Vegetation structure and edaphic factors in veredas reflect different conservation status in these threatened areas
Yul Rorta Frrira Nuns, Camila Silvira Souza,Islain Franilly Pinhiro Azvo, Oirli Sim~os Olivira,Liivan Almia Fraz~ao, Rúia Santos Fonsa, Runs Manol os Santos,Waltr Viana Nvs
a Programa de P'os-Graduaç~ao Em Bot^anica Aplicada,Departamento de Biologia Geral,Universidade Estadual de Montes Claros,Avenida Dr.Ruy Braga,S/N-Bairro Vila Mauric'eia, Montes Claros, Minas Gerais, 39401-089, Brazil
b Laborat'orio de Ecologia Vegetal, Universidade Estadual de Montes Claros, Avenida Dr. Ruy Braga, S/N - Bairro Vila Mauric'eia, Montes Claros, Minas Gerais, 39401-089, Brazil
c Instituto de Ci^encias Agr'arias - Universidade Federal de Minas Gerais, Avenida Universit'aria, 1.000 - Bairro Universit'ario, Montes Claros, Minas Gerais, 39404-547,Brazil
d Departamento de Ci^encias Florestais, Universidade Federal de Lavras, CP 3037, Lavras, Minas Gerais, 37200-000, Brazil
e Instituto Mineiro de Gest~ao das 'Aguas, Secretaria de Estado de Meio Ambiente e Desenvolvimento Sustent'avel, Avenida Jos'e Correia Machado, 900 - Bairro Ibituruna,Montes Claros, Minas Gerais, 39401-832, Brazil
Keywords:Cerrado Conservation Palm swamp Plant distribution Climate change Water balance effect Vereda drought Phytosociological analysis
ABSTRACT
1. Background
The Cerrado has a complex mosaic of distinct phytophysiognomies,with savanna and forest formations.In Cerrado humid areas,veredas are considered as hygrophilous communities,marked by the presence of the Mauritia flexuosa L.f. (buriti) palm tree emerging in the wetter zones(Araújo et al.,2002;Ribeiro and Walter,2008;Oliveira et al.,2009;'Avila et al.,2016).Veredas communities occupy hydromorphic soils,gleys,and organic turfs(Gleysols and Histosols),and are associated with relatively shallow water tables (Ramos et al., 2006; IUSS Working Group WRB,2015;Bijos et al.,2017).Veredas emerge from wet patches in the soil that are established by variations in the topography and alternation of layers of soil with distinct levels of permeability (Oliveira et al., 2009; Neves et al.,2015;Bijos et al.,2017).The vegetation is formed of a continuous herbaceous-shrubby layer and the gradient between more and less wet zones determines the variations in species composition (Araújo et al.,2002;Guimar~aes et al.,2002;Oliveira et al.,2009;Resende et al.,2013;Nunes et al., 2015). Thus, the herbaceous/shrubby component predominates in open areas and more humid soil, and the tree component occurs close to the water table expansion region (hygrophilous forests),in soil that is usually permanently saturated(Araújo et al.,2002;Oliveira et al.,2009; Nunes et al.,2015).
Studies in veredas are needed to fill the gaps in information about fauna, flora, ecology, hydrology, among others inherent to any ecosystem,in order to assess their impacts,when their biotic and abiotic functions are altered (Meirelles et al., 2004; 'Avila et al., 2016). The factors that are contributing to the mischaracterization of veredas are dams, with flooding and vegetation suppression, construction of unplanned roads, promoting the siltation of veredas courses, soil compaction mainly by cattle trampling,use of its floodplains as natural roads and the opening of roads with the use heavy machinery (Guimar~aes et al.,2002; Brasil et al., 2021). Furthermore, climate change and fires (Bond and Wilgen,1996)have been a great threat in the process of degradation of the veredas. Associated with climate change that causes drier years(with low rainfall),more warmer periods(Oliveira et al.,2017;Hofmann et al., 2021) and deforestation in these areas, the water balance is affected causing changes in hydrological processes and leading to drought of these areas in Cerrado(Oliveira et al.,2014,2015,2017).In addition, little attention has been paid to the consequences of climate change,land use and land cover on groundwater recharge.Furthermore,hypotheses have been proposed that groundwater levels may determine vegetation density and diversity (Orellana et al., 2012; Rossatto et al.,2012;Villalobos-Vega et al.,2014).The decrease in the level of the water table that causes the veredas drought indicates that it is the main impact causing modification of this formation('Avila et al.,2016).
These ecosystems are losing their vegetation originality. Open areas are more exposed to anthropogenic changes and their species are the most susceptible to environmental changes(Araújo et al.,2002;Oliveira et al.,2009).Further studies on the composition,richness,and diversity of plant species in veredas communities are essential for understanding relevant ecological processes and also for developing appropriate conservation policies (Junk et al., 2014; Rosolen et al., 2015; Neves et al.,2015;Bijos et al.,2017).Changes in veredas ecosystems,which represent important areas for the biodiversity maintenance in the Brazilian Cerrado,can lead to the emergence of new landscapes and ecosystems.These modified veredas may represent systems that differ in composition and/or function from present and past systems as a consequence of changing species distributions,environmental alteration through climate and land use change and shifting values about nature and ecosystems(Harris et al.,2006;Root and Schneider,2006).Thus,this formation has been losing its original characterization where common species in veredas are being replaced by species from other arboreal formations in Cerrado ('Avila et al.,2021).
