward primer, 0.6 μmol L-1common reverse primer,and double distilled water[22].Cycling conditions were as follows:95°C for 10 min,followed by 10 cycles of 95°C for 20 s and 61 °C (-0.6 °C per cycle) for 40 s and then 38 cycles of 95°C for 20 s and 55°C for 40 s.End-point fluorescence data analysis was performed manually using the embedded program Bio-Rad CFX96 Manager v3.1.The newly developed KASP markers were validated by analyzing polymorphisms between the parents and then used to estimate QTL effects in the RIL population. To check for polymorphisms for marker-assisted selection, the KASP markers were also assessed in 92 wheat cultivars grown in Sichuan Province.
3. Results
3.1. Phenotyping of stripe rust response
YBZR was susceptible to the current prevalent ChinesePstrace CYR34 at the seedling stage(Fig.S1A),whereas it exhibited strong resistance(FDS ≤5%)to mixedPstraces at the adult-plant stage in the field(Fig.S1B).Average FDS values of YBZR and TC29 across all test environments were 1% and 87.8%, respectively. The average FDS of the 117 RILs was 26.1% in all field tests, ranging from 0 to 100% in all environments. The frequency distribution of FDS data for each line in the YBZR × TC29 population in each environment was continuous with a pronounced skewness towards resistance,indicating quantitative inheritance (Fig. 1). In addition, a number of lines had a higher FDS than that of the susceptible parent TC29 in some environments, showing transgressive segregation for stripe rust resistance.The high estimated broad-sense heritability (H2= 0.87-0.93) of stripe rust resistance was observed in all field tests (Table 1), indicating that the APR in YBZR was highly heritable. FDS was significantly correlated among the six environments,with Pearson’s correlation coefficients ranging from 0.72 to 0.87 (P<0.01) (Table 2).
3.2. Linkage map construction and QTL analysis
After removing SNPs that were monomorphic or redundant the remaining 1385 bin markers were used for linkage map construction.The total length of the map was 3867.53 cM,with an average interval of 2.79 cM between adjacent markers. The map consisted of 27 linkage groups, including two groups each on chromosomes 5A, 1D, 2D, 3D, 4D, and 6D. Map lengths of the A, B and D subgenomes were 1339.49, 1212.80, and 1315.24 cM, respectively,with corresponding densities of 2.88, 2.51, and 3.02 cM/marker(Table S1).
Three QTL were detected on chromosomes 6A (QYr.YBZR-6AL),5B (QYr.YBZR-5BL), and 7D (QYr.YBZR-7DS) in the YBZR × TC29 RIL population (Fig. 2; Table 3), and the resistance conferred by these loci was contributed by YBZR.QYr.YBZR-6AL, flanked by markersAX-109842268andAX-110959446, was detected in all environments and explained 10.6%-14.7%of the phenotypic variation.QYr.YBZR-5BL, identified in three environments, was located betweenAX-111002705andAX-108929069and explained 10.2%-11.3% of the phenotypic variation. A second stable QTLQYr.YBZR-7DSflanked byAX-89737284andAX-109835322, explained 11.5%-21.2% of the phenotypic variation. This QTL was located within a 46.7-49.9 Mb genomic interval, with its peak around 47.7 Mb, close toYr18.
3.3. Effects of QTL combinations
To assess resistance effects conferred by each QTL alone and in combination, we divided the wheat lines according to the genotypes of the flanking markers for each QTL (Fig. 3; Table S2). The average FDS of lines carrying these loci (+QYr.YBZR-6AL, +QYr.YBZR-5BL, and +QYr.YBZR-7DS) were 14.1%, 17.1%, and 14.3%,respectively, whereas the FDS of those lacking these QTL (-QYr.YBZR-6AL, -QYr.YBZR-5BL, and -QYr.YBZR-7DS) were 37.1%,38.0%, and 43.6%, respectively (Fig. 3; Table S3). Differences in FDS between the two classes were used to estimate the respective resistance allelic effects, which varied from 55.0% to 67.2%(Table S3). In addition, RILs possessing all three resistance haplotypes exhibited a FDS that was only 10.0% of the average; these lines were more resistant to stripe rust than those with any single QTL or two-QTL combination (Fig. 3D).
