Novel evidence from Taxus fuana forests for niche-neutral process assembling community
Tinxing Li, Li Xu, Feng Wng, Weijun Zhng, Junpeng Dun, Xiolu Shen-Tu,Yoin Song,*, Runguo Zng, Ming Dong,**
a Key Laboratory of Hangzhou City for Ecosystem Protection and Restoration, College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou,311121, China
b Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry, Beijing, 100091, China
Keywords:Community assembly Environmental factors Limiting similarity Niche-neutral process Phylogenetic structure Randomness Rare and endangered species Taxus fuana
ABSTRACT
1. Introduction
Understanding the mechanisms underlying community assembly are among the core tasks of plant community ecology (Kraft et al., 2007;HilleRisLambers et al., 2012). This contributes to the understanding of community diversity and internal mechanisms of species coexistence;as well as the protection and restoration of biodiversity, which is of great significance for the establishment of management practices and formulation of conservation strategies (Chase, 2003;Myers and Harms,2009;Wiens et al., 2010; Lawley et al., 2013; Kraft et al., 2015; Tucker et al.,2017).Community assembly is commonly understood based on the niche theory and/or neutral theory (Tilman, 2004; Weiher et al., 2011). According to niche theory,community assembly is a deterministic process(Fenton and Bergeron, 2013; Wang et al., 2016; Zhou et al., 2021) in which species with similar niches are selected from habitats of the same type (Gianuca et al., 2014; dos Santos et al., 2014; Li et al., 2018).However, species with similar niches may not be allowed to coexist because of their mutual exclusion resulting from competition for resources of similar kinds(Takahashi and Tanaka,2016;Bracewell et al.,2017). Therefore, habitat filtering and limiting similarity are the main driving forces of community assembly under the framework of niche theory (Webb et al., 2002; Cornwell et al., 2006; Myers and Harms,2009).In contrast,the neutral theory holds that community assembly is a random process (Houlahan et al., 2007; Chase, 2010; Rosindell et al.,2012; Fenton and Bergeron, 2013) in which species randomly enter the community (Volkov et al., 2003). Accordingly, individuals in the community have the same immigration rate, emigration rate, and mortality rate, and niche theory has no influence on them (Hubbell, 2001).Correspondingly, according to the framework of the neutral theory,random effect and dispersion limitation are the major driving forces of community assembly (Hubbell, 2005; Kraft et al., 2008; Munoz and Huneman,2016;Ulrich et al.,2016).
Recently,niche theory and neutral theory have been found to not be completely mutually exclusive,but can be integrated and verified interactively (Mutshinda and O'Hara, 2011; Weiher et al., 2011; Fisher and Mehta,2014).Both describe the intrinsic features of the community from different perspectives. That is, neutral theory tends to the individual level, whereas niche theory focuses on the community level (Volkov et al., 2003; Tilman, 2004; Stokes and Archer, 2010; Fisher and Mehta,2014). It has been demonstrated that both niche and neutral processes work in the process of community assembly in many communities (Hu et al., 2016; Marteinsd'ottir et al., 2018; Liu et al., 2019; Zhou et al.,2021), and their relative importance might vary with the environment,research scale, and size of species pools (Cavender-Bares et al., 2009;Shipley et al., 2012; Wang et al., 2016; Conradi et al., 2017). For example, Pashirzad et al. (2018) found that the assemblages of plant communities in mountainous rangelands were influenced by both niche and neutral processes,and the relative importance of these two processes caused different distributions of herbaceous and shrub species assemblages on different environmental scales.Nevertheless,it is academically attractive but still unclear how the two processes are relatively important in shaping community assembly (Punchi-Manage et al., 2014; Vellend et al.,2014; Angulo et al.,2018).
Traditionally,community assembly mechanisms are based on species diversity(Bruelheide et al.,2011;Myers et al.,2013).Webb et al.(2002)combined species and traits to judge the process of community assembly by community phylogeny, and revealed the mechanisms of community assembly from the perspective of evolution. Kraft et al. (2007) amalgamated these findings with a neutral random process to further improve the research method of community assembly (Table 1). This indicates that the community assembly process can be inferred from the performance of community phylogeny (clustered, overdispersed, or random dispersion)and functional traits(conserved or convergent).That is,if the functional traits are conserved and the phylogeny is clustered, it can be deduced that the process of community assembly is mainly affected by habitat filtering in the niche process,whereas overdispersed and random dispersion of functional traits indicated limiting similarity and neutral assembly,respectively(Table 1).Current studies on community assembly mechanisms are primarily based on a combination of phylogeny and functional traits, resulting in relevant reports for various regions (e.g.,alpines, grasslands, temperate, and tropical forests) (Ding et al., 2012;Yan et al., 2013; Diniz et al., 2021; Huxley and Spasojevic, 2021; Xu et al., 2021). For example, Gastauer et al. (2017) showed that competition, density dependence, and environmental filtering were the driving forces behind the construction of the Brazilian Atlantic Forest. In addition, Liu et al. (2019) found that slope aspect was an important influencing factor in the assembly process of subalpine meadow communities,and its main driving forces varied with slope aspect. Zhou et al. (2021)verified that the combined effect of deterministic and stochastic factors was the dominant driving force for the community assembly of temperate forests. To date,many case studies have been conducted on community assembly (Chase, 2003; Weiher et al., 2011; G¨otzenberger et al., 2012;Pearson et al.,2018;Pontarp et al.,2019).However,little is known about community assemblies involving rare and endangered plants (Liu et al.,2020,2021).

Table 1Relationship between dominant driving force of community assembly, community phylogeny and functional traits(after Webb et al.,2002;Kraft et al.,2007).
Taxus fuana, syn. Taxus contorta grows in the west and southwest of the Himalayas from 2,500 to 3,400 m above sea level, specifically distributed from west to eastern Afghanistan and east to southwestern Tibet in China,northern India,and Nepal(Poudel et al.,2012;Song et al.,2020).T.fuana is a national key protected wild plant species(Wang et al.,2019). Moreover, it has been listed as an endangered species by IUCN(Poudel et al.,2014).The ecological amplitude of T.fuana is narrow,and the populations have a small number of individuals scattered in space;this species has been listed as one of the 120 plant species with extremely small populations(PSESP)in China(Zang et al.,2016;Song et al.,2020;Yang et al., 2020), and targeted for high priority conservation based on the Implementation Plan of Rescuing and Conserving China's PSESP(2011–2015) (Zang et al., 2016; Yang et al., 2020). Owing to its high medicinal and timber-use value, it is seriously affected by human activities and is becoming endangered (Shah et al., 2008a). Hence, it is necessary to comprehensively understand T. fuana and the ecosystem involved, thereby improving effective protection and management. To date, T. fuana has been studied in terms of its genetic patterns (Shah et al.,2008a,2008b;Poudel et al.,2014),population ecology(Song et al.,2020; Zhang et al., 2020), and community structure (Dan et al., 2020),respectively. However, little is known about the community assembly mechanisms of T.fuana(Poudel et al.,2012;Fatima et al.,2016;Yu et al.,2018), particularly in southwest Tibet (Li et al., 2014; Liu et al., 2019;Xiao et al., 2020; Cao et al., 2021). In addition, because of the special geographical location,and complex and changeable local environment of the Qinghai-Tibet Plateau, the community structure may change with differences in soil nutrients and topography (e.g., slope aspect, slope position, etc.) (Wang et al., 2012; Wu et al., 2012; Liu et al., 2019).Therefore,it is of great significance to study the community structure of T.fuana forests and their relationships with environmental factors.In this study, plant communities of T. fuana forests in Gyirong, Tibet, were investigated to answer the following questions: (1) Are both niche and neutral processes present in the community assembly of T.fuana forests?If so,which is the dominant driver?(2)What are the main environmental factors that affect the phylogenetic structure of the T. fuana forest community?