Assuming that the veredas are undergoing changes in species composition due to anthropogenic factors and climate change which leads to the drought of these formations (water balance effect), in this study, we evaluated the flora composition and described the current vegetation profile of two veredas under different levels of disturbances that are located in the north of Minas Gerais State,Brazil.We evaluated the species composition in the open areas and in the hygrophilous forests in the two veredas and verified whether the difference in species composition was influenced by soil fertility and acidity. We expected that: a) higher levels of disturbances (drought of these formations with water balance effect), affecting wood community differentiating the vegetation profile and species composition between the two veredas; b)soil fertility influences abundance, richness and diversity of species positively in these areas, and soil acidity negatively; c) the vereda with high water balance disturbance will have its open area more similar to other cerrado(savanna)vegetation types and its hygrophilous forest with a different species composition from the more conserved vereda (due drought of these formation). Thus, the results of species composition presented here will bring information about the modification of these ecosystems and the emergence of new possibly fragile ecosystems, susceptible to greater effects of climate change.
2. Methods
2.1. Study areas
We collected data in two Conservation Units: 'Area de Proteç~ao Ambiental do Rio Pandeiros (APA Rio Pandeiros) and Parque Estadual Veredas do Peruaçu(PEVP).The two areas are located in the north of the Minas Gerais State.Almescla vereda(15°20′54.9′′S; 44°53′84.5′′W),in the APA Rio Pandeiros,is located in the municipality of Bonito de Minas,and the Peruaçu vereda(15°01′10.5′′S;44°42′15.3′′W),in the PEVP,in the municipality of C^onego Marinho (Fig. 1). The region has a Aw type tropical climate according to Kӧppen’s classification,with dry winter and average annual temperature of 22.2–22.7°C, and rainfall ranging from 1,008 to 1,073 mm, respectively (Alvares et al., 2013; Azevedo et al.,2014).During the study period the two areas did not show differences in mean temperature and total precipitation (see Supplementary Material Fig.S1,P >0.05,F=2.70,df=138).The soil in the region is considered dystrophic and sandy (Nunes et al., 2015), classified as Hystosols (IUSS Working Group WRB, 2015). The vegetation in the study area is represented by Cerrado and Caatinga transition phytophysiognomies, culminating in a mosaic of riparian and dry forests,savanna,and palm swamps- veredas (Menino et al., 2012; 'Avila et al., 2021). All study sites are characterized by high and threatened biodiversity (Myers et al., 2000),and specifically the areas of veredas in the region are in the process of drought, with loss of springs (according to reports from residents and visual observations)and its landscape shows various anthropic activities,such as raising domestic animals,farming,grazing,harvesting wood and burning ('Avila et al., 2021). However, it is still possible to observe the presence of water above soil from the groundwater in some locals, and the vegetation presents a typical structure of hygrophilous environments,with the presence of two main zones (open areas, and hygrophilous forest).The Peruaçu vereda presents drought at an advanced stage,with loss of springs and retraction of the upper portion of the river (42 km).Furthermore, according to reports from the residents, the vereda has burnt sections,with high tree mortality leading to a change in landscape.This is evidenced due to changes in the vegetation composition at various points along its course and the approximation and invasion of typical cerrado species.
2.2. Vegetation sampling
For sampling the plant community in the studied areas, the plot method was used (Mueller-Dombois and Ellenberg, 1974). For a better characterization of the veredas sampled, these are defined in two zones linked to topography and soil drainage: hygrophilous forest and open area.In the Almescla vereda,the plots allocated were distributed in two transects parallel to the watercourse. In the transect one defined as the hygrophilous forest, 30 plots of 10 m × 20 m with a distance of 150 m between plots were distributed along the transect.The arboreal-shrubby individuals within these plots with DBH ≥5 cm(diameter at breast height– measured 1.30 m above ground level) had their height estimated and were identified and marked with numbered aluminum plates. Furthermore, within each of the 10 m × 20 m plots, smaller plots with a dimension of 5 m × 5 m were delimited for sampling the arboreal-shrubby individuals from the lower strata and/or juveniles.Thus,in these subplots all arboreal-shrubby individuals with DBH ≥3 cm had their height estimated, registered, and numbered with aluminum plates.In relation to the zone defined as the open area,two transects 20 m apart were allocated.In these transects,38 plots of 10 m×20 m were demarcated, 150 m between plots, arranged in parallel with the plots marked in the hygrophilous forest. All shrub-tree individuals at DBS(diameter at the base of the stem)≥5 cm were marked with aluminum plates, measured and identified. Likewise, sub-plots of 5 m × 5 m were plotted within the plots for sampling the shrub-sub-shrub individuals.Thus, all individuals with DBS ≥3 cm were marked, identified and measured.