3.4. Haplotype analysis in the interval of QYr.YBZR-6AL
AsQYr.YBZR-6ALwas a stable and potentially new QTL,the distribution ofQYr.YBZR-6ALin Chinese wheat landraces was assessed by haplotype analysis. Within theQYr.YBZR-6ALflanked by markersAX-109842268andAX-110959446(a physical distance of approximately 10 Mb)we identified nine informative SNP markers from the Wheat55K and Wheat660K SNP array data of the collection of 324 Chinese wheat landraces (Fig. 4A). There were five major haplotypes (n≥5) based on this SNP panel (Table S4).Among them, 9 accessions (2.8%) clustered with YBZR in Hap 1(Fig. 4A; Table S4). The average stripe rust severity of Hap 1 lines(5.9%) was lower than that of the other four haplotypes (Hap 2 = 17.5%, Hap 3 = 31.2%, Hap 4 = 18.8%, and Hap 5 = 19.4%)(Fig. 4B), indicating that Hap 1 was most representative of the resistance allele ofQYr.YBZR-6AL.Geographically,Hap 1 genotypes came from several different production zones in China (Fig. 4;Table S4).

Fig. 2. Graphical displays of locations of QTL for stripe rust resistance across all environments and average for the Yibinzhuermai × Taichung 29 RIL population. Markers surrounding the QTL are in underlined italic script.Genetic linkage maps for QYr.YBZR-6AL,QYr.YBZR-5BL and QYr.YBZR-7DS identified in the Yibinzhuermai×Taichung 29 F5:8 recombinant inbred line population. CZ and WJ denote Chongzhou and Wenjiang, respectively; 2016, 2017, 2018 and 2019 represent the RIL population grown during the 2015-2016, 2016-2017, 2017-2018 and 2018-2019 cropping seasons, respectively.
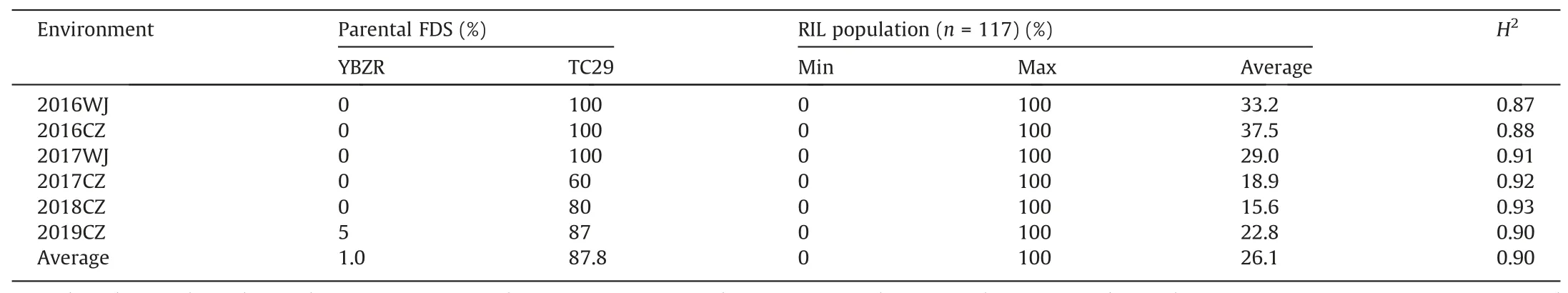
Table 1Mean stripe rust reactions of the parents and range of reactions and broad sense heritabilities (H2) for the Yibinzhuermai × Taichung 29 recombinant inbred line population grown in six environments.
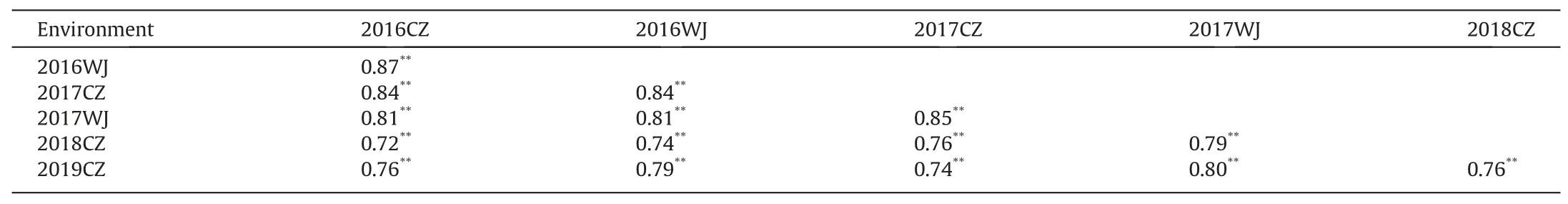
Table 2Correlation coefficients of mean final disease severities for the Yibinzhuermai × Taichung 29 recombinant inbred line population evaluated in six environments.