2. Materials and methods
2.1. Study site
The study site was located in the forest region of Gyirong Town(28°21′-28°29′N, 85°13′-85°21′E, 2,600–3,200 m above sea level).Because of its special geographical location in the Himalayas, the warm and humid air from the Indian Ocean is concentrated in this region (Qi et al.,2013;Zhang et al.,2020;Adhikari and Mejia,2021),which has a subtropical mountain monsoon climate,and the dominant wind direction is southeast. The mean annual temperature is 8–11°C, average annual precipitation is 800–1,000 mm, and average annual humidity exceeds 60%. The soil types include yellow-brown soil, brown soil, and dark brown soil(Song et al.,2020; Zhang et al.,2020).
2.2. Plant community survey
Plant community surveys were conducted from July to August 2018 and July to August 2019.According to our previous survey(Song et al.,2020),a total of 34 quadrants were set up to cover six known populations(Jilong,Jipu,Langjiu,Duofu,Kaire and Tangbo)of T.fuana(Fig.1).The tree, shrub, and herb layers of each quadrant were simultaneously investigated. The tree and shrub layers were divided according to the diameter at breast height (DBH) of woody plants. Woody plants with DBH ≥3 cm were considered tree layers, while those with DBH <3 cm were considered shrub layers.There was one tree-layer quadrant with an area of 20 m×20 m in each quadrant.The DBH and height of all woody plants with a DBH ≥3 cm were recorded. Two quadrants in the shrub layer with an area of 10 m×10 m were set and arranged on the diagonal of each quadrant.The height and number of woody plants with DBH <3 cm were recorded.Five quadrants in the herb layer with an area of 1 m×1 m were set and distributed in the corners and center of each quadrant.The average height,coverage,and abundance of herbaceous plants have been previously recorded (Condit, 1998; Kibet, 2011; Lopatin et al.,2016). At the same time, the latitude and longitude and topographic factors including altitude (AL), slope (SLOP), slope aspect (ASPE), and slope position(POSI)of each quadrant were recorded.Surface soil(0–30 cm) in the quadrant was sampled, air-dried, ground, and screened according to the requirements of the determination indices.The soil water content(SWC)of all air-dried samples was determined by the gravimetric method, and the pH was obtained by measuring the soil water leachate using a portable instrument (HQ40d18; Hach, China). Available phosphorus (AP, mg⋅kg-1) and available potassium (AK, mg⋅kg-1) were determined using a universal extract-colorimetric method. Soil organic carbon(SOC,mg⋅kg-1)was determined using the potassium dichromate oxidation spectrophotometric method, and available nitrogen (AN,mg⋅kg-1) was determined using the alkali diffusion method. Total phosphorus (TP, mg⋅kg-1), total potassium (TK, mg⋅kg-1), and total magnesium (TMg, mg⋅kg-1) were determined by acid digestion using inductively coupled plasma mass spectrometry (NexION 350X; PerkinElmer, USA). Total nitrogen (TN, mg⋅kg-1) and total carbon (TC,mg⋅kg-1) were determined using an elemental analyzer (Vario PYRO cube; Elemental,Germany)(Bao,2005;Sainepo et al.,2018).
2.3. Data analysis
The phylogenetic tree of the T.fuana forest community in the Gyirong area of Tibet was constructed using the online program PHYLOMATIC,and branch length estimates were included using the program PHYLOCOM version 4.2(Fig.S1-S4)(Webb et al.,2008).Net relatedness index(NRI)was calculated based on the phylogenetic tree of the community to measure the phylogenetic structure. The NRI is the standardized phylogenetic mean pairwise distance(MPD),which measures the phylogenetic structure of the community from the overall phylogenetic tree (Webb et al., 2008). Because the standard of species diversity measurement between different layers was not uniform, multi-degree weighting was not performed in the calculation process. The NRI was calculated according to Webb et al.(2008)as follows:
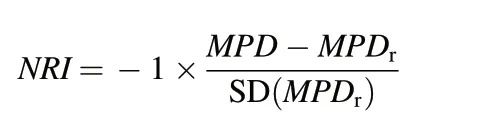
where MPD was the observed value of phylogenetic mean pairwise distance of species based on the phylogenetic tree, MPDrwas the mean phylogenetic pairwise distance randomly simulated by the null model,and the random value was automatically generated 999 times by software simulation.The SD represents the standard deviation.The positive and negative NRI values were used to determine the phylogenetic structure of the community. A positive NRI value indicated a clustered phylogenetic structure, whereas a negative NRI value indicated an overdispersed phylogenetic structure.When NRI was 0,the phylogenetic structure was completely random.When the NRI was greater than 1.96 or less than -1.96, it indicated that the phylogenetic structure deviated significantly from the null model(Kraft et al.,2007;Shivaprakash et al.,2018;Ding et al.,2019;Zhou et al.,2021).In this study we assumed that these functional traits were conserved, and under this assumption the mechanisms of community assembly were judged from the phylogenetic structure (Chazdon et al., 2003; Wiens and Graham,2005; Wiens et al.,2010).Therefore,the functional traits were not included in the analysis.If the phylogenetic structure was clustered, the community assembly process would be principally affected by habitat filtering. If the phylogenetic structure was overdispersed, it was dominated by limiting similarity. If the phylogenetic structure is random, it is influenced by a neutral effect(Webb et al.,2002;Kraft et al.,2007).NRI was calculated using the ses.mpd function of the picante package in R 3.6.2,and the null model“richness”was used(Kembel et al., 2010;Borcard et al.,2011;R Core Team,2019).

Fig. 1. Geographic distribution of study sites.
NRI values at the community, tree, shrub, and herb layers were calculated. To analyze the relationship between phylogeny and environmental factors, a multi-stepwise regression analysis was performed for the NRI and environmental factors. Moreover, residual analysis was carried out,and the Durbin-Watson test values were close to 2(Table S1);the residual plots did not show other special distributions(Fig.S5)(Leech et al., 2011). Then, the significant environmental factors in the multi-stepwise regression analysis were separately correlated with the corresponding NRI to infer the trend of the NRI with environmental factors. To meet the requirements of the calculation process, log10transformation was performed on environmental data (Borcard et al.,2011).In addition,a collinearity diagnosis of environmental factors was also done,which showed that the variance inflation factor(VIF)values of all environmental factors were less than 10,indicating that there was no multicollinearity among the environmental factors in this study (Jou et al., 2014). Multi-stepwise regression analysis of the NRI with environmental factors and isolated correlation analyses between the NRI and environmental factors that arrive at significance were conducted using SPSS 20.0(Leech et al.,2011).
3. Results
3.1. Community composition of T. fuana forests
A total of 235 vascular plant species belonging to 165 genera and 62 families were recorded in the 34 quadrants (Table S2). These included one pteridophyte species from one genus and family, six gymnosperm species from six genera and three families, and 228 angiosperm species from 158 genera and 58 families.Pteridophytes, gymnosperms, and angiosperms accounted for 0.43%,2.55%, and 97.02%of the total plants,respectively.Among them,26 Asteraceae species in 19 genera accounted for the largest proportion, accounting for 11.06% of the total plants,followed by Rosaceae with 13 genera and 23 species, accounting for 9.79%. The dominant species in the community were Pinus wallichiana,Quercus semecarpifolia, Thamnocalamus spathiflorus var. crassinodus, and Thalictrum reniforme. In addition, the tree layer included 56 species of plants, including P. wallichiana, Q. semecarpifolia, and T. fuana. There were 81 species of plants in the shrub layer, which contained seedlings and young trees of some tree layer species,including T. spathiflorus var.crassinodus,Desmodium sequax,and Rhamnus dumetorum.The herb layer included 141 species of plants,including T.reniforme,Anemone rivularis,and Rosa davurica.
3.2. Phylogenetic structure of T. fuana forests

Fig. 2. Difference of net relatedness index among different layers of T. fuana forest community (mean ± SE). Different letters showed significant differences among different layers (P <0.05).
The mean NRI values at the community level, tree, shrub, and herb layers were all less than 0, whereas NRI was significant only at the community level (<-1.96) (Fig. 2), which indicated that the phylogenetic structure at the community level was overdispersed, whereas the trend of overdispersed in each layer was weak.Meanwhile,the mean NRI values at the community level and each layer were significantly different as follows:community <tree layer <herb layer <shrub layer.Out of 34,17 quadrants at the community level had an NRI less than-1.96;while the number of quadrants less than -1.96 in the tree, shrub, and herb layer were 8,2 and 1,respectively.The NRI of the remaining quadrants was distributed within the range of -1.96 to 1.96 (Fig. 3). This means that the phylogenetic structure of the community was mainly influenced by the deterministic process, and the random process also played an important role(especially between separate layers).