Fig. 1. Location of the studied veredas, distribution of sampled plots, photographs, illustrations, and vegetation profile description of the Peruaçu and Almescla veredas.The photographs and illustrations show the open areas and the hygrophilous forest with the level of the groundwater and the height of the plant species.The data for the map construction were obtained from the Mapbiomas platform (Souza et al., 2020).
The procedure applied to the second area of study, the vereda Peruaçu,was similar to that described in the vereda Almescla.However,there were changes in some methods due to the advanced stage of drought in this ecosystem.Thus,in the transect defined as hygrophilous forest,30 plots of 10 m×20 m and 5 m×5 m were also allocated along with the subplots.However,there was no division in two transects in the open area as occurred in the Almescla. Thus, only one transect was established along the open area with 27 plots of 20 m×10 m along with the subplots.
To delineate the plots,a compass and measuring tape were used,and to demarcate them, PVC pipes were positioned at each plot vertex and their limits were marked with sisal wire.The individual’s identification was carried out in the field,given prior species knowledge,and through collected material(vegetative or reproductive)for identification through specialized literature, sent to specialists or comparison with existing material. Vouchers for all plant species were collected, identified, and deposited in the Montes Claros Herbarium(MCMG),of the Universidade Estadual de Montes Claros (UNIMONTES), and the Norte Mineiro Herbarium (ICA-UFMG) of the Universidade Federal de Minas Gerais(UFMG). The family names followed the Angiosperm Phylogeny Group(APG,2016),and species names were confirmed in the Plant list database(http://www.theplantlist.org/) and updated/corrected whenever necessary.
2.3. Groundwater level measurement
In addition to visual confirmation, the groundwater level (charged and uncharged)was measured in both veredas considering the open and hygrophilous areas over 20 months (July/2019 to August/2021). Measurements were not performed in November and December 2020 and January,March,April and June 2021 due to logistical issues.The water table was measured using three monitoring wells distributed along the two veredas studied. The wells were allocated and drilled at the ends of the transects.The perforations were carried out using a semi-mechanized auger.The holes were lined with tubes and the drilling depths exceeded the water table by approximately 2 m. The water table measurements were performed using an HS electronic water level meter(hydrosupplies,mode:ASNA-30).
2.4. Soil variables
Composite soil samples of 500 g from the topsoil (0–20 cm depth)were collected in all plots to evaluate the effect of soil variables on the species composition and structure.The soil texture(proportions of coarse sand(CS),fine sand(FS),silt,and clay)and the chemical variables[water pH (pH), Calcium Chloride pH (CaCl2pH), phosphorus (PMehlic), potassium (K), calcium (Ca), magnesium (Mg), aluminum (Al), hydrogen and aluminum (H + Al), effective cation exchange capacity (t), base saturation(V),aluminum saturation(m)]of each composite sample were performed at the Laboratory for Soil Analysis at the Institute of Agricultural Sciences (Universidade Federal de Minas Gerais) according to Teixeira et al.(2017).
2.5. Data analysis
We calculated the phytosociological parameters:absolute and relative density, dominance and frequency, and importance value (Mueller--Dombois and Ellenberg, 1974). All phytosociological parameters were conducted in R(Development Core Team,2021),and the code created to perform the analysis is available on the GitHub (https://gith ub.com/GustavoHeringer/phytosociology; Heringer et al., 2020). We estimated the sampling completeness for each sampled area by computing the Chao 1 species richness estimator (Chao, 1984; Colwell and Coddington,1994)using the iNEXT package(Hsieh et al.,2014)in R(Development Core Team,2021).With the same package,we also plotted rarefaction and extrapolation curves (Hsieh et al., 2014). The Shannon diversity index(H′)and Bray-Curtis dissimilarity(Magurran,2011)were calculated in the R program(Development Core Team,2021).
To assess the differences in species composition, first Nonmetric Multidimensional Scaling (NMDS) was calculated using the Bray-Curtis dissimilarity distance between species (Legendre and Legendre, 1998).The records contain combinations of continuous numerical data and we used the abundance of individuals in the plots for each species. The distance is always a number between 0 (identical) and 1 (maximally dissimilar) (Legendre and Legendre, 1998). The resulting dissimilarity matrix was used for computing the NMDS ordination with the function metaMDSiter in the vegan package in R,which identifies a stable solution using several random starts with smaller stress values (Borcard et al.,2011; Oksanen et al., 2016). In our analysis, we set the number of random starts as 2000, and examined whether solutions with two or three dimensions best describe the data. The optimal number of dimensions was two because the stress remained with acceptable value(Borcard et al., 2011). Finally, using the species-plot matrix at all sampled sites, we calculated a modularity analysis derived from ecological network theory.This analysis has the possibility to verify the affinity of some species for the areas and plots sampled.Thus,if certain plots occupy the same module, it means that these plots have a high species overlap showing greater similarity in species composition. We evaluate how the different plots differ in species composition using Modularity indices that quantify the prevalence of species within subsets of plots in the community and was calculated using the DIRTLPAwb +algorithm (Beckett, 2016) using the computeModules function in Bipartite package (Dormann et al., 2008). In addition to using the raw modularity,we used the Patefield null model,which fixes the matrix size and the marginal totals, which is species richness and species observation,while shuffling occurrence randomly(Patefield,1981).We consider the modularity and consequent separation of the plots in the different modules to be significant if the observed value was greater than those generated by the null model.
