Importance of Quercus spp. for diversity and biomass of vascular epiphytes in a managed pine-oak forest in Southern Mexico
Nyely Mrtínez-Mel'endez, Neptlí Rmírez-Mril,*, Jos'e G. Grí-Frno,Mnuel Jesús Ch-P'erez, Plo Mrtínez-Zurimendi
a Departamento de Conservaci'on de la Biodiversidad, El Colegio de la Frontera Sur (ECOSUR), San Crist'obal de Las Casas, 29290, Chiapas, Mexico
b Red de Ecología Functional, Instituto de Ecología, A.C. Xalapa, 91073, Veracruz, Mexico
c Departamento de Agricultura, Sociedad y Ambiente, El Colegio de la Frontera Sur (ECOSUR), Villahermosa, 86280, Tabasco, Mexico
Keywords:Bromeliads Chiapas Ferns Orchids Richness Sierra Madre Silvicultural development method (SDM)
ABSTRACT
1. Background
Tropical forests and ecosystems provide diverse goods and ecosystem services to human society (Bastian et al., 2014; Soh et al., 2019). However, due to a variety of anthropogenic activities,forests worldwide are currently undergoing degradation (FAOUNEP, 2020). For this reason,there is a need to adopt sustainable forest management practices that promote biodiversity conservation together with production of timber(Torres-Rojo et al.,2016;Ofoegbu and Speranza,2017;Grantham et al.,2020). Thus far, few so-called sustainable forestry management plans have avoided practices which harm non-target-particularly non-timberspecies(Costa and Magnusson,2003).These include vascular epiphytes,a highly diverse group of approximately 31,311 species worldwide(28,(2282,272 7angiosperms, 2 gymnosperms, 2,942 Polypodiopsida, and 142 Lycopodiopsida;Zotz et al.,2021).Approximately 30%of vascular plant species found in tropical mountain ecosystems are epiphytes (Küper et al.,2004).Therefore,any type of forest management creates ecological conditions that affect richness and diversity of this plant group, in turn modifying a range of ecological processes with effects on local and regional scales(Miller et al.,2011;Alroy,2017;Ding et al.,2017;Giam,2017;Hu et al.,2018).
Forestry management consists of programming harvesting each stand(a forest area with homogenous ecological and physical conditions) to achieve continual timber yield while maintaining considerable forestry mass. In this manner, a significant proportion of the forest’s structural attributes are preserved, allowing for provision of those ecosystem services which an unmanaged forest would provide.Nevertheless,different methods of forestry management, such as selective harvesting and by rotation with intermediate felling,modify the composition and structure of tree vegetation, generating secondary forests (Torras et al., 2012;Rutten et al., 2015; Faison et al., 2016; Ding et al., 2017; Shima et al.,2018; Martínez-Mel'endez et al., 2021). Extraction of trees leads to reduction in richness and abundance of different epiphyte species(Padmawathe et al., 2004; Jim'enez-Bautista et al., 2014). However, little is known of the diversity and biomass of epiphytes that remain in the landscape following silvicultural intervention.
In Mexico,two principal forestry management systems are used: the Mexican Method of Forest Management-implemented in the late 1970’s,and the Silvicultural Development Method (SDM) -implemented in the early 1980’s.The former prescribes harvesting selected trees in 1,000 to 2,000 km2forest stands over a cutting period ranging from 17 to 35 years to promote a variety of arboreal structures. SDM incorporate a 50-60-year rotation period in which extraction of most mature pine trees is permitted (Rosales et al., 1982). Most adult trees are cut to promote regeneration of a new cohort, leaving some of the most favored individuals as seed trees.The second stage consists of a release cutting(RC)of less desirable saplings or smaller trees in order to provide growth-space for favored trees of the same age class. Finally, thinning(TH) is carried out to reduce stand density, thereby improving growth and enhancing forest health (Moreno-S'anchez and Torres-Rojo, 2010;Torres-Rojo et al.,2016;Martínez-Mel'endez et al.,2021).
Implementation of SDM in different regions of Mexico has led to problems resulting from high heterogeneity in forest composition and structure, and in turn the need for improvements and adjustments(Moreno-S'anchez and Torres-Rojo, 2010). Aside from pines, SDM also allows for harvesting a small number of oaks (Quercus spp.) to produce charcoal(PMLO,2013).
Opening the canopy results in a variety of stages of regeneration and growth of timber species (Torres-Rojo et al., 2016; P'erez-L'opez et al.,2020; Martínez-Mel'endez et al., 2021). As epiphytes require a physical area to support themselves as well as given micro-climatic conditions for their establishment, they are extremely sensitive to tree elimination during forest management(Jim'enez-Bautista et al.,2014;Kr¨omer et al.,2014). Immediate consequences include: reduced mass with some isolated trees,modification of micro-environmental conditions, and reduction of possible sources of propagules and safe sites for germination,establishment, and development (Benzing, 1990; Barthlott et al., 2001;Kr¨omer and Gradstein,2003).
In Mexico, approximately 1,813 species of vascular epiphytes have been recorded (Espejo-Serna et al., 2021). Vascular epiphytes are key components of the ecosystems they inhabit due to their interaction with other plants(Mehltreter et al., 2006; Ackerman and Roubik,2012;García-Estrada et al., 2012; Aguilar-Rodríguez et al., 2014; Zotz, 2016).Furthermore, they constitute micro-ecosystems for many fauna species(Foissner,2010;McCracken and Forstner,2014).
Several ecological patterns and processes of epiphytes have been studied in a variety of ecosystems(e.g.,Merwin et al.,2003;Padmawathe et al.,2004;K¨oster et al.,2009;Werner and Gradstein,2009;Larrea and Werner, 2010), but few have been evaluated in managed forests, in particular those involving SDM (e.g., Jim'enez-Bautista et al., 2014).Silvicultural management often involves forest fragmentation,creating a microclimate which limits richness, diversity, and abundance of epiphytes,but this process is still poorly understood(Kr¨omer and Gradstein, 2003; K¨oster et al., 2009; Werner, 2011; Jim'enez-Bautista et al.,2014;Nfornkah et al.,2018).