3.3. Relationship between phylogenetic structure and environmental factors of T. fuana forests
Multi-stepwise regression analysis showed that environmental factors had significant effects on the phylogenetic structure of the T.fuana forest community (Table 2). It accounted for 44.38%, 46.52%, 24.04%, and 14.07%of the variation in phylogenetic structure at the community level,tree, shrub, and herb layers, respectively. The relationships between phylogenetic structure and the following environmental factors were significant at the community level: total potassium (TK), slope aspect(ASPE), available potassium (AK), and available phosphorus (AP). In addition, significant environmental factors in the tree layer were AK,ASPE, total phosphorus (TP), and slope position (POSI). Only one environmental factor was significant in both the shrub and herb layers, that is,altitude(AL)was significant in the shrub layer,and AP was significant in the herb layer (Table 2). In addition, environmental factors that reached significance in the multi-stepwise regression analysis were further analyzed in separate correlations with the corresponding NRI.The results showed that the NRI at the community level was significantly correlated with TK (r = 0.469, P = 0.005) (Fig. 4a) and ASPE (r =-0.384, P = 0.025) (Fig. 4b). However, there were no significant correlations between NRI and AK(r=0.337,P=0.051)(Fig.4c)or AP(r=-0.004,P =0.983) (Fig. 4d). The NRI of the tree layer was only significantly correlated with AK (r = 0.421, P = 0.013) (Fig. 4e) but was not significantly correlated with ASPE(r=-0.288,P=0.099)(Fig.4f),TP(r = -0.028, P = 0.877) (Fig. 4g), and POSI (r = -0.245, P = 0.163)(Fig. 4h). This indicated that the phylogenetic structure at the community level and tree layer was affected by multiple environmental factors.The NRI of the shrub layer was significantly associated with AL (r =-0.513, P = 0.002) (Fig. 4i), and showed lower altitudes greater than 0 and higher altitudes less than 0. This indicated that the phylogenetic structure tended to change from clustering to overdispersed with the increase in altitude,although majority of the NRIs of the shrub layer did not deviate from the range of the random threshold (-1.96 <NRI <1.96).The NRI of the herb layer was significantly associated with AP(r=0.408,P=0.017)(Fig.4j).
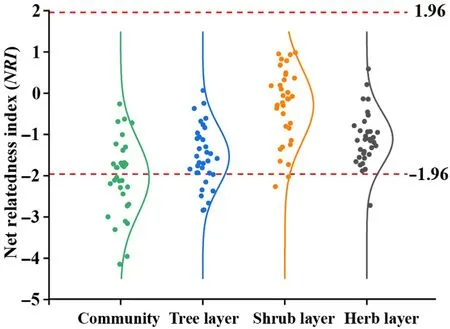
Fig.3. Distribution of net relatedness index(NRI)of T.fuana forest community.The curve was normal distribution curve. The NRI was significantly different to null model when it was greater than 1.96 or less than -1.96.
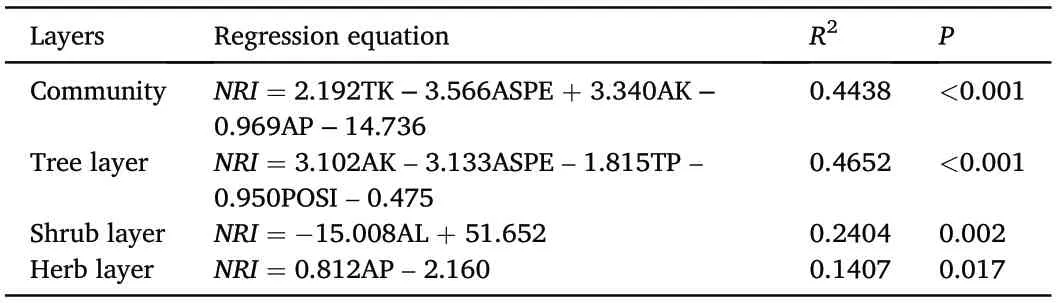
Table 2 Multi-stepwise regression analysis of net relatedness index (NRI) and environmental factors of T. fuana forest community.
4. Discussion
4.1. Community assembly process and driving forces of T. fuana forest community
Based on analysis of the phylogenetic structure, we can infer the assembly mechanisms of the community (Cavender-Bares et al., 2009;Pastore and Scherer, 2016). In this study, half of the NRI at the community level exceeded the range of the random threshold(-1.96 <NRI<1.96).However,most of the NRI in each layer did not exceed a random threshold. The tree layer accounted for a small part, whereas the shrub and herb layers were only individuals. Thus, it can be inferred that the community assembly process is affected by both deterministic and stochastic processes. Among the different community layers, the neutral process drove the community assembly of the shrub and herb layers,whereas the limiting similarity combined with the neutral process dominated the community assembly at the community level and tree layer.Some studies have suggested that the size of the species pool has an impact on community assembly (Fukami, 2004; Martínez-Villa et al.,2020;Catano et al.,2021),which,in turn,is reflected on a spatial scale(Swenson et al.,2007;Vamosi et al.,2009;Willis et al.,2010;Fisher and Mehta,2014;Münkemüller et al.,2014;Pashirzad et al.,2018).Species interactions and/or randomness may be more prominent on a small scale,whereas the regional process is more obvious on a large scale(Swenson et al., 2006; Horn et al., 2015; Leibold and Chase, 2017). In this study,species pool selection was different when a phylogenetic tree was constructed.The species pool at the community level included all species in the community.Accordingly,the species pool of the tree layer contained only the species that appeared in the tree layer. The selection of the species pool in the shrub and herb layers was similar to that of the tree layer.This study supports this view from the perspective of species pool size. Other studies have also shown that there is a correlation between the taxonomic level and phylogenetic structure (Cavender-Bares et al.,2004,2006;Vamosi et al.,2009).From a comparative analysis between the community level and different layers,our results support this view to a certain extent.
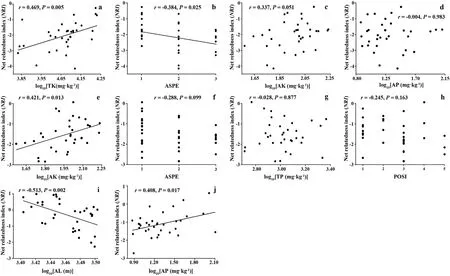
Fig.4. Relationship between net relatedness index(NRI)value at community level and total potassium(TK),slope aspect(ASPE),available potassium(AK),available phosphorus(AP)(A–D),relationship between NRI of tree layer and AK,ASPE,total phosphorus(TP),POSI(slope position)(E–H),relationship between NRI of shrub layer and altitude(AL)(I),and relationship between NRI of herb layer and AP(J).1 to 3 of ASPE represent half sunny slope,half shady slope,shady slope,respectively.1 to 5 of POSI represent lower slope, middle and lower slope, middle slope, middle and upper slope, upper slope, respectively. Regression lines were plotted for significant relationships with P <0.05.