To investigate the relation with the soil variables and the plots and veredas formations, principal component analysis (PCA) was performed with all soil variables.We use the first and second principal components(principal component 1 and principal component 2) resulting from the PCA as a generalized index that synthesizes the information of the soil variables. We calculated the PCA analysis using the “FactoMineR”package” (Husson et al., 2016) in R (Development Core Team,2021).
We evaluated the association between plots in the open areas and hygrophilous forests in the two veredas in the modular network. We conducted the Chi-square test to contrast the proportion of plots in the different areas sampled across the modules. We then illustrated the proportion/contribution of each area to each module graphically using Pearson residuals from the Chi-square test with the corrplot package in R(Wei and Simko, 2017). Then, we fitted Linear Models with a Poisson distribution, tanking richness, Shannon diversity index (H′) and abundance as the predictor’s variables and areas as response variables-fixed factors. We also fitted Linear Models with a Poisson distribution,taking PCA analysis axes for soil and NMDS as the predictor’s variables and species richness,abundance and Shannon diversity index(H′)as response variables - fixed factors (Zuur et al., 2009). All statistical analyses were conducted in R (Development Core Team,2021).
3. Results
Altogether, 2,268 individuals of 91 species belonging to 36 families were recorded(Table S1).The family Fabaceae was the most represented(17 species), followed by Melastomataceae (nine), Malpighiaceae and Vochysiaceae with seven species each. In the hygrophilous forests,Peruaçu presented 43 species, while Almescla had 37 species. For open areas, Peruaçu 26 species and Almescla 32 species. Thus, all sampled areas differed in terms of species richness(F=6.87;p <0.001),diversity(F=2.40;p=0.05),and abundance in the plots(F=11.46;p <0.0001;Figs. 1 and 2).
In the sampled areas, the species varied in their importance value(IV), being in the Peruaçu hygrophilous forest Tapirira guianensis Aubl.(Anacardiaceae), Xylopia emarginata Mart. (Annonaceae), Cecropia pachystachya Tr'ecul(Urticaceae)and the palm Mauritiella armata(Mart.)Burret(Arecaceae)the species with the highest IV values.In the Almescla hygrophilous forest, Tapirira guianensis Aubl. (Anacardiaceae), Mauritia flexuosa L.f.(Arecaceae),Mauritiella armata(Mart.)Burret and Cecropia pachystachya Tr'ecul(Urticaceae)had the highest IV values. In this way,almost all the species are repeated in the two areas in relation to their importance value, except for Xylopia emarginata being a species with higher importance value only in the vereda Almescla(Table S1).
In relation to the open areas,in Peruaçu Stryphnodendron adstringens(Mart.) Coville (Fabaceae), Tachigali subvelutina (Benth.) Oliveira-Filho(Fabaceae), Senna cana (Nees & Mart.) H.S. Irwin & Barneby (Fabaceae) and Macairea radula (Bonpl.) DC. (Melastomataceae) had the highest IV values, and Byrsonima pachyphylla A. Juss. (Malpighiaceae),Xylopia aromatica (Lam.) Mart. (Anonnaceae), Macairea radula (Bonpl.)DC. (Melastomataceae) and Curatella americana L. (Dilleniaceae) were the species with the highest VI values in the Almescla open areas(Table S1). The rarefaction curve for the hygrophilous forest areas exhibited a tendency toward stabilization.However,for open areas,the rarefaction curves did not stabilize, as in these areas there are few tree species compared to hygrophilous forest(Fig.2).
We identified that the groundwater level differs between the two veredas(p <0.0001;F=128.5;df=0.62).In Almescla,the groundwater presents variations in the charged and uncharged levels due to variation in precipitation, showing still normal conditions. However, for the Peruaçu,the groundwater level is in deficit(see Supplementary Material Fig.S2).In the chemical soil analyses,the first PCA axis contained 31.4%of the variation, and the second comprised 28.7%. The values that contributed most to the variation and had the highest positive correlation with the first axis were m%,Al,H+Al,Clay,Sand,Silt and Mg(Fig.3ad).In addition,the first axis had high pH values(negative coordinates in PC1) and high H + Al values (positive coordinates in PC1; Fig. 3c).Therefore, we consider the first PCA axis as a soil acidity gradient. The second axis of the PCA showed a positive correlation with sand,Mg and V% and negatively correlated with H + Al, P, K, Al, silt, Ca, pH and V%,considered in the soil PCA a fertility gradient, which the positive coordinates comprise the most fertile soils(Fig.3d).