The objective of this study was to evaluate diversity and accumulated biomass of the community of epiphytes present on remnant oak trees(Quercus spp.)left standing following timber harvest(PMLO,2013).We focus on these remnant oaks because they allow us to record changes in the epiphyte community following forest management.We selected two silvicultural treatments included in the SDM (RC and TH) and - as a control -an old-secondary forest (SF)without harvesting. We evaluated the diversity and biomass of vascular epiphytes in the three forest conditions to address the following questions:(1)How do epiphyte diversity and biomass vary in each forest conditions? (2) How does tree harvest affect diversity and biomass of different epiphyte species groups (bromeliads, orchids, ferns, and other families)? and (3) How variable is epiphyte diversity and biomass for each oak species present in each forest condition? We hypothesize that the diversity and biomass of epiphytes are lower under SDM than in the old-growth secondary forest.
2. Materials and methods
2.1. Study area
Fieldwork was conducted in the Los Ocotones forest stand,which has a surface area of 1,838.4 ha,located in the municipality of Cintalapa de Figueroa in Chiapas,southern Mexico(16°47′31.09′′to 16°47′48.97′′N and 94°01′29.53′′to 94°02′4.43′′W),at an elevation of 800–1,500 m asl.The forest stand is in the NW extreme of the Sierra Madre of Chiapas,on the border with Oaxaca,and forms part of the Selva Zoque-La Sepultura Priority Terrestrial Region of Mexico(Arriaga et al.,2000,Fig.1).Mean annual precipitation is 1,250 mm and mean annual temperature is 22°C(García,2003).Predominant soil types are eutric regosol,chromic cambisol, and lithosol, with a silt texture and a lithic phase (PMLO, 2013).Pine-oak forest dominates the study site (Breedlove, 1981), principally
Pinus oocarpa, P. maximinoi, Quercus peduncularis, Q. sapotifolia and Q. glaucescens.
The Los Ocotones forest is managed by a private company which has a management plan regulated by the Mexican Forestry Commission(Comisi'on Nacional Forestal, PMLO, 2013). SDM forestry management has been practiced in the site since the late 1990s. The Los Ocotones forestry management plan includes timber extraction following a 45-year rotation, including five cutting periods (one every nine years): regeneration cutting, release cutting, and three thinning (Martínez-Mel'endez et al., 2021). The first cutting period began in 2004. During the cutting periods,different treatments may be applied simultaneously in sectors of the managed area depending on the physiognomic characteristics of the trees, regardless of the preceding treatment according to the order of rotation of the SDM, so as to regulate the forest mass of all the stands according to that prescribed in the SDM (R. Ramos, pers. comms.). The main target species are Pinus oocarpa and P. maximinoi for timber, and Quercus spp.to produce charcoal.
2.2. Study site selection


Fig. 1. Map of location of the Los Ocotones forest in Chiapas, Mexico, and the sampling sites (square: release cutting treatment; triangle: thinning treatment; circle:secondary forest treatment).

Thinning(TH),corresponds to the fourth period of the rotation.The study area within this stand underwent two thinning periods, in 2007 and 2016 (PMLO, 2013). Sampling of epiphytes in this stand was conducted two years after the final cutting. In general, TH involves intermediate cutting,which is more significant than RC as it is carried out to control stand density at different points in the growth cycle. TH favors trees with optimal characteristics for future harvest while eliminating the others, and is carried out during a late forest successional stage to promote rapid advance to the subsequent phase of the SDM sequence treatments.TH cutting intensity is 75%for pines,20%for oaks,and 3%for other species. Based on data from 2013 to 2017 (PMLO, 2013),average timber volume harvested was 31.9 m3⋅ha-1for pine and 8.8 m3⋅ha-1for oak.
Secondary forest (SF): In the Los Ocotones forest management plan,areas without silvicultural treatment are considered “conservation zones”. While unregulated extraction of P. oocarpa, P. maximinoi, and P.chiapensis was practiced in these areas in the 1960s(Del Carpio,2006),there has been no timber harvest since(R.Ramos,pers.comms.).Due to this history of management,in this study,we considered these areas to be old-secondary forest and used them as control sites for comparison of epiphyte data.
2.3. Epiphyte sampling
Epiphyte sampling was conducted in 2018. Twelve circular 0.1 ha plots were established in each forest conditions(12 in RC,12 in TH and 12 in SF, for a total of 36 plots). Plots ranged in altitude range from 1,053–1,200 m asl. Epiphytes were sampled in oaks which survived the silvicultural treatments, because oaks are more effective hosts than are pines(Wolf,2005).In each plot,five host trees of Quercus spp.(hereafter,host trees) with a DBH ≥20 cm were selected (60 individuals for each treatment, for a total of 180). For each individual host tree, species of Quercus and DBH were recorded,and the basal area(m2)was calculated(Newton, 2007). With this data, mean basal area (m2⋅ha-1± standard deviation) was calculated for each host tree species per plot and per treatment.
Using tree climbing equipment (Perry, 1978) and binoculars (6.5 ×32 Kingbird Binocular,Eagle Optics,USA),we recorded epiphyte species and their number of individuals present on each of the 180 selected host trees from trunk to outer crown.Epiphyte species were identified in the field by a single person (the first author, due to her experience) and to maximize consistency. As we aimed for non-destructive sampling, a limited amount of epiphytes was collected, measured, and weighed,which facilitated adjusting estimation of visual parameters with greater precision.We classified epiphytes into four groups:bromeliads,orchids,ferns (including lycopods), and others. The first three are the most abundant epiphyte groups in the study area.Bromeliaceae,Orchidaceae,and the pteridophytes make up approximately 60%of epiphytes present in this Neotropical region(Zotz,2016;Mendieta-Leiva et al.,2020).The group of“others”included species of less abundant angiosperm families(e.g., Araceae, Gesneriaceae, Piperaceae and Rubiaceae), and was included to avoid bias toward Orchidaceae, Bromeliaceae and pteridophytes,which have received greater attention than other equally significant families (Zotz,2016).
Density of bromeliads was evaluated by number of rosettes, and for ferns number of fronds. Plants that were not interconnected, such as those of the families Araceae, Gesneriaceae, and Rubiaceae, were recorded as individual organisms. Pseudobulbs of orchids and stems of Peperomia were quantified (Martínez-Mel'endez et al., 2008). Upon detecting intertwined groups of species, individual species were distinguished by their vegetative and reproductive structures. Dominance of epiphytes was determined by estimating their biomass (Wolf et al.,2009). Given variation in size among epiphyte species, they vary with respect to their contribution to total epiphyte biomass; therefore, we made an adjustment between size and weight to estimate biomass for each species(Benavides et al.,2006;Wolf,2005).For this,10 specimens per size category were collected for each bromeliad species in the vicinity of the sampling sites. However, for orchids, ferns and others, only 10 specimens were collected per species as there were not enough specimens to represent each size category. For bromeliad specimens, average biomass (dry weight in kg, without accumulated dead inorganic material)was obtained per size class and species(Wolf et al.,2009),while for the other epiphytes we obtained only total biomass per species. Plant samples were dehydrated in a drying oven(292A,Felisa®,Mexico)until reaching a constant weight. Once dried, they were weighed using an analytical balance(Ohaus PA 214,Pioneer,Japan).Mean biomass of the 10 specimens was then multiplied by the number of specimens recorded in the field.Biomass(±SD)was obtained per epiphyte species,epiphyte group,sampled host tree,and host tree species.