The NRI distribution varied among the layers,although most NRI did not exceed the range of the random threshold(-1.96 <NRI <1.96).The NRI of the tree and herb layers was mainly distributed in the-1 to-1.5 range, showing a tendency for overdispersed in the phylogenetic structure.In addition,the NRI of the shrub layer was nearly half positive and half negative, indicating that there were two trends in its phylogenetic structure: overdispersed and clustering. This means that, although the individual layer was mainly affected by the random process,the relative importance of the deterministic process for community assembly in each layer was different (Kembel and Hubbell, 2006). Habitat filtering and limiting similarity are believed to be the two basic driving forces of community assembly processes, based on niche theory (Webb et al.,2002; Kraft et al., 2007; Pashirzad et al., 2018). According to the hypothesis in this study that traits were conserved, the phylogenetic structure of the tree and herb layers tended to be overdispersed due to limited similarity (competitive exclusion).Habitat filtering and limiting similarity had different relative importance in the shrub layer; thus, its phylogenetic structure showed a trend of overdispersed and clustering.This may be related to the characteristics of the local community,that is,the tree layer was predominant in the community in most areas and in the uppermost layer of the community. The competitive exclusion(limiting similarity) effect was greater than that in the other layers. Thus, the phylogenetic structure of the tree layer shows the trend of overdispersed(Zhang et al.,2020).As mentioned above,because of the predominance of the tree layer in the community and its position in the community,it may act as an environmental filter(especially in areas with large canopy density)to cluster the phylogenetic structure of the shrub layer.In other areas(such as high altitudes),the shrub layer grew fully with the decline of the tree layer advantage,and the interspecific competition intensified,so the phylogenetic structure showed an overdispersed trend.Similar to the results in this study,the species richness of the herb layer was higher than that of other layers, which easily led to competition for the same resource (Donoghue, 2008; Vockenhuber et al., 2011). Therefore, the phylogenetic structure of the herb layer tends to be overdispersed.
4.2. Effects of environmental factors on phylogenetic structure of T. fuana forest
The process of community assembly may vary owing to the heterogeneity of habitat conditions (Freestone and Inouye, 2006; Honorio Coronado et al.,2015;L'opez-Angulo et al.,2018;Asefa et al.,2019;Yue and Li, 2021). The multi-stepwise regression analysis in this study showed that the NRI value at the community level was significantly affected by TK, ASPE, AK, and AP; however, the individual correlation analysis showed that only TK and ASPE reached a significant level. In addition,the NRI of the tree layer was significantly affected by AK,ASPE,TP,and POSI,but only AK reached a significance level in the individual correlation analysis.Therefore,it can be speculated that the phylogenetic structure at the community level and tree layer was affected by multiple environmental factors rather than a single environmental factor. Owing to tree layer species having a greater body size and longer growth cycles than shrub and herb layer ones, the phylogenetic structure of the tree layer may not react very strongly to a single environmental factor in small regions (Odum, 1983). The community level was a comprehensive one containing or integrating tree,shrub,and herb layers.Thus,phylogenetic structure at the community level may be more responsive to the synergy of multiple environmental factors. It may also be that the single environmental change in this study was not sufficient to cause different responses at the tree layer species and community level(Zhou et al.,2021).The NRI of the shrub layer was significantly affected only by the altitude.However, altitude explained 24.04% of the variation in the NRI, which meant that altitude was an important environmental factor affecting the phylogenetic structure of shrub layer among the selected environmental factors. Furthermore, the phylogenetic structure of the shrub layer showed a trend of clustering and overdispersed at lower and higher altitudes, respectively. The effect of altitude on community assembly has been demonstrated previously (Cornwell and Ackerly, 2009; Xu et al.,2017; Zhou et al., 2021). Altitude is an environmental factor of great relevance (K¨orner, 2007). Other environmental factors may differ with altitude changes,leading to diverse species distributions(Gaston,2000;Nottingham et al., 2015). According to the field survey, at lower altitudes,the tree layer has a major advantage in the community,and with the characteristics of high canopy density and strong ability to intercept light,the growth of shrub layer species may be restricted.Consequently,pressure from the tree layer as an environmental filter caused the phylogenetic structure of the shrub layer to cluster. With an increase in altitude, the superiority of the tree layer and the competition for resources(e.g.,light and space)weaken the growth of species in the shrub layer,and interspecific competition intensifies.Therefore,it is likely that the phylogenetic structure tends to be overdispersed. Xiao et al. (2020)found that the phylogenetic structure of the alpine shrub community in the Qinghai-Tibet Plateau was more clustered.A possible explanation is that the main sources of the impact were different.The growth pressure of the alpine shrub community largely came from the harsh environment,as only adapted species could survive, so the overall phylogenetic structure was clustered. However, in this study, environmental and interspecific interactions(especially the latter)were important factors in the shrub layer.This presumably led to differences in the trends.The NRI of the herb layer was only significantly affected by AP, with an explanation of 14.07%, suggesting that most of the variation was affected by other environmental factors (rainfall, temperature, and light) that were not included in this study. This could depend on the characteristics of herb plants, such as small individuals, short life history cycles, and greater species richness (Odum, 1983; Donoghue,2008; Gibson, 2009).Therefore,competitive exclusion between species was relatively obvious,which may have led to the trend of overdispersed in the phylogenetic structure(García et al.,2008;Hawkins et al.,2011).
5. Conclusions
In this study, the phylogenetic structure of the T. fuana forest community and its relationship with environmental factors were analyzed,and it was found that the community assembly was affected by both niche and neutral processes. The niche process was more significant at the community level,whereas the neutral process was more significant in the three different layers.In other words,limiting similarity and neutral processes were the main assembly mechanisms at the community and tree layers,and the neutral process was the dominant force in the shrub and herb layers. Environmental factors had a significant influence on community assembly.The effects of multiple environmental factors at the community level and tree layer were more obvious than those of a single environmental factor. The influence of altitude on the shrub layer was greater than that of other environmental factors. The herb layer may have been affected by environmental factors other than those in this study. The process of community assembly in T. fuana forests was discussed, which is of great significance for understanding species coexistence and the maintenance of community diversity.
Ethics approval and consent to participate
Not applicable.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2016YFC0503100); and the National Natural Science Foundation of China (Grant Nos. 31670429 and 31400346).
Authors’contributions
Y.S., R.Z. and M.D. contributed to the conception of the study; T.L.,L.X., F.W., W.Z., J.D., X.S.-T. performed the experiment and collected data;T.L.analyzed the data and wrote the manuscript.All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.The data are not publicly available due to privacy restriction.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank all the students from Hangzhou Normal University for their kindly helps in field. We also thank the anonymous reviewers for thoughtful and valuable comments.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100035.
ReferencesAdhikari,P.,Mejia,J.F.,2021.Influence of aerosols on clouds,precipitation and freezing level height over the foothills of the Himalayas during the Indian summer monsoon.Clim. Dynam. 57, 395–413. https://doi.org/10.1007/s00382-021-05710-2.
Angulo, D.F., Tun-Garrido, J., Arceo-G'omez, G., Munguía-Rosas, M.A., Parra-Tabla, V.,2018.Patterns of phylogenetic community structure of sand dune plant communities in the Yucatan Peninsula: The role of deterministic and stochastic processes in community assembly. Plant Ecol. Divers. 11, 515–526. https://doi.org/10.1080/17550874.2018.1534289.
Asefa, M., Brown, C., Cao, M., Zhang, G.C., Ci, X.Q., Sha, L.Q., Li, J., Lin, L.X., Yang, J.,2019. Contrasting effects of space and environment on functional and phylogenetic dissimilarity in a tropical forest.J.Plant Ecol.12,314–326.https://doi.org/10.1093/jpe/rty026.
Bao, S.D., 2005. Soil Agricultural Chemistry Analysis. China Agriculture Press, Beijing.
Borcard,D.,Gillet,F.,Legendre,P.,2011.Numerical Ecology with R.Springer,New York.
Bracewell,S.A.,Johnston,E.L.,Clark,G.F.,2017.Latitudinal variation in the competitioncolonisation trade-off reveals rate-mediated mechanisms of coexistence. Ecol. Lett.20, 947–957. https://doi.org/10.1111/ele.12791.
Bruelheide, H., B¨ohnke, M., Both, S., Fang, T., Assmann, T., Baruffol, M., Bauhus, J.,Buscot, F., Chen, X.Y., Ding, B.Y., Durka, W., Erfmeier, A., Fischer, M., Geissler, C.,Guo, D.L., H¨aerdtle, W., He, J.S., Hector, A., Kr¨ober, W., Schmid, B., 2011.Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 81, 25–41. https://doi.org/10.1890/09-2172.1.
Cao,J.H.,Qi,R.,Liu,T.,Li,B.,Gao,B.Q.,Chen,X.L.,Zhao,Y.,Zhao,Z.G.,2021.Patterns of species and phylogenetic diversity in Picea purpurea forests under different levels of disturbance on the northeastern Qinghai-Tibetan Plateau. Global. Ecol. Conserv. 30,e1779. https://doi.org/10.1016/j.gecco.2021.e01779.