Thus, the first axis of the PCA (soil acidity gradient) explained the variation in richness, abundance and diversity of species in the open areas of Peruaçu and Almescla (richness: F = 5.53, r = 0.13, p = 0.02;abundance:F=9.81,r=0.17,p=0.003;Shannon index:F=3.88,r=0.10,p=0.05;Table 1),but it was not related to richness,abundance and diversity in the hygrophilous forest (richness: F = 3.33, r = 0.10, p =0.07;abundance:F=3.29,r=0.10,p=0.07;Shannon index:F=2.95,r= 0.10, p = 0.09). The second axis of the PCA (fertility gradient) was related to richness and abundance of open areas, but not to species diversity(richness:F=5.53,r=0.13,p=0.04;abundance:F=12.04,r=0.20,p=0.001;Shannon index:F=3.88,r=0.10,p=0.14).In addition,the second axis of the PCA did not show a relation for the hygrophilous forest with any of the response variables analysed(richness:F=0.01,r=0.01,p=0.92;abundance:F=2.62,r=0.20,p=0.11;Shannon index:F=0.44,r= 0.01,p =0.51;Table 1).

Fig.2. Rarefaction and extrapolation curve using the Chao 1 estimator of species richness and sampling effort calculated as the ratio of observed and estimated species richness for the sampled areas (Peruaçu and Almescla veredas) and richness, Shannon index and abundance comparisons between areas. HFA = hygrophilous forest Almescla; HFP = hygrophilous forest Peruaçu; OAA = Open area Almescla; OAP = Open area Peruaçu.

Fig. 3. Principal component analysis(PCA)from soil data(a)and scree plot showing the percentage of explained variances in each dimension (b).Contribution and Pearson correlations of the soil variables to the variances of first(c)and second PCA dimensions(d).HFA=hygrophilous forest Almescla;HFP=hygrophilous forest Peruaçu; OAA = Open area Almescla; OAP = Open area Peruaçu.
The NMDS of the species composition had a stress value of 0.10 and identified a difference in species composition and dissimilarity between the sampled areas (Fig. 4a-b). Thus, the open areas and hygrophilous forest plots differed in relation to their species composition in Almescla and Peruaçu.That way,the first axis of the NMDS analysis was correlated with the richness and abundance of species in the open areas, but not with the species diversity(richness:F = 6.21,r =0.11,p = 0.02;abundance: F = 140.50, r = 0.76, p <0.0001;Shannon index: F = 1.15, r =0.03,p=0.29;Table 1).Regards the hygrophilous forest,the first axis of the NMDS was related to species richness and abundance but not with diversity of species(richness:F=5.64,r=0.20,p=0.02;abundance:F= 143.70, r = 0.71, p <0.001; Shannon index: F = 0.15, r = 0.02, p =0.70). On the other hand, axis 2 only for the abundance of species,was related to open areas(richness:F=0.58,r=0.01,p=0.45;abundance:F=4.44,r=0.10,p=0.04;Shannon index:F=0.05,r=0.02,p=0.83),and for hygrophilous forest, none of the response variables analysedshowed a significant relationship with the second axis of the NMDS(richness:F=0.14,r=0.14,p=0.70;abundance:F=0.03,r=0.20,p=0.87;Shannon index:F =0.05,r=0.03,p =0.82;Table 1).
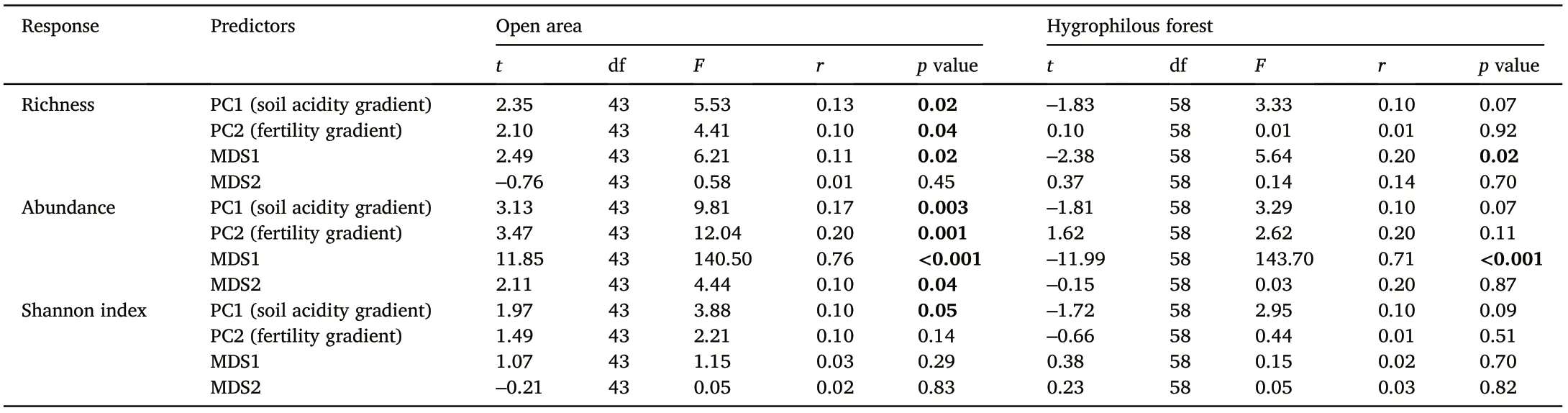
Table 1 Results from Generalized Linear Models testing the effect of soil variables and NMDS results in richness, abundance, and species diversity in the vereda Peruaçu and Almescla.