Samples of each unknown epiphyte species were collected and herborized. For their identification, scientific collections of the herbarium(CH)of El Colegio de la Frontera Sur were consulted,as well as experts in epiphyte taxonomy. The updated taxonomic nomenclature was verified in Plants of the World Online (http://www.plantsoftheworldonlin e.org/). Epiphyte designation was verified considering the global list of vascular epiphytes EpiList 1.0(Zotz et al.,2021).
2.4. Analysis
Diversity of epiphytes for each treatment was calculated using Hill numbers (Jost, 2006). The three components of effective number of species were obtained:0D = observed richness,1D = common or abundant species,and2D=hyper-abundant species(equivalent to the inverse of the Simpson’s Diversity Index).This analysis was conducted for each treatment using data for species abundance per plot. Accumulation curves for the species were generated using the rarefaction (interpolation)and extrapolation(prediction)procedures.We chose these methods as the number of individuals may vary within each treatment,potentially introducing bias to the estimation of species diversity in plots with more individuals. In this method of standardization, species accumulation curves are generated,with 95%confidence intervals calculated using the bootstrapping method,such that species diversity may be quantified and visually compared in multiple groups (Hsieh et al., 2016). For this analysis,we used the iNEXT library(Hsieh et al., 2016).
Mean values(mean±1 SD)of1D and biomass of epiphytes per host tree were analyzed per group of species(bromeliads,orchids,ferns,and others)and per host tree species(Quercus spp.).To compare these values among treatments,a simple analysis of variance(ANOVA)was used.To determine whether significant differences existed in host tree basal area among treatments,an ANOVA was performed.Normality of the residuals was verified for each model;if the ANOVA models proved significant(p<0.05),Tukey tests were used to detect differences among treatments.In order to analyze the relationship between1D and epiphyte biomass on the one hand with host tree basal area on the other, linear regression models were fitted, and the normality of the residuals verified. We decided to use host tree basal area rather than DBH as it has been demonstrated that tree surface area is more closely correlated with basal area than with DBH(West et al.,1999),and it also has a stronger linear relationship to epiphyte diversity and biomass(Werner et al.,2012).To visually examine the patterns of abundance of the epiphyte species and identify the dominant species in each treatment, we constructed rank abundance curves(Magurran,2004).Epiphyte abundance is expressed as the logarithm of the biomass(kg)recorded in each of the 12 plots of each treatment.To analyze beta diversity,the Jaccard coefficient of similarity was used (Magurran,2004). This analysis compared the composition of epiphyte species among the plots of each treatment and among treatments. Mean values of similarity among plots were obtained through paired comparisons using the CommEcol and vegan libraries (Oksanen,2016;Sanches,2019,respectively).To observe overlapping of the species among the treatments, a Venn diagram was constructed (Magurran,2004).
To evaluate the relationships of1D and epiphyte biomass among group of species(bromeliads,orchids,ferns,and others)and treatments,we used generalized linear models(GLM;Crawley,2013).These models considered treatment, host tree basal area, and the interaction between the two as predictive variables, and the1D and epiphyte biomass as response variables. Two general models were constructed: one for each response variable(1D and epiphyte biomass),and one for each group of species (bromeliads, orchids, ferns, and others), for a total of eight models. All models had a gamma distribution, and the normality of the residuals and overdispersion were verified. If the categorical variable treatment was significant in the model,Tukey tests were conducted using the multcomp library (Hothorn et al., 2019). An alpha value of p <0.05 was used to determine the significance level.All analyses were conducted using the R software,version 3.6.0(R Development Core Team,2019).
3. Results
3.1. Composition and structure
We recorded a total of 24,268 individuals of vascular epiphytes,corresponding to 67 species (35 orchids, 16 bromeliads, 10 ferns-including lycopods-and 6 others),in 35 genera and 10 families found in the 180 host trees within the 36 study plots.We identified 39 species in the release cutting(RC)treatment,44 in the thinning(TH),and 52 in the control (SF; Table S1). Bromeliads were present in 99% of host trees(179),orchids in 56%(100),ferns in 40%(71),and others in 19%(34).In general,five host trees species were identified:Quercus glaucescens(59 individuals), Q. sapotifolia (57 individuals), Q. peduncularis (26 individuals),Q.elliptica(20 individuals)and Q.calophylla(18 individuals).All five species were found in all plots. However, the number of individuals per species varied among treatments: Q. elliptica and Q.glaucescens were more frequent in RC,while Q.peduncularis was most frequent in NT. Basal area of the sampled host trees presented no significant differences among treatments(F2,177=1.56,p >0.05,Table 1).
3.2. Diversity of epiphytes
Mean epiphyte richness per host tree(0D)varied significantly among treatments (F2,177= 10.83, p <0.001). The greatest epiphyte richness was found in SF (0DSF= 7.43 ± 4.32), followed by TH (0DTH= 5.05 ±3.96), and RC (0DRC= 4.10 ± 3.13).1D of orchids varied among treatments(F2,177=12.72,p <0.001),being highest in SF and lowest in RC(Table 2).Diversity of bromeliads(F2,177=1.69,p >0.05),ferns(F2,177= 2.50, p >0.05), and others (F2,177= 3.04, p ≥0.05) did not vary significantly among treatments(Table 2).
In general,1D of epiphytes did not vary among host tree species(F4,175= 0.52, p >0.05).1D of epiphytes differed significantly among the different treatments in Q. glaucescens (F2,56= 6.16, p <0.01) and in Q.sapotifolia(F2,54=5.75,p <0.01).1D for both host tree species was highest in the control site (Table 3).