Catano,C.P.,Grman,E.,Behrens,E.,Brudvig,L.A.,2021.Species pool size alters speciesarea relationships during experimental community assembly. Ecology 102, e3231.https://doi.org/10.1002/ecy.3231.
Cavender-Bares, J., Ackerly, D.D., Baum, D.A., Bazzaz, F.A., 2004. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 163, 823–843. https://doi.org/10.1086/386375.
Cavender-Bares, J., Keen, A., Miles, B., 2006. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology 87, S109–S122.https://doi.org/10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2.
Cavender-Bares, J., Kozak, K.H., Fine, P.V.A., Kembel, S.W., 2009. The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715. https://doi.org/10.1111/j.1461-0248.2009.01314.x.
Chase, J.M., 2003. Community assembly: When should history matter? Oecologia 136,489–498. https://doi.org/10.1007/s00442-003-1311-7.
Chase, J.M., 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391. https://doi.org/10.1126/science.1084783.
Chazdon, R.L., Careaga, S., Webb, C., Vargas, O., 2003. Community and phylogenetic structure of reproductive traits of woody species in wet tropical forests.Ecol.Monogr.73, 331–348. https://doi.org/10.1890/02-4037.
Condit,R.,1998.Tropical Forest Census Plots:Methods and Results from Barro Colorado Island,Panama and a Comparison with Other Plots.Springer,Berlin.https://doi.org/10.1007/978-3-662-03664-8.
Conradi, T., Temperton, V.M., Kollmann, J., 2017. Resource availability determines the importance of niche-based versus stochastic community assembly in grasslands.Oikos 126, 1134–1141. https://doi.org/10.1111/oik.03969.
Cornwell, W.K., Ackerly, D.D., 2009. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr.79, 109–126. https://doi.org/10.1890/07-1134.1.
We are all born with wide-eyed, enthusiastic wonder as anyone knows who has ever seen an infant s delight at the jingle of keys or the scurrying of a beetle.
Cornwell,W.K.,Schwilk,L.D.,Ackerly,D.D.,2006.A trait-based test for habitat filtering:Convex hull volume. Ecology 87, 1465–1471. https://doi.org/10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2.
Dan, Z., Li, B.Z., Yin, Z.J., 2020. Analysis of the characteristics and floristic elements of communities containing Taxus contorta Griffith in Zhumulangmafeng National Nature Reserve, Tibet. Plant Sci. J. 38, 58–67. https://doi.org/10.11913/PSJ.2095-0837.2020.10058.
Ding, Y., Zang, R.G., Letcher, S.G., Liu, S.R., He, F.L., 2012. Disturbance regime changes the trait distribution,phylogenetic structure and community assembly of tropical rain forests.Oikos 121,1263–1270.https://doi.org/10.1111/j.1600-0706.2011.19992.x.
Ding, Y., Zang, R.G., Lu, X.H., Huang, J.H., Xu, Y., 2019. The effect of environmental filtering on variation in functional diversity along a tropical elevational gradient.J. Veg. Sci. 30, 973–983. https://doi.org/10.1111/jvs.12786.
Diniz, 'E.S., Gastauer, M., Thiele, J., Meira-Neto, J.A.A., 2021. Phylogenetic dynamics of tropical Atlantic forests.Evol.Ecol.35,65–81.https://doi.org/10.1007/s10682-020-10094-6.
Donoghue, M.J., 2008. Phylogenetic perspective on the distribution of plant diversity.Proc. Natl. Acad. Sci. U.S.A. 105, 11549–11555. https://doi.org/10.1073/pnas.0801962105.
dos Santos, N.D., da Costa, D.P., Kinoshita, L.S., Shepherd, G.J., 2014. Windborne: can liverworts be used as indicators of altitudinal gradient in the Brazilian Atlantic Forest? Ecol. Indicat. 36, 431–440. https://doi.org/10.1016/j.ecolind.2013.08.020.
Fatima, N., Kondratyuk, T.P., Park, E.J., Marler, L.E., Jadoon, M., Qazi, M.A., Mehboob Mirza,H.,Khan,I.,Atiq,N.,Chang,L.C.,Ahmed,S.,Pezzuto,J.M.,2016.Endophytic fungi associated with Taxus fuana (West Himalayan Yew) of Pakistan: Potential bioresources for cancer chemopreventive agents. Pharm. Biol. 54, 2547–2554. https://doi.org/10.3109/13880209.2016.1170154.
Fisher, C.K., Mehta, P., 2014. The transition between the niche and neutral regimes in ecology. Proc. Natl. Acad. Sci. U.S.A. 111, 13111–13116. https://doi.org/10.1073/pnas.1405637111.
Freestone,A.L.,Inouye,B.D.,2006.Dispersal limitation and environmental heterogeneity shape scale-dependent diversity patterns in plant communities. Ecology 87,2425–2432. https://doi.org/10.1890/0012-9658(2006)87[2425:dlaehs]2.0.co;2.
Fukami, T., 2004. Community assembly along a species pool gradient: Implications for multiple-scale patterns of species diversity. Popul. Ecol. 46, 137–147. https://doi.org/10.1007/s10144-004-0182-z.
García,M.B.,Pic'o,F.X.,Ehrl'en,J.,2008.Life span correlates with population dynamics in perennial herbaceous plants. Am. J. Bot. 95, 258–262. https://doi.org/10.3732/ajb.95.2.258.
Gastauer, M., Saporetti-Junior, A.W., Valladares, F., Meira-Neto, J.A.A., 2017.Phylogenetic community structure reveals differences in plant community assembly of an oligotrophic white-sand ecosystem from the Brazilian Atlantic Forest.Acta Bot.Bras. 31, 531–538. https://doi.org/10.1590/0102-33062016abb0442.
Gaston,K.J.,2000.Global patterns in biodiversity.Nature 405,220–227.https://doi.org/10.1038/35012228.
Gianuca, A.T., Dias, R.A., Debastiani, V.J., Duarte, L.D.S., 2014. Habitat filtering influences the phylogenetic structure of avian communities across a coastal gradient in southern Brazil. Austr. Ecol. 39, 29–38. https://doi.org/10.1111/aec.12042.
Gibson, D.J., 2009. Grasses and Grassland Ecology. Oxford University Press, Oxford.
G¨otzenberger, L., de Bello, F., Bråthen, K.A., Davison, J., Dubuis, A., Guisan, A., Lepˇs, J.,Lindborg, R., Moora, M., P¨artel, M., Pellissier, L., Pottier, J., Vittoz, P., Zobel, K.,Zobel, M., 2012. Ecological assembly rules in plant communities: Approaches,patterns and prospects. Biol. Rev. 87, 111–127. https://doi.org/10.1111/j.1469-185X.2011.00187.x.
Hawkins, B.A., Rodríguez, M.'A., Weller, S.G., 2011. Global angiosperm family richness revisited: Linking ecology and evolution to climate. J. Biogeogr. 38, 1253–1266.https://doi.org/10.1111/j.1365-2699.2011.02490.x.
HilleRisLambers, J., Adler, P.B., Harpole, W.S., Levine, J.M., Mayfield, M.M., 2012.Rethinking community assembly through the lens of coexistence theory. Annu. Rev.Ecol. Evol. Syst. 43, 227–248. https://doi.org/10.1146/annurev-ecolsys-110411-160411.
Honorio Coronado, E.N., Dexter, K.G., Pennington, R.T., Chave, J., Lewis, S.L.,Alexiades, M.N., Alvarez, E., de Oliveira, A.A., Amaral, I.L., Araujo-Murakami, A.,Arets,E.J.M.M.,Aymard,G.A.,Baraloto,C.,Bonal,D.,Brienen,R.,Cer'on,C.,Cornejo Valverde, F., Di Fiore, A., Farfan-Rios, W., Feldpausch, T.R., Higuchi, N.,Huamantupa-Chuquimaco, I., Laurance, S.G., Laurance, W.F., L'opez-Gonzalez, G.,Marimon, B.S., Marimon-Junior, B.H., Monteagudo Mendoza, A., Neill, D., Palacios Cuenca, W., Pe~nuela Mora, M.C., Pitman, N.C.A., Prieto, A., Quesada, C.A., Ramirez Angulo, H., Rudas, A., Ruschel, A.R., Salinas Revilla, N., Salom~ao, R.P., de Andrade, A.S., Silman, M.R., Spironello, W., ter Steege, H., Terborgh, J., Toledo, M.,Gamarra, L.V., Vieira, I.C.G., Torre, E.V., Vos, V., Phillips, O.L., 2015. Phylogenetic diversity of Amazonian tree communities. Divers. Distrib. 21, 1295–1307. https://doi.org/10.1111/ddi.12357.