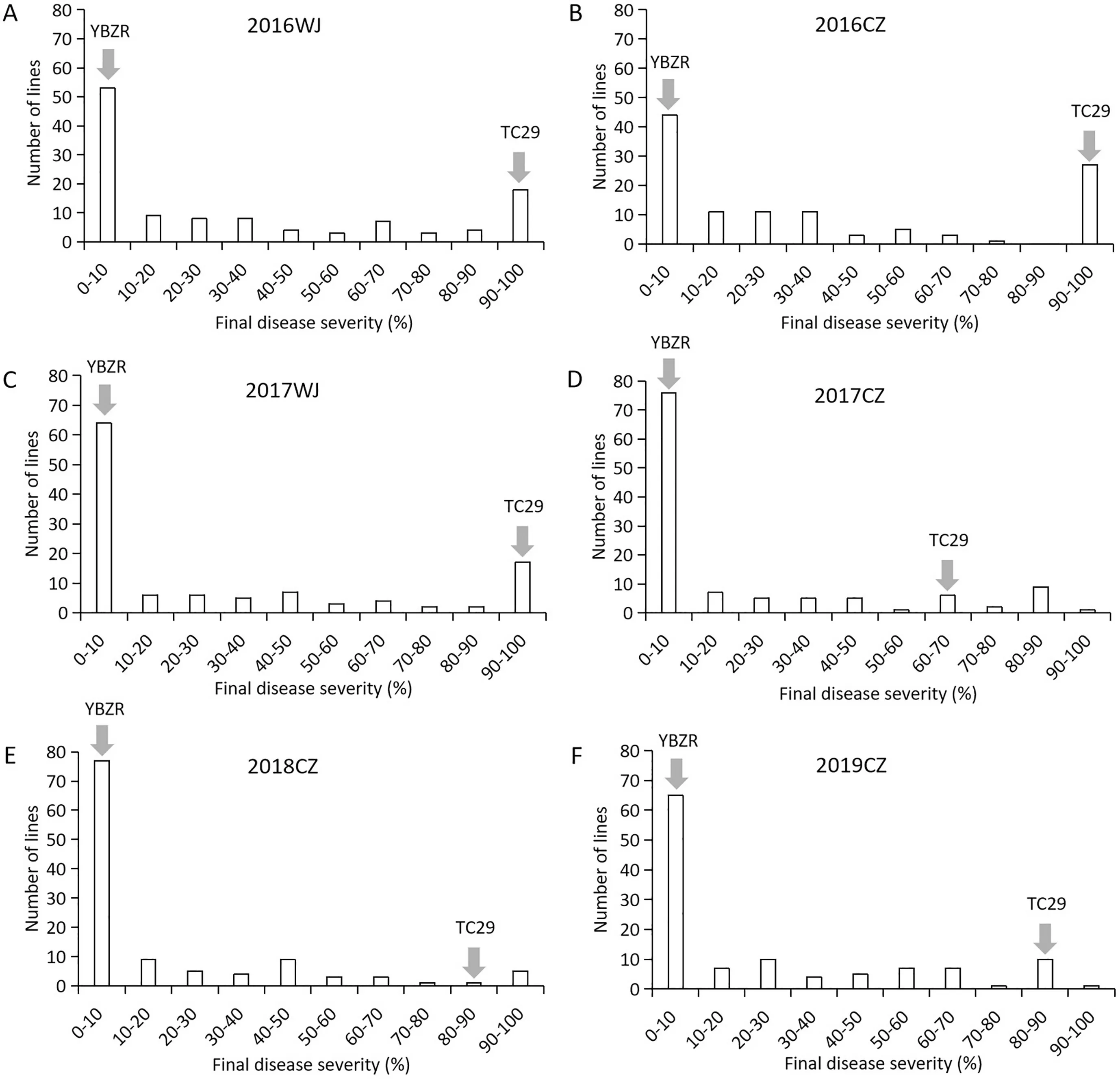
Fig.1. Frequency distributions of final disease severity for the Yibinzhuermai(YBZR)×Taichung 29(TC29)recombinant inbred line population grown at Wenjiang(WJ)in(A)2015-2016; (C) 2016-2017; Chongzhou (CZ) in (B) 2015-2016; (D) 2016-2017; (E) 2017-2018; and (F) 2018-2019. Arrows indicate values of the parental lines.