True diversity (0D) showed that the SF treatment had the greatest richness of epiphyte species (52), compared to TH (44) and RC (39;Fig. 2). The number of most common species (1D) was lower in RC (15 species) than in TH (19) and SF (18; Fig. 2). The number of dominant species(2D)was greater in TH and SF(12 each)than in RC(nine;Fig.2).There was a slight relationship between1D of epiphytes and basal area of host trees(1DRCr2=0.06,p <0.05;1DTHr2=0.02,p >0.05;1DSFr2=0.02,p >0.05;Fig.3a).
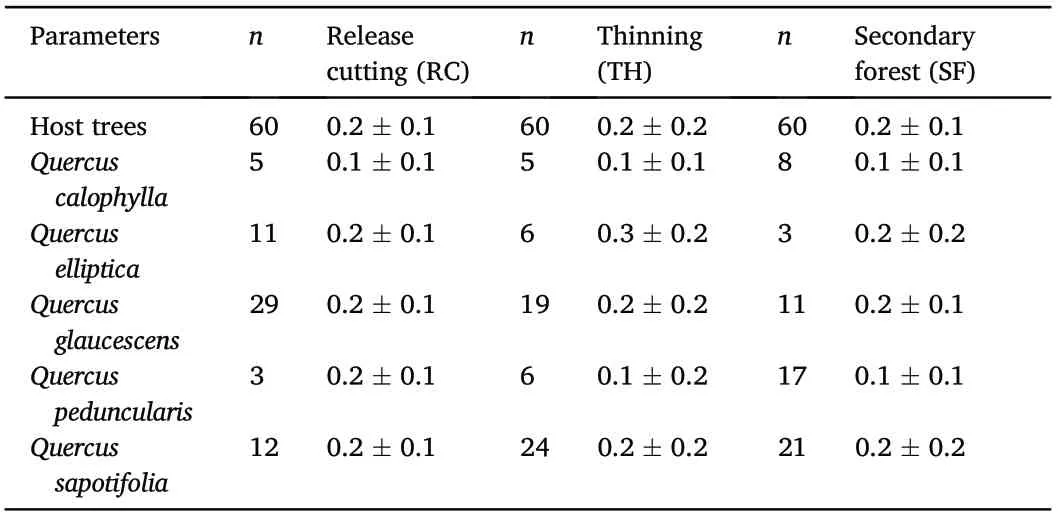
Table 1 Basal area (m2⋅ha-1, mean ± SD) of host tree species sampled in three silvicultural treatments in the Los Ocotones forest, southern Mexico.

Table 2 Epiphyte 1D value per tree (mean ± 1 SD) in sampled host trees (n = 180) for three silvicultural treatments in the Los Ocotones forest, southern Mexico.
3.3. Beta diversity
The Jaccard similarity analysis indicated that the plots within each treatment share over 50%of the epiphyte species present in each forest condition. Plots of the RC and TH sites presented greater similarity among each other (TH IJ= 0.70 ± 0.20 and RC IJ= 0.68 ± 0.20) than with those of SF,which were more heterogeneous(SF IJ=0.59±0.20).On the other hand,over 60%of species are shared among the tree forest conditions(TH vs RC IJ=0.68±0.19,TH vs SF IJ=0.67±0.17,RC vs SF IJ=0.68±0.17).
In SF, orchids, bromeliads, and others had greater species richness than ferns,while in TH ferns had the greatest richness(Fig.4).A total of 68.7%of the species of bromeliads,50%of others,34.3%of orchids,and 30% of ferns were shared among the three treatments (Fig. 4). The number of exclusive species in each treatment was 14 in SF,8 in TH,and 5 in RC (Table S1). Orchids, bromeliads, and others presented more exclusive species in SF(9,3,and 2 species respectively)than in the other treatments. Ferns presented more exclusive species in TH than in the other treatments(Table S1).
None of the orchid species are threatened, but of the 11 species of bromeliads shared among the three treatments, two are threatened(Tillandsia tricolor and T. seleriana) and one under special protection(T.festucoides)according to Mexican law(SEMARNAT,2010).The three treatments share the fern Serpocaulon triseriale (Polypodiaceae); the bromeliad T.concolor is present in RC.None of the species in the group of others are threatened.
3.4. Biomass
Total dry biomass of the epiphyte species calculated in the sampled host trees (n = 180) was 46.7 kg. A slight relationship existed between epiphyte biomass (B) and host tree basal area (BTHr2= 0.12, p <0.01;BRCr2=0.01,p >0.05;BSFr2=0.25,p <0.001;Fig.3b).Mean epiphyte biomass per host tree was 0.26 kg ± 0.34, pooling all treatments. Bromeliads made up 57.9%of total biomass,orchids 31.2%,ferns 7.3%,and others 3.7%. The species with the greatest contribution to biomass by species group were Tillandsia seleriana(63%of bromeliads),Camaridium densum (59% of orchids), Serpocaulon triseriale (52% of ferns), and Peperomia obtusifolia(40%of others).
Mean epiphyte biomass per host tree varied among treatments (F2,177= 3.06, p <0.05), being highest in SF and lowest in TH (Table 4).Biomass of bromeliads did not vary among treatments(F2,177=2.52,p >0.05),nor did that of orchids(F2,177=2.32,p >0.05)or ferns(F2,177=1.72,p >0.05).However,a bit greatest amount of biomass was observed in RC than SF. The biomass of others differed significantly among treatments(F2,177=3.92,p <0.05),being highest in SF and lowest in TH(Table 4).
Meanwhile,mean biomass of epiphytes recorded on the sampled host tree species varied slightly among treatments(F4,175=2.70,p <0.05).Quercus peduncularis hosted the greatest biomass of epiphytes on only three trees in RC, followed by Q. sapotifolia,while Q. calophylla was the species with the lowest epiphyte biomass (Table 5). Furthermore,epiphyte biomass per host tree species and treatment was greatest on Q.glaucescens in SF(F2,56=3.45,p <0.05)and on Q.peduncularis in RC(F2,23=6.38,p ≤0.01;Table 5).

Table 3 Epiphyte 1D per host tree species (mean ± 1 SD)sampled (n =180 trees) in three silvicultural treatments in the Los Ocotones forest, southern Mexico.

Fig. 2. Epiphyte species accumulation curves in three silvicultural treatments in the Los Ocotones forest, southern Mexico. Continuous lines indicate interpolation(rarefaction) and discontinuous lines denote extrapolation. Diversity is represented by Hill numbers: 0D = observed richness; 1D = common species; 2D = dominant species. Bands correspond to 95% confidence intervals. RC = release cutting treatment, TH = thinning treatment, SF = secondary forest treatment.