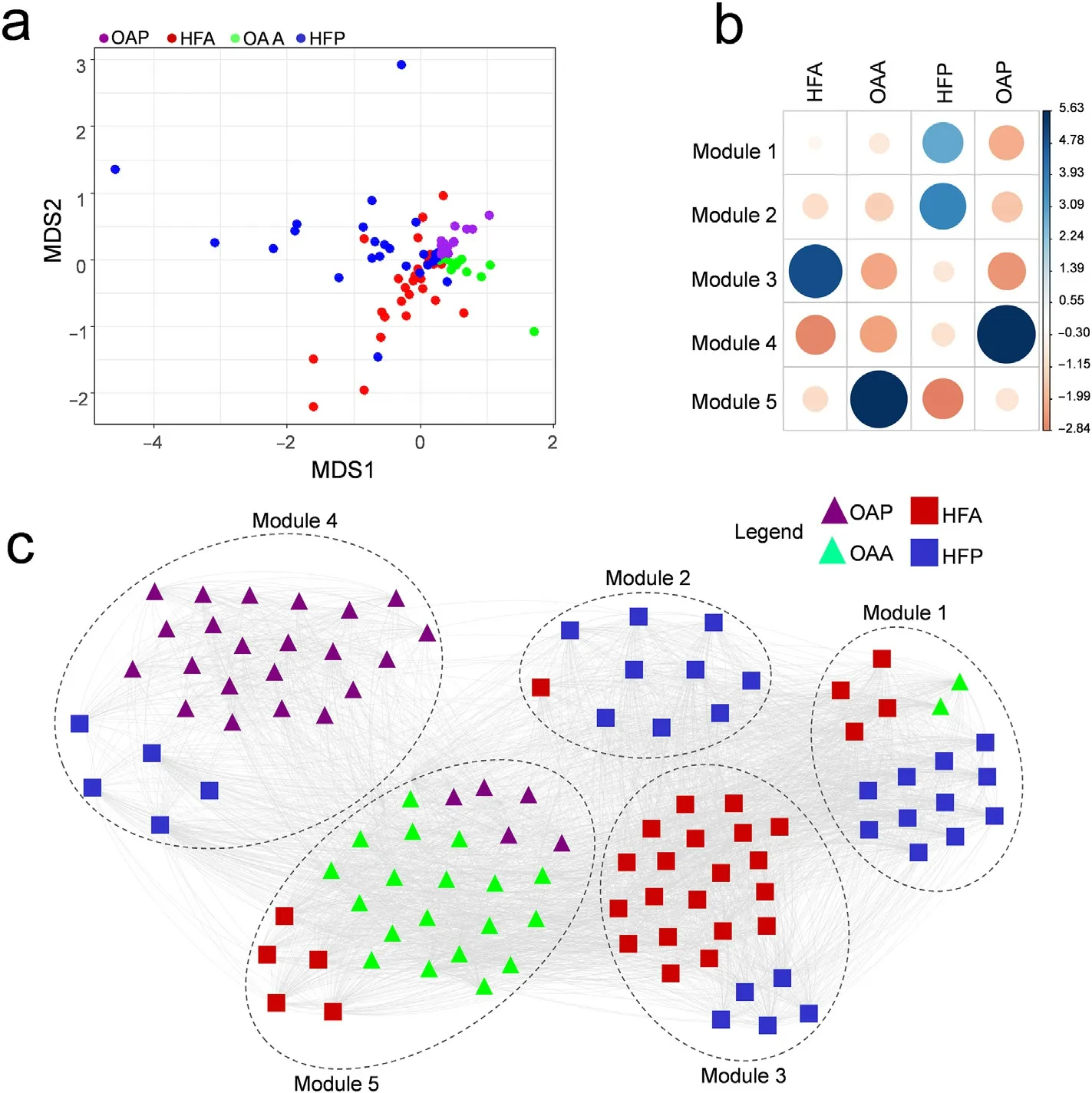
Fig. 4. NMDS results for the plots and areas sampled in the two veredas (a); correlation plot showing the association power of the plots composition and the five detected modules in the modularity analysis (b).Blue circles indicate a positive association, expressed by positive Pearson residual values, while red circles indicate a negative association, or negative Pearson residual values; network combining the plots recovered from the two veredas. The modular network structure (c), discriminating the open areas and hygrophilous forests distribution. Each module is delimited by circles with dotted lines. The lines connecting the different sampled areas mean that these sites share plant species with each other. Thus, the greater the number of shared species, the greater the number of connections. HFA = hygrophilous forest Almescla;HFP=hygrophilous forest Peruaçu;OAA=Open area Almescla; OAP = Open area Peruaçu. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
This difference in species composition was consolidated by modularity analysis,which was significant(Q=0.52;delta Q=0.48;Fig.4cd) and identified 5 modules. Some of the modules included plots from more than one area (Fig. 4c), but in general, the plots of the same area tend to occupy the same module, showing a definition in the species composition between the different modules (χ2= 158.55, df = 12, p <0.0001;Fig.4c).For instance,modules 1 and 2 were strongly associated with Peruaçu hygrophilous forest plots, module 3 with Almescla hygrophilous forest plots,module 4 with open areas Peruaçu and module 5 with open areas of Almescla plots(Fig.4c).