Horn, S., Hempel, S., Ristow, M., Rillig, M.C., Kowarik, I., Caruso, T., 2015. Plant community assembly at small scales:Spatial vs.environmental factors in a European grassland. Acta Oecol. 63, 56–62. https://doi.org/10.1016/j.actao.2015.01.004.
Houlahan, J.E., Currie, D.J., Cottenie, K., Cumming, G.S., Ernest, S.K.M., Findlay, C.S.,Fuhlendorf, S.D., Gaedke, U., Legendre, P., Magnuson, J.J., McArdle, B.H.,Muldavin, E.H., Noble, D., Russell, R., Stevens, R.D., Willis, T.J., Woiwod, I.P.,Wondzell, S.M., 2007. Compensatory dynamics are rare in natural ecological communities. Proc. Natl. Acad. Sci. U.S.A. 104, 3273–3277. https://doi.org/10.1073/pnas.0603798104.
Hu, G., Feeley, K.J., Yu, M.J., 2016. Habitat fragmentation drives plant community assembly processes across life stages. PLoS One 11, e159572. https://doi.org/10.1371/journal.pone.0159572.
Hubbell, S.P., 2005. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 19, 166–172. https://doi.org/10.1111/j.0269-8463.2005.00965.x.
Hubbell, S.P., 2001. The Unified Neutral Theory of Biodiversity and Biogeography.Princeton University Press, Princeton and Oxford.
Huxley, J.D., Spasojevic, M.J.,2021. Area not geographic isolation mediates biodiversity responses of alpine refugia to climate change. Front. Ecol. Evol. 9, 633697. https://doi.org/10.3389/fevo.2021.633697.
Jou, Y.J., Huang, C.C.L., Cho, H.J., 2014. A VIF-based optimization model to alleviate collinearity problems in multiple linear regression. Comput. Stat. 29, 1515–1541.https://doi.org/10.1007/s00180-014-0504-3.
Kembel, S.W., Cowan, P.D., Helmus, M.R., Cornwell, W.K., Morlon, H., Ackerly, D.D.,Blomberg, S.P., Webb, C.O., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. https://doi.org/10.1093/bioinformatics/btq166.
Kembel,S.W.,Hubbell,S.P.,2006.The phylogenetic structure of a neotropical forest tree community. Ecology 87, 86–99. https://doi.org/10.1890/0012-9658(2006)87[86:TPSOAN]2.0.CO;2.
Kibet, S., 2011. Plant communities, species diversity, richness, and regeneration of a traditionally managed coastal forest,Kenya.For.Ecol.Manag.261,949–957.https://doi.org/10.1016/j.foreco.2010.11.027.
K¨orner, C., 2007. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22,569–574. https://doi.org/10.1016/j.tree.2007.09.006.
Kraft, N.J.B., Cornwell, W.K., Webb, C.O., Ackerly, D.D., 2007. Trait evolution,community assembly,and the phylogenetic structure of ecological communities.Am.Nat. 170, 271–283. https://doi.org/10.1086/519400.
Kraft, N.J.B., Godoy, O., Levine, J.M., 2015. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. U.S.A. 112,797–802. https://doi.org/10.1073/pnas.1413650112.
Kraft, N.J.B., Valencia, R., Ackerly, D.D., 2008. Functional traits and niche-based tree community assembly in an Amazonian forest.Science 322,580–582.https://doi.org/10.1126/science.1160662.
Lawley, V., Parrott, L., Lewis, M., Sinclair, R., Ostendorf, B., 2013. Self-organization and complex dynamics of regenerating vegetation in an arid ecosystem: 82 years of recovery after grazing. J. Arid Environ. 88, 156–164. https://doi.org/10.1016/j.jaridenv.2012.08.014.
Leech, N.L., Barett, K.C., Morgan, G.A., 2011. IBM SPSS for Intermediate Statistics Use and Interpretation. Routledge, New York.
Leibold, M.A., Chase, J.M., 2017. Metacommunity Ecology. Princeton University Press,Princeton.
Li,X.H.,Zhu,X.X.,Niu,Y.,Sun,H.,2014.Phylogenetic clustering and overdispersion for alpine plants along elevational gradient in the Hengduan Mountains Region,southwest China. J. Systemat. Evol. 52, 280–288. https://doi.org/10.1111/jse.12027.
Li, Y.Z., Shipley, B., Price, J.N., Dantas, V.D., Tamme, R., Westoby, M., Siefert, A.,Schamp, B.S., Spasojevic, M.J., Jung, V., Laughlin, D.C., Richardson, S.J., Le Bagousse-Pinguet, Y., Sch¨ob, C., Gazol, A., Prentice, H.C., Gross, N., Overton, J.,Cianciaruso, M.V., Louault, F., Kamiyama, C., Nakashizuka, T., Hikosaka, K.,Sasaki, T., Katabuchi, M., Dussault, C.F., Gaucherand, S., Chen, N., Vandewalle, M.,Batalha, M.A., 2018. Habitat filtering determines the functional niche occupancy of plant communities worldwide. J. Ecol. 106, 1001–1009. https://doi.org/10.1111/1365-2745.12802.
Liu, H.D., Chen, Q., Chen, Y.F., Xu, Z.Y., Dai, Y.C., Liu, Y., Jiang, Y., Peng, X., Li, H.Y.,Wang, J.,Liu, H.,2020. Effects of biotic/abiotic factors on the seedling regeneration of Dacrydium pectinatum formations in tropical montane forests on Hainan Island,China. Global Ecol. Conserv. 24, e1370. https://doi.org/10.1016/j.gecco.2020.e01370.
Liu,H.D.,Liu,H.,Chen,Y.F.,Xu,Z.Y.,Dai,Y.C.,Chen,Q.,Ma,Y.K.,2021.Identifying the patterns of changes in α-and β-diversity across Dacrydium pectinatum communities in Hainan Island, China. Ecol. Evol. 11, 4616–4630. https://doi.org/10.1002/ece3.7361.
Liu, M.X., Che, Y.D., Jiao, J., Li, L.R., Jiang, X.X., 2019. Exploring the community phylogenetic structure along the slope aspect of subalpine meadows in the eastern Qinghai-Tibetan Plateau,China.Ecol. Evol.9,5270–5280. https://doi.org/10.1002/ece3.5117.
Lopatin,J.,Dolos,K.,Hern'andez,H.J.,Galleguillos,M.,Fassnacht,F.E.,2016.Comparing Generalized Linear Models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens.Environ.173, 200–210. https://doi.org/10.1016/j.rse.2015.11.029.
L'opez-Angulo,J.,Swenson,N.G.,Cavieres,L.A.,Escudero,A.,2018.Interactions between abiotic gradients determine functional and phylogenetic diversity patterns in Mediterranean-type climate mountains in the Andes. J. Veg. Sci. 29, 245–254.https://doi.org/10.1111/jvs.12607.
Marteinsd'ottir, B., Svavarsd'ottir, K., Th'orhallsd'ottir, T.E., 2018. Multiple mechanisms of early plant community assembly with stochasticity driving the process. Ecology 99,91–102. https://doi.org/10.1002/ecy.2079.
Martínez-Villa,J.A.,Gonz'alez-Caro,S.,Duque, 'A.,2020.The importance of grain and cutoff size in shaping tree beta diversity along an elevational gradient in the northwest of Colombia. For. Ecosyst. 7, 2. https://doi.org/10.1186/s40663-020-0214-y.