Fig. 3. Relationship between epiphyte alpha diversity (1D) and biomass and host tree basal area (m2) in three silvicultural treatments in the Los Ocotones forest,southern Mexico.The shaded area in each graph indicates the 95%confidence interval.Regression values(r),explained variance(r2),and significance(p)are shown in each graph.
The rank abundance curves for biomass of epiphyte species showed little variation in the dominant species among treatments (Fig. 5).
Tillandsia seleriana and Camaridium densum dominated all treatments,but presented the greatest biomass in TH. Co-dominant species were the orchid Maxillariella elatior in RC,and the bromeliads Tillandsia festucoides in TH and Catopsis occulta in SF. The curves also showed the exclusive presence of T. filifolia in TH and of Serpocaulon triseriale in SF, both species within the five most co-dominant species(Fig.5).
3.5. Relationship between vascular epiphyte diversity, biomass, treatment,and host tree size
The GLM showed that1D of vascular epiphytes is significantly related to the silvicultural treatment (p <0.001). Diversity in SF was significantly greater than that of RC and TH(SF vs RC,p <0.001;SF vs TH,p <0.05). Epiphyte biomass was related to host tree basal area (p <0.001;Table 6). This coincides with mean values and the ANOVA tests that indicate that diversity and biomass differ significantly among treatments and that the greatest values are found in SF.Moreover,epiphyte diversity and biomass are not significantly related to the interaction between host tree basal area and treatment(p >0.05;Table 6).
At the level of species group, diversity of bromeliads showed a significant interaction between host tree basal area and treatment (p <0.05).Diversity of orchids was related to treatment(p <0.001),but not to host tree basal area(p >0.05;Table 6).Diversity of orchids in SF was greater than those of RC(p <0.001)and TH(p <0.001).No significant relationship was found between diversity of ferns or that of others with any factor (p >0.05; Table 6). Rather, epiphyte biomass was directly related to host tree basal area for all the species’groups(bromeliads,p <0.01; orchids p <0.01; ferns p <0.001, others p <0.01), but was not significantly related to treatment or to the interaction host trees basal area×treatment(p >0.05;Table 6).
4. Discussion
4.1. Local diversity of epiphytes
Total richness of epiphytes in the Los Ocotones forest (67 species)represents 5.6% of the richness of epiphytes reported for Mexico(Espejo-Serna et al., 2021). In the present study, orchids were the most species-rich group(35 species),followed by bromeliads(16 species).This coincides with some of the studies conducted in tropical forests in which Orchidaceae and Bromeliaceae have been found to be the most diverse families (Zotz, 2016; Mendieta-Leiva et al., 2020). However, it contradicts a study of a disturbed pine-oak forests of the same region(Chiapas,Mexico) that indicate that ferns are more diverse than bromeliads, surpassed only by orchids(Wolf and Flamenco-Sandoval,2005).

Fig. 4. Venn diagram showing the total number of species, number of species per group of epiphytes in each treatment (RC=release cutting, TH =thinning,SF = secondary forest), and number of species shared among three silvicultural treatments in the Los Ocotones forest, southern Mexico. Total number of epiphyte species recorded for each treatment is presented in parentheses.
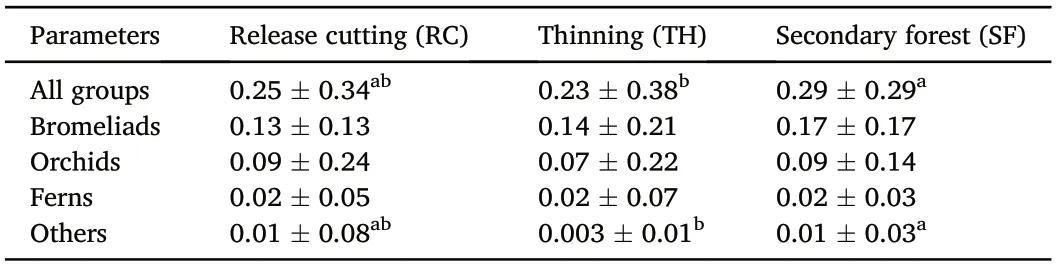
Table 4 Epiphyte biomass(kg)per tree(mean±1 SD)in sampled host trees(n=180)in three silvicultural treatments in the Los Ocotones forest, southern Mexico.
Generally, a positive relationship has been found among epiphyte biomass, diversity, and size of the host tree, as larger trees provide a greater surface area on their trunk and branches on which to host epiphytes (Gradstein et al., 2003; Wolf et al., 2009; Werner et al., 2012;Nfornkah et al.,2018).In this study,epiphyte diversity and biomass were slightly related to host tree basal area.We consider this to be a result of the fact that the treatments vary with respect to the structure of their vegetation (Martínez-Mel'endez et al., 2021). Even if they are the same size, each tree varies with respect to its conditions of coverage, luminosity, exposure, as well as other variables which affect the quantity of epiphytic biomass that it may host.The largest host tree in our study was recorded in SF,while most others were small.This variability within the SF reflects the history of forestry and agricultural use of the study region(Del Carpio,2006).While its previous management was irregular,it has not been exploited since the current management plan was implemented in 2004 (PMLO, 2013), and therefore we consider SF to be an old-secondary forest undergoing recovery.