4. Discussion
Our results showed that the studied veredas were floristically contrasting in terms of the arboreous-shrubby species composition,richness,and diversity. These results have already been reported for the tree stratum when comparing different sites of Cerrado sensu stricto,possibly in response to the interactions among climate,altitude,and soil fertility(Oliveira-Filho et al., 1989; Ratter et al., 2003; Silva et al., 2006). In addition,our results demonstrate that open areas are more likely to vary in species richness,abundance and diversity due to changes in soil acidity and fertility.
The studied veredas were relatively rich in tree species when compared with the other palm swamps(Guimar~aes et al.,2002;Resende et al., 2013) and other phytophysiognomies dominated by herbaceous-shrubby vegetation, such as moist grasslands (Munhoz and Felfili,2008).Differently,the species richness was lower than that found in other phytophysiognomies of the Brazilian savannas, such as dry shrub-grassland (Munhoz and Felfili, 2006), possibly due to differences in water content,physical and chemical properties of the soil,and other biotic factors (Bijos et al., 2017). The herbaceous-shrubby layer is a principal component of humid ecosystems, although at the sites where they occur there are diverse, characteristic, and exclusive flora that include a predominance of typical species morphologically and physiologically adapted to survival in saturated environments(Blom,1999).
However, the high richness of tree species draws attention to an expansion of species from the Cerrado in the open areas of the studied veredas, which leads to a mischaracterization of this formation. This expansion of species from the Cerrado may be due to the open area’s drainage,which allows for a decrease in species adapted to this condition and the emergence of tree species arising from Cerrado. This can be reinforced by the vegetation profile of the Peruaçu open areas,which has a wide distribution of larger shrub and arboreal species.Stryphnodendron adstringens, Tachigali subvelutina and Senna cana trees are characteristic species of cerrado areas that had high importance values in the open areas of vereda Peruaçu.In the vereda Almescla,despite the species being more characteristic in open areas in the veredas,such as Melastomataceae and Malpighiaceae (Byrsonima pachyphylla and Macairea radula),
Curatella americana has already been expanding its individuals in open areas.This reflects the warning for conservation strategies.Regarding the hygrophilous forest areas, despite the specific characteristics and the greater abundance of tree species compared to the open areas,the results found here show divergence in relation to the composition of tree species between the two veredas. This is reinforced by the more advanced drought in the Peruaçu vereda,indicating possible future changes in this forest formation. Thus, an expansion of species typical of less saturated environments or a tendency towards a monodominance of species more resistant to changes in the level of the water table(vereda drought)will also occur in these areas of hygrophilious forest (Orellana et al., 2012;Rossatto et al.,2012;Villalobos-Vega et al.,2014;Oliveira et al.,2017).The presence of species common in drier areas such as Astronium fraxinifolium Schott,Senegalia langsdorffii(Benth.)Seigler&Ebinger,Guarea kunthiana A. Juss. and Guarea macrophylla Vahl already indicates the effect of drying on these forest areas.
The Brazilian Cerrado includes forest, savanna, and grassland formations, so that both forest and savanna communities experience the same climate regime (Ribeiro and Walter, 2008). Soil moisture and fertility levels,as well as geographic proximity,are also important factors contributing to species distributions in those environments(Silva Júnior et al., 2001). Swamp forests, as well as palm swamps, are humid formations within the Cerrado biome, with different surrounding plant communities that will influence their species compositions(Teixeira and Assis,2011;Menino et al.,2012; 'Avila et al.,2016).Thus,in relation the edaphic factors, like the flood, the greater availability of nutrients is generally related to lower species richness and a higher number of individuals (Huston, 1980; Enright et al., 1994; Slik et al., 2010; 'Avila et al., 2016). A higher nutrient availability allows faster growth with more biomass accumulation and more individuals, which can trigger exclusive competition leading to reduced species richness(Enright et al.,1994). Despite the negative relationship between species richness and soil fertility in large part of the studies, soil fertility can positively or negatively affect richness and dominance of species(Dupr'e et al.,2002).This may explain the fact that open areas have a higher incidence of certain species of Melastomataceae and Malpighiaceae,for example.
