Responses of soil CH4 fluxes to nitrogen addition in two tropical montane rainforests in southern China
Fantao Wu, Chanhui Pn,c,*, Chuanyao Wan, Huai Chn, Wiuo Liu,Zhihao Liu, Hui Wan, Hon Li, Dxian Chn, Yi Li, Shiron Liu
a Center for Ecological Forecasting and Global Change, College of Forestry, Northwest Agriculture and Forestry University, Yangling, 712100, China
b State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau, College of Forestry, Northwest Agriculture and Forestry University, Yangling, 712100,China
c Department of Biology Sciences, Institute of Environment Sciences, University of Quebec at Montreal, C.P. 8888, Succ. Centre-Ville, Montreal, QC, H3C 3P8, Canada d Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, 610041, China
e School of Architecture and Urban Planning, Chongqing University, Chongqing, 400044, China
f Jianfengling National Key Field Observation and Research Station for Forest Ecosystem,Research Institute of Tropical Forestry,Chinese Academy of Forestry,Guangzhou,510520, China
g Research Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry, Beijing, 100091, China
Keywords:Atmospheric nitrogen deposition Greenhouse gases Soil CH4 flux Tropical rainforest
ABSTRACT
1. Introduction
The increase of greenhouse gas (GHG) concentrations in the atmosphere is the main driver of global climate change.Methane(CH4)is one of the most important GHGs with a greenhouse effect 28-fold higher than that of carbon dioxide(CO2)within a 100-year timeframe(IPCC,2013),contributing approximately 20% to the observed global warming(induced by long-lived GHGs) since the pre-industrial period (Kirschke et al., 2013). Forest ecosystems cover 30% of the Earth's land area(McLauchlan et al., 2017) and have a substantial effect on global CH4cycles and budgets (Covey and Megonigal, 2019; Feng et al., 2020). In particular, forest soils are primary sinks of atmospheric CH4(Yu et al.,2017; Feng et al., 2020) and have been the focus of research on CH4consumption by soils in recent years(Jones et al.,2016;Ni and Groffman,2018;Yu et al.,2021).
The CH4exchange between soils and the atmosphere is the result of the interplay of several physiochemical and biological processes in the soil (e.g., gas diffusion and microbial activities involved in CH4metabolism)(Bradford et al.,2001;Smith et al.,2018;Tveit et al.,2019).Soil CH4fluxes are closely associated with two groups of microorganisms(i.e.,methanogens and methanotrophs)(Teh et al.,2005;Aronson et al.,2013; Feng et al., 2020). In soils, CH4is produced by anaerobic methanogens and consumed by aerobic methanotrophs (Le Mer and Roger,2001;Rowings et al.,2012).The different environmental requirements of these two microorganisms, especially in terms of pH, soil temperature,soil moisture, nutrient availability, and gas diffusion, determine the balance of methanogenic and methanotrophic activities in the soil, and thus the net CH4flux of a given soil (Aronson and Helliker, 2010; Luo et al.,2013).
Many studies have reported that changes in soil conditions (e.g.,climate change,nitrogen(N)status,and forest management)may change the balance between the production and consumption pathways of CH4in forest soils, turning them into larger sinks or even sources of CH4(Zhang et al., 2008; Blankinship et al., 2010; Krause et al., 2013). In particular,in the context of the rapid increase of atmospheric N deposition,the impacts of N deposition on soil CH4flux and thus the global CH4cycle have attracted increasing scientific attention (Jang et al., 2011;Peng et al.,2019; Song et al.,2020;Li et al.,2021).
On a global basis, anthropogenic atmospheric N deposition onto terrestrial ecosystems has increased by more than three-fold since the beginning of the industrial revolution(Galloway et al.,2004).Nitrogen is one of the most important nutrients for most forest ecosystems (Matson et al.,2002;Liu et al.,2012). The increase of N inputs will have a great impact on ecosystem processes, including increasing plant productivity(Lu et al., 2011), changing ecosystem carbon (C) and N pools (Matsushima and Chang,2007;Gu et al.,2015),and affecting the exchange of soil GHGs from forests(Krause et al.,2013;Geng et al.,2017;Fan et al.,2020).
A number of studies have found that the addition of inorganic N can repress CH4uptake capacity in forest soils (Wang and Ineson, 2003;Jassal et al., 2011; Kim et al., 2012), possibly because higher N concentrations in the soil inhibit the activities of methanotrophs and affect enzymes involved in CH4oxidation (Gulledge et al., 2004; Jang et al.,2011;Aronson et al.,2013).However,other studies have reported that N addition had no impact on soil CH4uptake(Li et al.,2015),or sometimes even promoted CH4uptake (Xu et al., 2014). These inconsistent results have been attributed to soil properties (Zhang et al., 2008; Jang et al.,2011; Jones et al., 2016), forest type (Zhang et al., 2012), and the type and level of the N additions(Yang et al.,2017b).Importantly,most of the aforementioned studies were carried out in subtropical forests and temperate forests,and the information on the response of soil CH4fluxes to N additions in tropical forests is very limited. To improve the assessment of the global CH4budget and its future predictions, more detailed investigations about the effects of N addition on soil CH4fluxes in tropical forests are urgently needed.