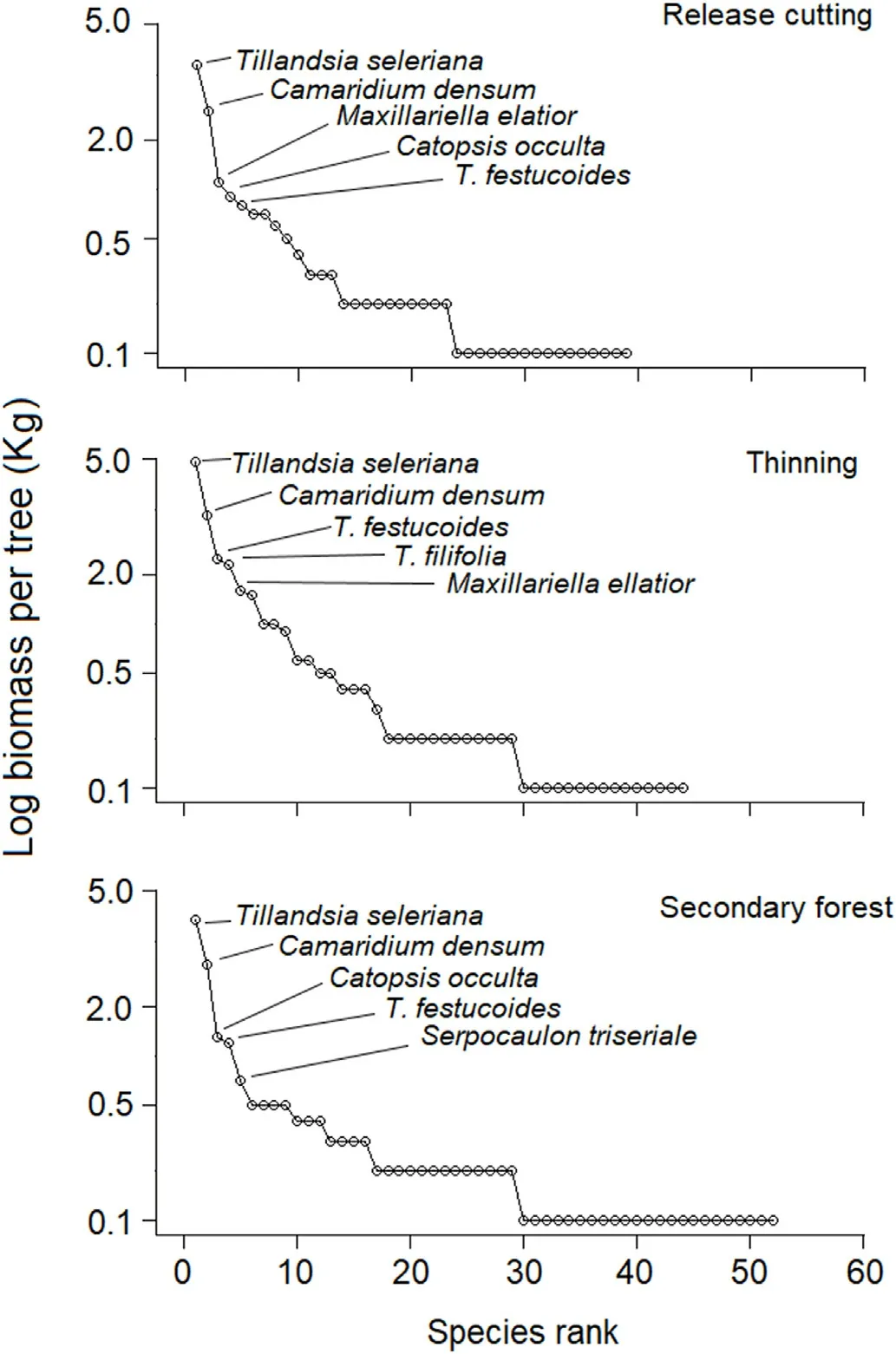
Fig. 5. Rank abundance curves based on biomass of vascular epiphyte species recorded for three silvicultural treatments in the Los Ocotones forest, southern Mexico.
We found that diversity of epiphytes depends more on treatment type than on host tree size,possibly due to variation in structural conditions of each treatment.Our calculations of true diversity showed that richness of epiphytes (0D) was greatest in SF and lowest in RC. In addition, the treatment RC was also the least diverse in terms of common (1D) and dominant (2D) species. The more benign structural conditions of SF as compared to RC and TH have facilitated establishment of epiphytes.This is related to size,density, and diversity of oak species present(see Martínez-Mel'endez et al.,2021),which could provide a variety of bark types for successful epiphyte establishment. This is not the case with pines -which predominate in the study sites, as they are considered less adequate host trees for some epiphytic families due to the low level ofwater retention of their bark and its tendency to detach from the tree,as well as its presence of resins and phenolic compounds (Callaway et al.,2002;Hietz and Hietz-Seifert,1995;Wolf,2005).However,other studies mention that some pine species-including P.ayacahuite-could favor the establishment of some orchids(Jim'enez-Bautista et al.,2014).Although the basal area of the sampled oaks did not vary significantly among the silvicultural treatments studied,the diversity of epiphytes per tree in SF was greater than in the other sites. It is evident that removal of trees in managed sites leads to a decrease in richness of epiphytes as a result of the elimination of their host trees (Kr¨omer and Gradstein, 2003; K¨oster et al.,2009);however,long-term studies involving continual monitoring will help to comprehend the response of epiphyte communities on remnant trees.
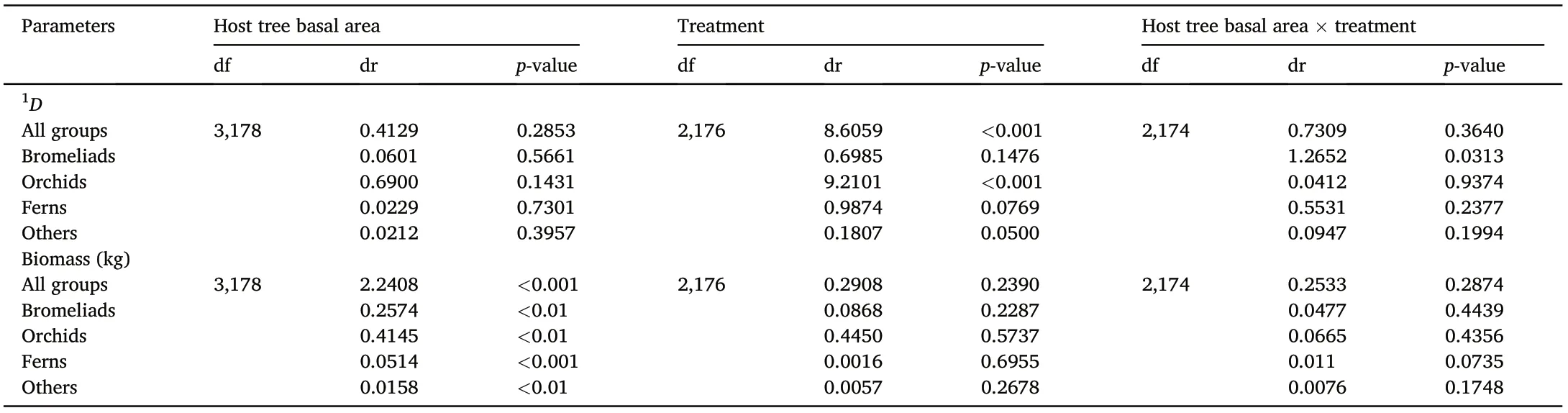
Table 6 Relationship between the alpha diversity(1D)and biomass(kg)of epiphytes and among host tree basal area(m2),silvicultural treatment,and the interaction between these two factors.