Münkemüller, T., Gallien, L., Lavergne, S., Renaud, J., Roquet, C., Abdulhak, S.,Dullinger, S., Garraud, L., Guisan, A., Lenoir, J., Svenning, J., Van Es, J., Vittoz, P.,Willner,W.,Wohlgemuth,T.,Zimmermann,N.E.,Thuiller,W.,2014.Scale decisions can reverse conclusions on community assembly processes.Global Ecol.Biogeogr.23,620–632. https://doi.org/10.1111/geb.12137.
Munoz, F., Huneman, P., 2016. From the neutral theory to a comprehensive and multiscale theory of ecological equivalence. Q. Rev. Biol. 91, 321–342. https://doi.org/10.1086/688098.
Mutshinda, C.M., O'Hara, R.B., 2011. Integrating the niche and neutral perspectives on community structure and dynamics. Oecologia 166, 241–251. https://doi.org/10.1007/s00442-010-1831-x.
Myers, J.A., Chase, J.M., Jim'enez, I., Jørgensen, P.M., Araujo-Murakami, A., Paniagua-Zambrana, N., Seidel, R., 2013. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 16, 151–157.https://doi.org/10.1111/ele.12021.
Myers,J.A.,Harms,K.E.,2009.Seed arrival,ecological filters,and plant species richness:A meta-analysis. Ecol. Lett. 12, 1250–1260. https://doi.org/10.1111/j.1461-0248.2009.01373.x.
Nottingham, A.T.,Turner, B.L.,Whitaker, J., Ostle, N.J.,Mcnamara,N.P., Bardgett, R.D.,Salinas, N., Meir, P., 2015. Soil microbial nutrient constraints along a tropical forest elevation gradient: A belowground test of a biogeochemical paradigm.Biogeosciences 12, 6071–6083. https://doi.org/10.5194/bg-12-6071-2015.
Odum, E.P., 1983. Basic Ecology. Saunders College Publishing, New York.
Pashirzad, M., Ejtehadi, H., Vaezi, J., Shefferson, R.P., 2018. Spatial scale-dependent phylogenetic signal in species distributions along geographic and elevation gradients in a mountainous rangeland. Ecol. Evol. 8, 10364–10373. https://doi.org/10.1002/ece3.4293.
Pastore, A.I., Scherer, B.P., 2016. Changes in community phylogenetic structure in a North American forest chronosequence. Ecosphere 7, e1592. https://doi.org/10.1002/ecs2.1592.
Pearson,D.E.,Ortega,Y.K.,Eren, ¨O.,Hierro,J.L.,2018.Community assembly theory as a framework for biological invasions.Trends Ecol.Evol.33,313–325.https://doi.org/10.1016/j.tree.2018.03.002.
Pontarp, M., Br¨annstr¨om, Å., Petchey, O.L., 2019. Inferring community assembly processes from macroscopic patterns using dynamic eco-evolutionary models and Approximate Bayesian Computation (ABC). Methods Ecol. Evol. 10, 450–460.https://doi.org/10.1111/2041-210X.13129.
Poudel, R.C., M¨oeller, M., Li, D.Z., Shah, A., Gao, L.M., 2014. Genetic diversity,demographical history and conservation aspects of the endangered yew tree Taxus contorta (syn. Taxus fuana) in Pakistan. Tree Genet. Genomes 10, 653–665. https://doi.org/10.1007/s11295-014-0711-7.
Poudel,R.C.,M¨oeller,M.,Gao,L.M.,Ahrends,A.,Baral,S.R.,Liu,J.,Thomas,P.,Li,D.Z.,2012.Using morphological,molecular and climatic data to delimitate yews along the Hindu Kush-Himalaya and adjacent regions. PLoS One 7, e46873. https://doi.org/10.1371/journal.pone.0046873.
Punchi-Manage,R.,Wiegand,T.,Wiegand,K.,Getzin,S.,Savitri,Gunatilleke C.V.,Nimal Gunatilleke, I.A.U., 2014. Effect of spatial processes and topography on structuring species assemblages in a Sri Lankan dipterocarp forest.Ecology 95,376–386.https://doi.org/10.1890/12-2102.1.
Qi,W.,Zhang,Y.L.,Gao,J.G.,Yang,X.C.,Liu,L.S.,Khanal,N.R.,2013.Climate change on the southern slope of Mt. Qomolangma (Everest) Region in Nepal since 1971.J. Geogr. Sci. 23, 595–611. https://doi.org/10.1007/s11442-013-1031-9.
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Rosindell, J., Hubbell, S.P., He, F.L., Harmon, L.J., Etienne, R.S., 2012. The case for ecological neutral theory. Trends Ecol. Evol. 27, 203–208. https://doi.org/10.1016/j.tree.2012.01.004.
Sainepo, B.M., Gachene, C.K., Karuma, A., 2018. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Bal. Manag. 13, 4. https://doi.org/10.1186/s13021-018-0091-7.
Shah, A., Li, D.Z., Gao, L.M., Li, H.T., M¨oeller, M., 2008a. Genetic Diversity within and among populations of the endangered species Taxus fuana (Taxaceae) from Pakistan and implications for its conservation.Biochem.Systemat.Ecol.36,183–193.https://doi.org/10.1016/j.bse.2007.09.012.
Shah, A., Li, D.Z., M¨oller, M., Gao, L.M., Hollingsworth, M.L., Gibby, M., 2008b.Delimitation of Taxus fuana Nan Li & R.R. Mill (Taxaceae) based on morphological and molecular data. Taxon 57, 211–222. https://doi.org/10.2307/25065961.
Shipley, B., Paine, C.E.T., Baraloto, C., 2012. Quantifying the importance of local nichebased and stochastic processes to tropical tree community assembly. Ecology 93,760–769. https://doi.org/10.1890/11-0944.1.
Shivaprakash, K.N., Ramesh, B.R., Umashaanker, R., Dayanandan, S., 2018. Functional trait and community phylogenetic analyses reveal environmental filtering as the major determinant of assembly of tropical forest tree communities in the Western Ghats biodiversity hotspot in India. For. Ecosyst. 5, 25. https://doi.org/10.1186/s40663-018-0144-0.
Song, Y.B., Xu, L., Duan, J.P., Zhang, W.J., Shen-Tu, X.L., Li, T.X., Zang, R.G., Dong, M.,2020.Sex ratio and spatial pattern of Taxus fuana,a wild plant with extremely small populations in Tibet. Biodivers. Sci. 28, 269–276. https://doi.org/10.17520/biods.2019102.
Stokes, C.J., Archer, S.R., 2010. Niche differentiation and neutral theory: an integrated perspective on shrub assemblages in a parkland savanna. Ecology 91, 1152–1162.https://doi.org/10.1890/08-1105.1.
Swenson, N.G., Enquist, B.J., Thompson, J., Zimmerman, J.K., 2007. The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities.Ecology 88, 1770–1780. https://doi.org/10.1890/06-1499.1.
Swenson, N.G., Enquist, B.J., Pither, J., Thompson, J., Zimmerman, J.K., 2006. The problem and promise of scale dependency in community phylogenetics. Ecology 87,2418–2424. https://doi.org/10.1890/0012-9658(2006)87[2418:TPAPOS]2.0.CO;2.
Takahashi, K., Tanaka, S., 2016. Relative importance of habitat filtering and limiting similarity on species assemblages of alpine and subalpine plant communities.J.Plant Res. 129, 1041–1049. https://doi.org/10.1007/s10265-016-0852-x.
Tilman, D., 2004. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition,invasion,and community assembly.Proc.Natl.Acad.Sci. U.S.A. 101, 10854–10861. https://doi.org/10.1073/pnas.0403458101.
Tucker, C.M., Cadotte, M.W., Carvalho, S.B., Davies, T.J., Ferrier, S., Fritz, S.A.,Grenyer, R., Helmus, M.R., Jin, L.S., Mooers, A.O., Pavoine, S., Purschke, O.,Redding, D.W., Rosauer, D.F., Winter, M., Mazel, F., 2017. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 92,698–715. https://doi.org/10.1111/brv.12252.