3.5. Development of KASP markers for QTL validation and markerassisted selection
SNP markers flankingQYr.YBZR-6ALwere selected for conversion to KASP markers (Table S5). Six markers were polymorphic between YBZR and TC29 as well as between the resistant (Rbulk) and susceptible (S-bulk) DNA bulks. KASP markersKASP_AX-109842268andKASP_AX-110959446were validated by exhibiting the same genotypes as the original SNP markers in the individual RILs (Table S2; Fig. S2). To check the specificity and
polymorphism of the linked SNP markers flankingQYr.YBZR-6ALfor use in marker-assisted selection,KASP_AX-109842268andKASP_AX-110959446were screened on a panel of 92 wheat cultivars (Table S6). Most of the lines amplified the alleles associated with susceptibility (76.1% and 96.7%, respectively). In addition,only one line (Xifu 14) (1.1%) carried the YBZR-allele for both markersKASP_AX-109842268(C:C) andKASP_AX-110959446(A:A).Thus,KASP_AX-109842268andKASP_AX-110959446can be used as diagnostic markers forQYr.YBZR-6ALto incorporate this QTL into breeding populations.
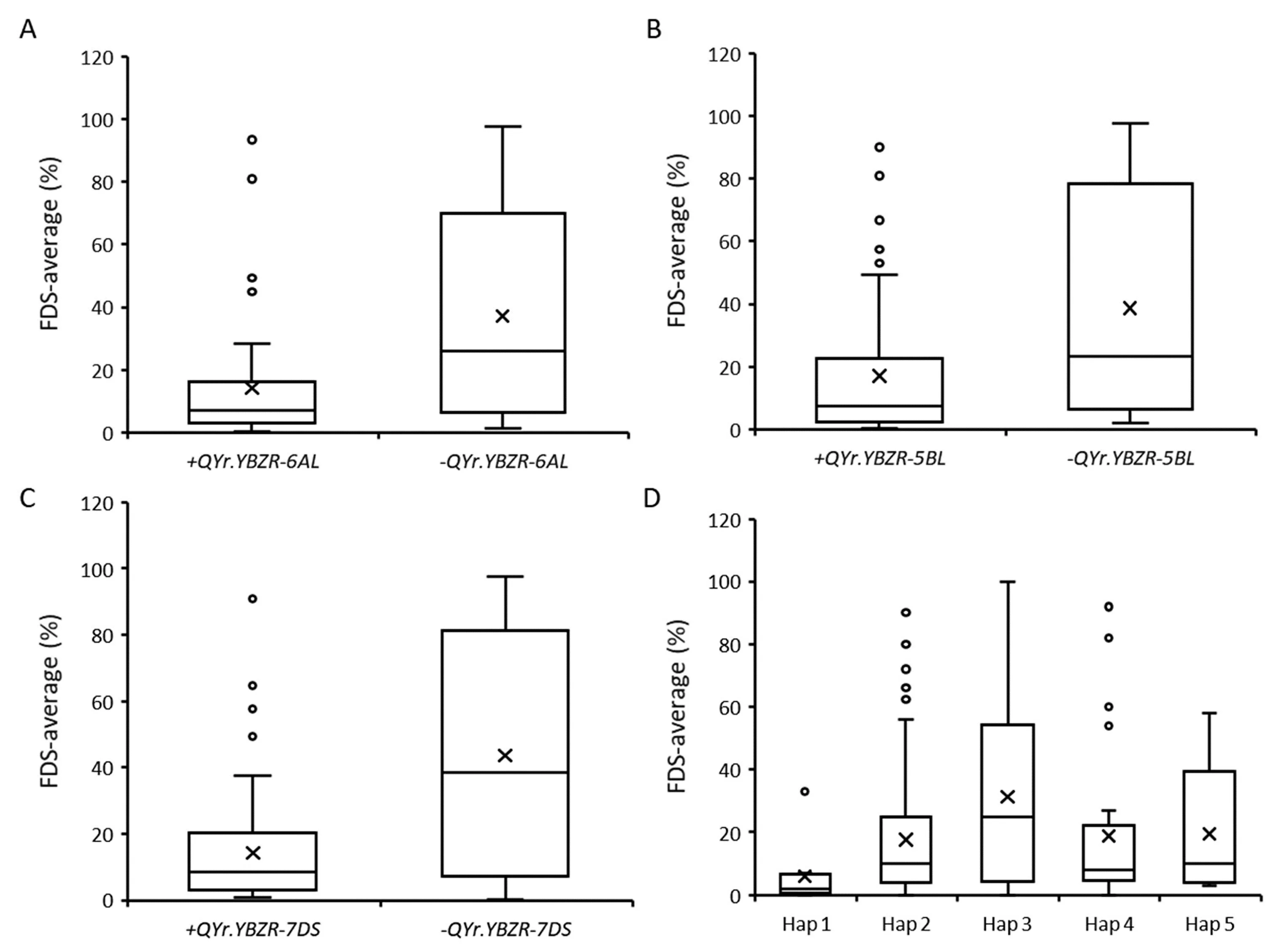
Fig.3. Box plots for average final disease severity associated with the three QTL(6AL,5BL,and 7DS)identified in the Yibinzhuermai×Taichung 29 recombinant inbred line population.
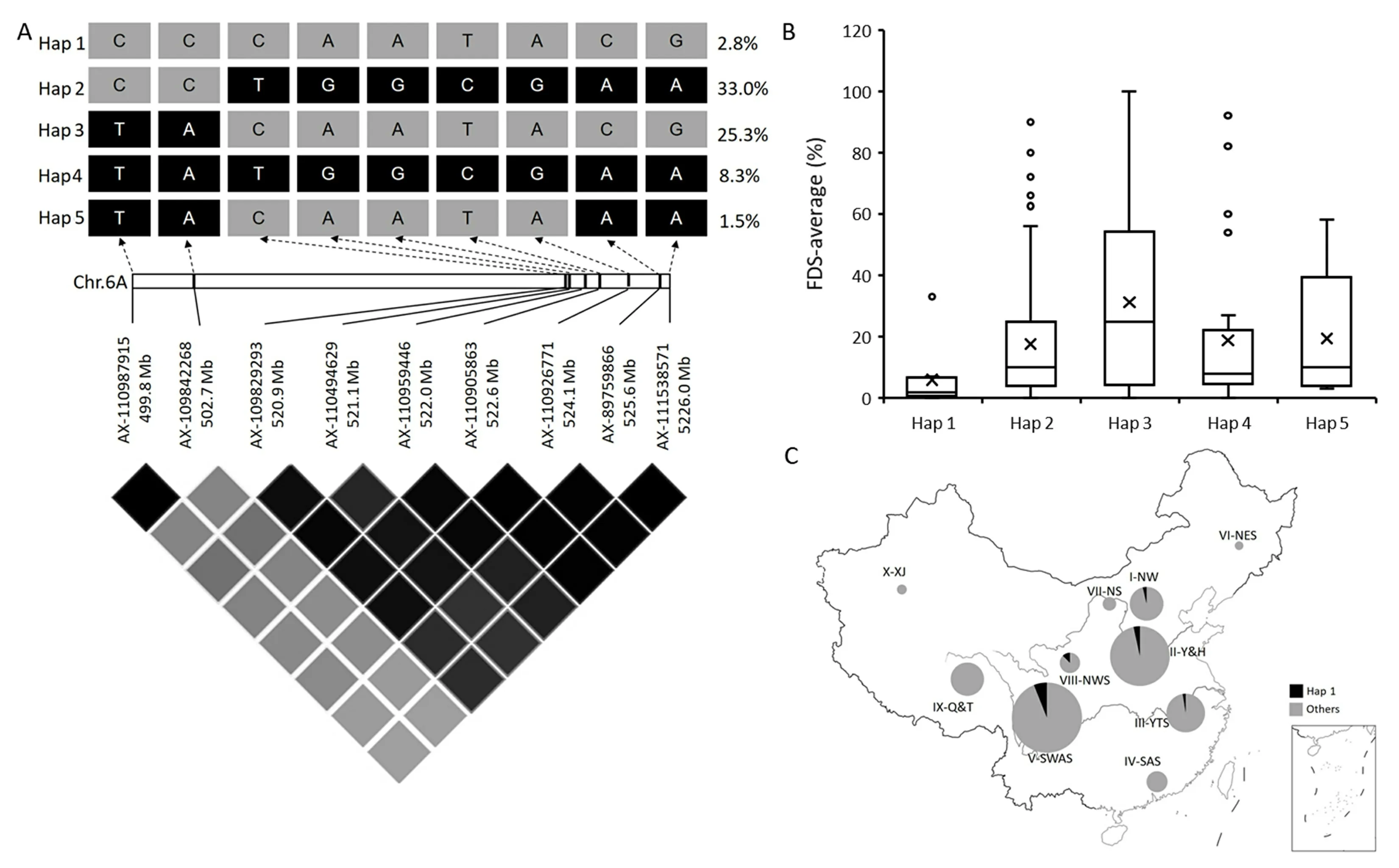
Fig.4. Haplotype analysis of QYr.YBZR-6AL associated with resistance to stripe rust in 324 Chinese wheat landraces.(A)LD heatmap surrounding QYr.YBZR-6AL. (B)Boxplot displays the average final disease severity of the accessions carrying resistance haplotype.