4.2. Beta diversity
A high level of similarity of epiphyte species among the three treatments was observed(ca.60%).However,this result contradicts a recent study by Guzm'an-Jacob et al. (2020) as well as other studies (e.g.,Hietz-Seifert et al., 1996; Barthlott et al., 2001; Benavides et al., 2006;Larrea and Werner, 2010) which have reported changes in composition of vascular epiphytes across land-use or habitat types. In the present study,between all pairs of treatments the composition of epiphytes was similar (67%–68%). This indicates that during the silvicultural interventions, many remnant trees - including oak species - have been spared(Martínez-Mel'endez et al.,2021),which helps to conserve a high percentage of the “original” epiphyte species. This result may be attributed to the large proportion of bromeliad species shared among the three treatments. Bromeliads have been reported to be highly capable of adapting to anthropogenic disturbance and dry climates(Benzing,2000;Wolf, 2005; Kr¨omer et al., 2014; Amici et al., 2019). Similarly, abundance of xeromorphic or atmospheric bromeliads and absence of shade-tolerant hygrophilous ferns have been observed in disturbed habitats of eastern Mexico(Kr¨omer et al.,2014),although some species of Polypodiaceae are able to withstand harsh microclimates (Hietz and Briones, 1998).
The proportion of species of orchids and bromeliads shared among the three treatments was high(70%–90%),while 30%–70%of species of the other two groups were shared. This indicates that in our study site,several species at risk remain following silvicultural treatments,although long-term monitoring throughout the management rotation would be necessary to predict the fate of these threatened species.
The species exclusive to each treatment suggest that they are adapted to the current arboreal structure and composition, which in SF has allowed for persistence of 14 epiphyte species that were not recorded in RC or TH.This suggests that modifications to stand structure as a result of silvicultural treatments in the managed sites did not favor permanence of these epiphytes (Larrea and Werner, 2010). Most of these exclusive species were orchids,reflecting the fact that this group is highly adapted to zones with open canopies and strong light intensity originated by anthropic disturbance(Kr¨omer et al.,2014;Hern'andez-P'erez and Solano,2015). Future studies in more advanced successional stages of the intervened sites would help to determine whether changes in forest structure would favor establishment and permanence of these epiphyte species.
4.3. Epiphyte biomass

Bromeliads presented the greatest total biomass (comparable to studies by Wolf et al.,2009 and Díaz et al.,2010),which was 50%greater than that of orchids.Similar trends have been recorded in other montane pine-oak forests of Chiapas (Wolf and Flamenco-Sandoval, 2006). The high values of biomass of bromeliads in the present study are attributed to the high abundance of T.seleriana in the three treatments.
Total biomass of epiphytes varied among treatments and was greatest in SF.In a study of managed forests of southern Mexico(Jim'enez-Bautista et al.,2014),greater abundance of epiphytes has been found in conserved sites than in silvicultural treatments; although no biomass data are reported. In the present study, biomass of bromeliads, orchids, and ferns did not vary significantly among treatments but biomass tended to increase from the most (RC) to the least (SF) disturbed conditions. It has been found that conversion of the forests to landscapes with anthropogenic activities, such as timber harvesting, reduces richness of epiphyte species, limits seed sources, and modifies structural parameters such as amount of biomass of the most diverse epiphyte groups(Flores-Palacios and García-Franco,2004,2008;Cascante-Marín et al.,2009).This is due to direct removal of host trees and consequent changes in light and temperature regimes upon transformation of the habitat(Jim'enez-Bautista et al., 2014; Hern'andez-P'erez and Solano, 2015). On the other hand, the group “others” contributed 3.7% of total epiphyte biomass, although the quantity of this biomass varied significantly among treatments, being highest in SF and RC. We consider that the presence of the large-sized Peperomia obtusifolia contributed to this result in both forest management conditions.
Changes in dominance of some species can be a useful measure of anthropic disturbance(Kr¨omer et al.,2014).Our results indicate that the effects of management are evident in the dominance of epiphyte species upon comparing forest conditions. Tillandsia seleriana and Camaridium densum were the most dominant in terms of biomass in the three forest conditions, but the biomass of co-dominant species was less uniform among treatments. The dominance of T. seleriana is probably due to its atmospheric habit usually related to the presence of CAM photosynthesis that provides C3species with greater tolerance to drought (Benzing,2000;Crayn et al.,2015).
In all treatments,orchids were less abundant than bromeliads.Some authors hold that orchids are susceptible to disturbance (Turner et al.,1994; Barthlott et al., 2001; Kr¨omer et al., 2014). We found that Camaridium densum was the second most dominant species in all three forest conditions studied,and Maxillariella elatior was co-dominant in RC and TH. The drought resistance observed in some epiphytic orchids is partly due to their CAM metabolism associated with succulence and development of bulbs or pseudobulbs. The succulent leaves and numerous pseudobulbs that form large colonies in C. densum allow the species to store water and persist in dry environments for large periods(Zotz,2016),a condition that prevails in oak forests of the Los Ocotones,regardless of the silvicultural treatment. Furthermore, C. densum is characterized by its long-extended rhizomes with numerous pseudobulbs.The other 35 orchid species recorded were smaller than C.densum but far less abundant, and therefore their contribution to biomass was generally less than that of bromeliads. Exceptions were Elleanthus capitatus, Epidendrum clowesii, Brassia verrucosa, C. densum, M. elatior, and Nemaconia striata,which can reach 40–130 cm depending on the species but were not present in all treatments; for example, E. capitatus was recorded only in TH,and E.clowesii and B.verrucosa only in SF(Table S1).
Tillandsia festucoides and C.occulta were co-dominant species,while it has been reported that T.festucoides has a low capacity for colonization in the presence of other Tillandsia species(Einzmann and Zotz,2017).The co-dominance of T. festucoides in all treatments could be related to its production of anthocyanins, which gives it the reddish foliar coloration observed in the field that allows it to tolerate and filter solar radiation-a physiological adaptation present in Bromeliaceae (Benzing, 2000).Meanwhile,the presence of C. occulta showed an interesting contrast in RC and SF;it was more abundant in SF,but its capacity to dominate in RC reflects physiological characteristics of the genus that allow it to adapt to adverse conditions. The genus Catopsis owes its success to its heliophile habit, with white or whitish pruinose rosettes(Palací et al.,2004). This allows it to tolerate exposure to the sun,since the plants inhabit the more exposed parts of the tree crowns (N. Martínez-Mel'endez, pers. obs.).However,it does not mean that they will be more drought tolerant.They may take advantage of their position in the tree to capture rainwater,favoring their persistence and drought resistance(Graham and Andrade,2004), also due to their tank-type habit. Meanwhile, T. filifolia was less abundant,but its atmospheric habit-thin cuticle and epidermis covered in absorbent trichomes-allows it to capture atmospheric moisture in the form of mist and dew.Furthermore,its small foliar area and volume help to reduce water loss(Benzing,2000).