Ulrich, W., Zaplata, M.K., Winter, S., Schaaf, W., Fischer, A., Soliveres, S., Gotelli, N.J.,2016. Species interactions and random dispersal rather than habitat filtering drive community assembly during early plant succession. Oikos 125, 698–707. https://doi.org/10.1111/oik.02658.
Vamosi, S.M., Heard, S.B., Vamosi, J.C., Webb, C.O., 2009. Emerging patterns in the comparative analysis of phylogenetic community structure. Mol. Ecol. 18, 572–592.https://doi.org/10.1111/j.1365-294X.2008.04001.x.
Vellend, M., Srivastava, D.S., Anderson, K.M., Brown, C.D., Jankowski, J.E.,Kleynhans,E.J.,Kraft,N.J.B.,Letaw,A.D.,Macdonald,A.A.M.,Maclean,J.E.,Myers-Smith,I.H.,Norris,A.R.,Xue,X.X.,2014.Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123, 1420–1430. https://doi.org/10.1111/oik.01493.
Vockenhuber,E.A.,Scherber,C.,Langenbruch,C.,Meißner,M.,Seidel,D.,Tscharntke,T.,2011. Tree diversity and environmental context predict herb species richness and cover in Germany's largest connected deciduous forest. Perspect. Plant Ecol. Evol.Systemat. 13, 111–119. https://doi.org/10.1016/j.ppees.2011.02.004.
Volkov, I., Banavar, J.R., Hubbell, S.P., Maritan, A., 2003. Neutral theory and relative species abundance in ecology. Nature 424, 1035–1037. https://doi.org/10.1038/nature01883.
Wang, J.M., Wang, Y., Feng, J.M., Chen, C., Chen, J., Long, T., Li, J.Q., Zang, R.G.,Li, J.W., 2019. Differential responses to climate and land-use changes in threatened Chinese Taxus species. Forests 10, 766. https://doi.org/10.3390/f10090766.
Wang, X.G., Wiegand, T., Kraft, N.J.B., Swenson, N.G., Davies, S.J., Hao, Z.Q., Howe, R.,Lin, Y.C., Ma, K.P., Mi, X.C., Su, S.H., Sun, I.F., Wolf, A., 2016. Stochastic dilution effects weaken deterministic effects of niche-based processes in species rich forests.Ecology 97, 347–360. https://doi.org/10.1890/14-2357.1.
Wang, Z.R., Yang, G.J., Yi, S.H., Chen, S.Y., Wu, Z., Guan, J.Y., Zhao, C.C., Zhao, Q.D.,Ye, B.S., 2012. Effects of environmental factors on the distribution of plant communities in a semi-arid region of the Qinghai-Tibet Plateau. Ecol. Res. 27,667–675. https://doi.org/10.1007/s11284-012-0951-7.
Webb, C.O., Ackerly, D.D., Kembel, S.W., 2008. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24,2098–2100. https://doi.org/10.1093/bioinformatics/btn358.
Webb, C.O., Ackerly, D.D., McPeek, M.A., Donoghue, M.J., 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Systemat. 33, 475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.150448.
Weiher, E.,Freund,D.,Bunton, T.,Stefanski, A.,Lee,T.,Bentivenga,S., 2011.Advances,challenges and a developing synthesis of ecological community assembly theory.Philos. Trans. R. Soc. B. 366, 2403–2413. https://doi.org/10.1098/rstb.2011.0056.
Wiens, J.J., Ackerly, D.D., Allen, A.P., Anacker, B.L., Buckley, L.B., Cornell, H.V.,Damschen, E.I., Jonathan Davies, T., Grytnes, J.A., Harrison, S.P., Hawkins, B.A.,Holt, R.D., McCain, C.M., Stephens, P.R., 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. https://doi.org/10.1111/j.1461-0248.2010.01515.x.
Wiens,J.J.,Graham,C.H.,2005.Niche conservatism:Integrating evolution,ecology,and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431.
Willis, C.G., Halina, M., Lehman, C., Reich, P.B., Keen, A., McCarthy, S., Cavender-Bares, J., 2010. Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography 33, 565–577.https://doi.org/10.1111/j.1600-0587.2009.05975.x.
Wu, X.D., Zhao, L., Chen, M.J., Fang, H.B., Yue, G.Y., Chen, J., Pang, Q.Q., Wang, Z.W.,Ding, Y.J., 2012. Soil organic carbon and its relationship to vegetation communities and soil properties in permafrost areas of the central western Qinghai-Tibet Plateau,China. Permafr. Periglac. 23, 162–169. https://doi.org/10.1002/ppp.1740.
Xiao, Y.M., Yang, L.C., Nie, X.Q., Li, C.B., Xiong, F., Zhou, G.Y., 2020. Phylogenetic patterns of shrub communities along the longitudinal and latitudinal gradients on the northeastern Qinghai-Tibetan Plateau. J. Mt. Sci. 17, 1106–1114. https://doi.org/10.1007/s11629-019-5561-1.
Xu, J.S., Chen, Y., Zhang, L.X., Chai, Y.F., Wang, M., Guo, Y.X., Li, T., Yue, M., 2017.Using phylogeny and functional traits for assessing community assembly along environmental gradients: A deterministic process driven by elevation. Ecol. Evol. 7,5056–5069. https://doi.org/10.1002/ece3.3068.
Xu,Y.,Liu,J.J.,Li,H.N.,Liu,J.,Burgess,K.S.,Ge,X.J.,2021.The effects of evolutionary and environmental variance on estimates of phylogenetic diversity in temperate forest plots. J. Plant Ecol. 14, 96–107. https://doi.org/10.1093/jpe/rtaa078.
Yan, Y.J., Yang, X., Tang, Z.Y., 2013. Patterns of species diversity and phylogenetic structure of vascular plants on the Qinghai-Tibetan Plateau. Ecol. Evol. 3,4584–4595. https://doi.org/10.1002/ece3.847.
Yang, J., Cai, L., Liu, D.T., Chen, G., Gratzfeld, J., Sun, W.B., 2020. China’s conservation program on Plant Species with Extremely Small Populations (PSESP): Progress and perspectives. Biol. Conserv. 244, 108535. https://doi.org/10.1016/j.biocon.2020.108535.
Yu,C.N.,Luo,X.J.,Zhan,X.R.,Hao,J.,Zhang,L.,Song,Y.B.,Shen,C.J.,Dong,M.,2018.Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol. 18,197. https://doi.org/10.1186/s12870-018-1412-4.
Yue, J., Li, R., 2021. Phylogenetic relatedness of woody angiosperm assemblages and its environmental determinants along a subtropical elevational gradient in China. Plant Divers 43, 111–116. https://doi.org/10.1016/j.pld.2020.08.003.
Zang, R.G., Dong, M., Li, J.Q., Chen, X.Y., Zeng, S.J., Jiang, M.X., Li, Z.Q., Huang, J.H.,2016.Conservation and restoration for typical critically endangered wild plants with extremely small population.Acta Ecol.Sin.36,7130–7135.https://doi.org/10.5846/stxb201610082011.
Zhang, Y.Q., Li, Z.C., Song, L.G., Hou, L.Y., Sun, Q.W., 2020. Natural distribution and community ecological characteristics of Taxus fuana. Acta Ecol. Sin. 40, 1999–2009.https://doi.org/10.5846/stxb201809282117.
Zhou, W.S., Zhang, Y.X., Zhang, S., Yakimov, B.N., Ma, K.M., 2021. Phylogenetic and functional traits verify the combined effect of deterministic and stochastic processes in the community assembly of temperate forests along an elevational gradient.Forests 12, 591. https://doi.org/10.3390/f12050591.
- Forest Ecosystems的其它文章
- Vegetation structure and edaphic factors in veredas reflect different conservation status in these threatened areas
- Black locust coppice stands homogenize soil diazotrophic communities by reducing soil net nitrogen mineralization
- Responses of soil CH4 fluxes to nitrogen addition in two tropical montane rainforests in southern China
- Importance of Quercus spp. for diversity and biomass of vascular epiphytes in a managed pine-oak forest in Southern Mexico
- Using machine learning algorithms to estimate stand volume growth of Larix and Quercus forests based on national-scale Forest Inventory data in China
- Effects of fertilization on the growth dominance of Inland Northwest forests of the United States