According to the Brazilian environmental legislation, veredas forests are situated in“Areas of Permanent Preservation”and their suppression could only occur in special cases (Kurtz et al., 2013, 2015). Unfortunately,this has not always been enforced,which led to the destruction of a large portion of these forests in Brazil.Swamp forests are a very fragile vegetation (Scarano et al., 1998; Kurtz et al., 2013, 2014), and particularly sensitive to changes in the flooding regime. When these changes occur due to human activity and become permanent,in addition to rapid degradation,the forests show no natural recovery(Scarano et al.,1998; Kurtz et al., 2014). However, external factors are responsible for changes in these formations.With the effect of climate change that causes drying years (with low rainfall), more warmer periods (Oliveira et al.,2017;Hofmann et al.,2021),the water balance is affected by changes in hydrological processes and leading to drought of these areas in the Cerrado,this process being the main responsible for the modification of these formations.In addition to these changes,it is evident that land use and land cover change in different physiognomies of the undisturbed closed has the potential to modify the groundwater recharge dynamics.These impacts on recharge will depend on the extent of the change in closed physiognomies or cropland expansion areas. Thus, the patterns and aspects discussed above are important to guide the future efforts of conservation and management of veredas remnants in and the restoration of disturbed areas. Furthermore, these results make clear the change in the structure of the vegetation in these veredas.
5. Conclusions
Thus, assuming that veredas are changed in species composition possibly due to local anthropogenic factors which leads to the drought of these formations(water balance effect),we demonstrated that the studied veredas were floristically different in terms of the arboreous-shrubby species composition, richness, and diversity. These differences are mainly due to local scale effects that cause drier and warmer periods and to the expansion of the drought in these formations.These anthropogenic effects cause a vegetation modification with the expansion and increase in the abundance of species typical of other areas of Cerrado and resistant to less saturated soils, leading to the collapse of the veredas. Finally,future studies should investigate other veredas on a broader scale to detect changes in the water table that lead to a consequent change in vegetation structure. In this way, we will have subsidies for the conscientious management and conservation of these areas that represent equilibrium places for the Cerrado biome.
Funding
This research was supported by the Long-term Ecological Research Network (PELD-VERE) of the Conselho Nacional de Desenvolvimento Científico e Tecnol'ogico (CNPq 441440/2016-9; 441583/2020-2;308877/2019-5), Coordenaç~ao de Aperfeiçoamento de Pessoal de Nível Superior (CAPES 88887.136273/2017–00), and Fundaç~ao de Amparo`a Pesquisa do Estado de Minas Gerais (FAPEMIG APQ-04816-17; CRAPPM-00539-18; APQ-04816-17). We also thanks FAPEMIG (FAPEMIG/RED-00253-16) for the support and for the second author scholarship.
Competing interests
The authors declare no competing financial interests.
Author contributions
YRFN, IFPA and WVN conducted the conception and design of the work. YRFN, IFPA, OSO, LAF, RSF and WVN conducted data collection.RSF and RMS proceeded identification of the species. CSS conducted statistical analysis and draft of the work. All the authors read and approved the final manuscript.
Availability of data and materials
The authors confirm that if the manuscript be accepted, the data supporting the results will be archived in an appropriate public repository and the data DOI will be included at the end of the article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Yule Roberta Ferreira Nunes reports financial support and administrative support were provided by Montes Claros State University. Yule Roberta Ferreira Nunes reports financial support, equipment, drugs, or supplies,and travel were provided by National Council for Scientific and Technological Development. Yule Roberta Ferreira Nunes reports financial support,equipment,drugs,or supplies,and travel were provided by Minas Gerais State Foundation of Support to the Research.Yule Roberta Ferreira Nunes reports financial support, equipment, drugs, or supplies,and travel were provided by Coordination of Higher Education Personnel Improvement. Yule Roberta Ferreira Nunes reports financial support,equipment, drugs, or supplies, and travel were provided by Brazil Ministry of Science Technology and Innovation.
Acknowledgements
Postgraduate Program in Applied Botany,to the field assistance of the Plant Ecology Laboratory(Universidade Estadual de Montes Claros)and logistical support from the Instituto Estadual de Florestas, in the Minas Gerais State. We also thank Daniel Alcantara for the help with the map construction.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100036.
- Forest Ecosystems的其它文章
- Black locust coppice stands homogenize soil diazotrophic communities by reducing soil net nitrogen mineralization
- Responses of soil CH4 fluxes to nitrogen addition in two tropical montane rainforests in southern China
- Importance of Quercus spp. for diversity and biomass of vascular epiphytes in a managed pine-oak forest in Southern Mexico
- Novel evidence from Taxus fuana forests for niche-neutral process assembling community
- Using machine learning algorithms to estimate stand volume growth of Larix and Quercus forests based on national-scale Forest Inventory data in China
- Effects of fertilization on the growth dominance of Inland Northwest forests of the United States