(C)Frequencies of resistance allele of QYr.YBZR-6AL in Chinese wheat landraces in ten major agro-ecological production zones of China(The map was downloaded at the website http://bzdt.ch.mnr.gov.cn,and the map content approval number is GS(2016)1569).
4. Discussion
Chinese wheat landraces are an underutilized genetic resource for improvement of stripe rust resistance in wheat breeding.In this study, we identified three QTL for APR to stripe rust on chromosome arms 6AL,5BL and 7DS in the Chinese landrace cultivar YBZR using high-density molecular markers and multiple environmental field tests.These QTL were designatedQYr.YBZR-6AL,QYr.YBZR-5BL,andQYr.YBZR-7DS, respectively.QYr.YBZR-6ALandQYr.YBZR-7DSwere stable across all six environments, explaining 10.6% to 14.7%and 11.5%to 21.2%of the phenotypic variation,respectively.Our results indicated that the most resistant haplotype ofQYr.YBZR-6ALwas rare in a panel of Chinese wheat landraces and had not been used in modern wheat breeding. Thus,QYr.YBZR-6ALshould be useful for diversifying sources of genetic resistance in breeding populations and the KASP markers developed in this study will assist that process.
The positions of SNP markersAX-109842268andAX-110959446flankingQYr.YBZR-6ALare 502.7 to 521.9 Mb on chromosome arm 6AL of the Chinese Spring IWGSC RefSeq v1.0. FiveYrgenes have been mapped on chromosome 6AL(Table S7),includingYr38fromAegilops sharonensis[38],Yr42fromAegilops neglecta[39],YrHuafromPsathyrostachys huashanicaaccession 0503383 [40],yrLM168from CIMMYT wheat cultivar Milan [41], andYrP10090from