Epiphyte biomass per host tree species varied between TH and SF.The highest mean biomass of epiphytes per host tree was recorded on Q.peduncularis(0.34 kg per host tree)in RC,although it had a relatively low number of individuals.This species of oak with dense branching,and fissured bark(Romero et al.,2015)allows for establishment of epiphyte seeds and roots (N. Martínez-Mel'endez, pers. Obs.). According to field observations, Q. glaucescens does not present structural characteristics(e.g. the presence of horizontal thick branches, and rough bark) that would make it a good host tree.Indeed,it was one of the species with the least epiphyte biomass in this study although this host species was the most abundant in RC.Nevertheless,the environmental conditions could have determined that epiphyte biomass be greater in SF than in RC,which has a more open canopy.
4.4. Relationship between vascular epiphyte diversity, biomass, treatment and host tree size
Diversity(1D)of epiphytes was related more to the treatment than to host tree basal area, with the greatest diversity in SF.1D was related to treatment only in orchids,and this group is strongly affected by anthropic disturbances(Kr¨omer et al.,2014).While micro-environmental changes among treatments were not measured in this study,there is evidence that canopy openness contributes to increasing air temperatures in forests under management, while closed-canopy maintains lower temperatures in unmanaged areas (Ehbrecht et al., 2019); indeed, some reports indicate an increase of approximately 1°C and reduction in air humidity in disturbed montane forests as compared to undisturbed forest (Ramírez-Marcial et al., 2001; Carvajal-Hern'andez et al., 2017). In the present study, bromeliad diversity was related to the interaction between host tree basal area and the treatment.In this manner,the response depends on the combination of the levels of both factors.This can be explained by the fact that while host tree basal area does not vary among treatments,the proportion of most abundant trees per species does vary(e.g.,almost 50% of trees were Q. glaucescens in RC, 40% Q. peduncularis in TH, and 35% Q. sapotifolia in SF), which may determine the number of species they host.
While biomass per epiphyte group was unrelated to treatment,it was related to host tree basal area. Tree size has been found to be an important predictor of epiphyte biomass (Taylor and Burns, 2015;Wagner et al., 2015). It is known that some host tree traits (e.g., characteristics of the bark, shape and arrangement of branches) may favor colonization and growth of epiphytes(Wagner et al.,2015;Wagner and Zotz, 2020). The characteristics of Q. peduncularis - such as its dense branching and fissured bark (N. Martínez-Mel'endez, pers. obs.) - may favor a greater biomass of epiphytes in some tree individuals,but this has yet to be explored through host specificity studies.
Finally,although this study found epiphyte diversity to be related to treatment type and epiphyte biomass to tree size,there is a need for longterm studies that evaluate changes in structural attributes of epiphytes to better comprehend the effects of forestry management on epiphyte communities. Since this study was conducted shortly following management prescriptions for RC and TH, it would be wise to monitor subsequent effects as well as whether the epiphyte community persists,together with possible changes in the epiphyte biomass. This would contribute to determining to what extend forestry use may be integrated with biodiversity conservation.
5. Conclusions
This study demonstrates that forest management influences the diversity of vascular epiphytes. Composition and abundance of epiphyte species varies among treatments. The presence of threatened or endangered bromeliad species in the different treatments suggests that despite disturbance, certain conditions are maintained which allow for persistence of these species. The presence of remnant oak trees left standing following silvicultural interventions contributes to establishment and development of epiphyte communities and should be fomented in the sites both with and without forestry intervention in this pine-oak forest.Forest management through SDM is fulfilling its objectives,contributing to reconciling production, regeneration, and conservation of a considerable number of epiphyte species.
Author contributions
NMM, NRM, JGGF, MJCP, and PMZ designed the study; NMM conducted the fieldwork; NMM, NRM, JGGF,MJCP, and PMZ analyzed the results;NMM and NRM wrote the first draft of the article;and all authors contributed equally to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study received research funding from the Rufford Foundation(Grant No.25259–1)and from federal funds allotted to ECOSUR(NRM).
Availability of data and materials
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Consejo Nacional de Ciencia y Tecnología(CONACYT)for the doctoral scholarship awarded to NMM(No.341343/618822) and the Rufford Foundation (Grant 25259-1). We thank V.G'omez and A.G'omez for their permission to conduct the fieldwork in the Los Ocotones forest, and A. Espejo (bromeliads), C. R. Beutelspacher(orchids), and S. Valencia (Quercus) for their help with taxonomic determination.We thank J.R.Ramos Moreno,J.Jim'enez Guill'en and J.G'omez Gir'on for guiding the field visits and M. F. Cruz Jim'enez, V. D.Castillo Amaya,J.W.L'opez Santiago,A.L'opez Cruz,V.García Mendoza,L. E. S'anchez Cuesta, S. M. Isidro, R. I. P'erez L'opez, E. A. Montoya Cabrera,A.Osorio Gonz'alez,L.F.Gonz'alez Martínez,E.Reyes Grajales,and E.G'omez P'erez for assistance with fieldwork.Finally,our thanks to D.A.Jim'enez-L'opez for designing the location map,K.MacMillan and A.Greenberg for English translation and review of style, respectively, and two anonymous reviewers for their comments that helped improve the manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.fecs.2022.100034.
- Forest Ecosystems的其它文章
- Vegetation structure and edaphic factors in veredas reflect different conservation status in these threatened areas
- Black locust coppice stands homogenize soil diazotrophic communities by reducing soil net nitrogen mineralization
- Responses of soil CH4 fluxes to nitrogen addition in two tropical montane rainforests in southern China
- Novel evidence from Taxus fuana forests for niche-neutral process assembling community
- Using machine learning algorithms to estimate stand volume growth of Larix and Quercus forests based on national-scale Forest Inventory data in China
- Effects of fertilization on the growth dominance of Inland Northwest forests of the United States