CIMMYT wheat accession P10090[42].On the basis of origin and resistance type,QYr.YBZR-6ALis different from the ASR genesYr38,Yr42,andYrHuathat were transferred to common wheat from alien species. Both yrLM168andYrP10090confer APR to stripe rust and were identified from CIMMYT-derived lines. These genes are in the centromere region (107.1-446.5 Mb) [41,42] and do not overlap the interval encompassingQYr.YBZR-6AL.In addition,at least 23 QTL for stripe rust resistance have been located on 6AL using biparental populations and genome-wide association studies; however, most of these QTL are quite distal (greater than50 Mb) fromQYr.YBZR-6AL(Table S7).Four QTL have been reported in the interval between 450 and 550 Mb on chromosome arm 6AL(Fig.5).QYr.wsu-6Awas a minor QTL located at ~461.3 Mb[43].Qyr-6A-1,the most closely linked QTL toQYr.YBZR-6ALwas located between 514.0 and 522.2 Mb[44].QYr-6A-1was identified in aT.dicoccoidesFig.5.Comparison ofQYr.YBZR-6ALwith previously identified QTL for resistance to stripe rust based on the reference genome of bread wheat (IWGSC, RefSeq v1.0).accession (pau4656) and confers ASR to stripe rust, and is thus unlikely to be identical to a QTL for APR in a Chinese wheat landrace. The minor unstableQYr.caas-6ALin the cultivar Zhong 892 was located in the region 531.0 to 572.6 Mb [45].QYr.ufs-6A, a minor QTL for stripe rust resistance in the wheat cultivar Avocet was located at 545.1 to 605.3 Mb[46].Given the resistance source,type, degree of effectiveness, and physical location of each gene/QTL,QYr.YBZR-6ALappears to be novel.
QYr.YBZR-5BL,flanked byAX-111002705andAX-108929069,and located in the chromosome 5BL interval 519.0-542.7 Mb overlapped the recently reported QTLQYr.AYH-5BL(521.7-539.0 Mb)[22].QYr.AYH-5BLis a major QTL for stripe rust resistance in the Sichuan landrace Anyuehong. KASP markers developed forQYr.AYH-5BLwere positively associated withQYr.YBZR-5BLgenotyping when tested on the YBZR × TC29 RIL population. Thus,QYr.YBZR-5BLandQYr.AYH-5BLare likely the same.
QYr.YBZR-7DSwas flanked by SNP markersAX-109857040andAX-110052753,corresponding to the region 46.7-49.9 Mb on chromosome 7D and overlapping withYr18(47.4 Mb).Yr18is a common resistance gene in Chinese wheat landraces [47-50]. In the present study, application of the diagnostic markercssfr5and leaf tip necrosis in some environments indicated that YBZR carriedYr18([11], Fig. S1; Table S2). Hence,QYr.YBZR-7DSis likelyYr18.
CRediT authorship contribution statement
Mei Deng:Visualization,Writing-original draft.Li Long:Visualization, Writing - original draft.Yukun Cheng:Visualization,Writing - original draft.Fangjie Yao:Investigation, Data curation.Fangnian Guan:Investigation,Data curation.Yuqi Wang:Investigation, Data curation.Hao Li:Investigation, Data curation.Zhien Pu:Methodology.Wei Li:Methodology.Qiantao Jiang:Methodology.Yuming Wei:Formal analysis, Software, Resources.Jian Ma:Methodology.Houyang Kang:Formal analysis, Software,Resources.Pengfei Qi:Formal analysis, Software, Resources.Jirui Wang:Formal analysis, Software, Resources.Youliang Zheng:Conceptualization.Yunfeng Jiang:Formal analysis,Project administration,Writing-review&editing.Guoyue Chen:Conceptualization,Funding acquisition,Project administration,Writing-review& editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFD0100100),the International Science and Technology Cooperation and Exchanges Programs of Science and Technology Department of Sichuan Province (2019YFH0063) and the Applied Basic Research Programs of Sichuan Province(2021YJ0297).The authors are grateful to Prof. Qiuzhen Jia (Plant Protection Research Institute, Gansu Academy of Agricultural Sciences, Lanzhou, Gansu, China) for providing Pst urediniospores, and Prof. Lihui Li and Xiuquan Li (Chinese Academy of Agricultural Sciences, Beijing, China) for providing seed of wheat landraces.
Appendix A. Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2021.10.011.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Revisiting the role of delta-1-pyrroline-5-carboxylate synthetase in drought-tolerant crop breeding
- A new gain-of-function OsGS2/GRF4 allele generated by CRISPR/Cas9 genome editing increases rice grain size and yield
- Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat
- Corrigendum to ‘‘De novo design of future rapeseed crops: Challenges and opportunities” [Crop J. 10 (2022) 587-596]
- Improving the resistance of the rice PTGMS line Feng39S by pyramiding blast, bacterial blight, and brown planthopper resistance genes