Tropical forests soils are generally considered important sinks for atmospheric CH4(Kiese et al.,2003;Liu et al.,2019;Zhao et al.,2019),playing a vital role in regulating the Earth’s climate. In this work, a primary tropical montane rainforest (PTMR) and a secondary tropical montane rainforest (STMR) within the Jianfengling National Natural Reserve in southern China were used to study the responses of soil CH4flux to N addition in tropical forests.Due to increased human activity,the reserve is facing an increasing rate of N deposition.After about 4 years of simulated N deposition (Du et al., 2013), which is generally considered enough time to alter the microbial community and N cycle for tropical forests(Cusack et al.,2016),we measured soil CH4fluxes using the static chamber-gas chromatography method (Blankinship et al., 2010; Krause et al.,2013).The main objectives of this work were to(1)quantify CH4fluxes at the PTMR and STMR sites,and(2)better understand the impacts of N additions on CH4fluxes within two tropical montane rainforest types in southern China.
2. Materials and methods
2.1. Study area and experimental sites
Two tropical montane rainforest sites (PTMR and STMR) in the Jianfengling National Natural Reserve (18°23′–18°50′N,108°36′–109°05′E) were selected to conduct the CH4flux measurements.The 470 km2reserve has a tropical monsoon climate,with a mean annual rainfall of 2,449 mm, of which about 80%–90% falls in the wet season (May–October) and 10%–20% in the dry season (November–April) (Chen et al., 2010). The mean annual temperature of the reserve is 19.8°C, ranging from 14.8°C (January) to 23.3°C (June)(Yang et al.,2017a).Mean annual relative humidity is 88%(Chen et al.,2010). At the PTMR site, the forest has never been disturbed by human activities and mainly consists of long-lived tree species such as Livistona saribus, Lithocarpus fenzelianus, and Castanopsis patelliformis, etc (Tang et al.,2018).At the STMR site,the forest has developed naturally after a clear-cut in the 1960–1970s, and mainly includes Alniphyllum fortunei,Castanopsis tonkinensis, and Schefflera octophylla (Xu et al., 2009; Zhou,2013;Tang et al.,2018).The soils are well-drained lateritic yellow soils(Wang et al.,2021),with a porosity of 56%at the PTMR and 50%at the STMR sites.Detailed characteristics of the two forest sites are presented in Table 1.Total inorganic N deposition via rainfall for the reserve is 6.7 kg N⋅ha-1⋅year-1(1:1 NO3-/NH4+ratio)(Wang et al.,2021).
2.2. Experimental treatments
The simulated N deposition experiment was conducted in September 2010 at the PTMR and STMR sites.There were four N addition treatments of 0(N0),25(N25),50(N50),and 100(N100)kg N⋅ha-1⋅year-1,which represented a control(without N addition),a low N addition,a medium N addition,and a high N addition treatment,respectively(Du et al.,2013;Zhou,2013;Tang et al.,2018).NH4NO3was used as the N source in this experiment. For each treatment, we dissolved the NH4NO3fertilizer in 100 L water and sprayed it onto the soil surface monthly (Zhou, 2013).The N0 treatment received 100 L of water without any NH4NO3fertilizer added. Further details have been described by Zhou (2013) and Tang et al. (2018). In January 2015, twelve (3 replicates × 4 treatments)scattered plots (10 m × 10 m) at about 10 m intervals were randomly established within each forest.During the CH4flux measurement periodof this study(January 2015 to December 2018),the monthly N addition amount and method were the same as those described above.

Table 1 General information for the experimental tropical rainforests. PTMR: primary tropical montane rainforest; STMR:secondary tropical montane rainforest.
2.3. Field measurements
The soil CH4flux was sampled using a static chamber and analyzed using gas chromatography. Gas samples were collected twice a month and adjusted in case of extreme weather.At each sampling plot,a static chamber was established at the beginning of the experiment. The chamber consisted of a removable cover (without bottom, 20 cm diameter×40 cm height)and a fixed base frame(permanently anchored 7 cm into the soil) (Bai et al., 2014; Wu et al., 2021). During sampling, the removable cover was inserted into the fixed frame.For each plot,four gas samples were collected with vacuum tubes(10 mL)at 0,10,20,and 30 min following closing the chamber. The air temperature inside the chamber was recorded during sampling using a mercury thermometer.A gas chromatograph was used to analyze the concentration of CH4in the samples. CH4flux was calculated from linear regression of the gas concentrations against time according to Kim et al. (2012).
Soil temperature and moisture at a 5-cm depth were measured near each chamber during gas sampling using a digital thermometer(Saiyasi,Dandong, China) and a moisture probe meter (Delta-T Devices, Cambridge,UK),respectively.
We collected soil samples(0–10 cm)from three separate soil cores in each plot in June and December of 2016,2017,and 2018.The three soil cores within each plot were pooled into one sample. Afterward, the samples were passed through a mesh sieve to remove litter and roots,and then maintained at 4°C until processing.Soil ammonium-N(NH4+-N)and nitrate-N (NO3--N) were extracted with 2 M KCl and analyzed using a continuous-flow analyzer(Seal Analytical,Norderstedt,Germany).
2.4. Statistical analysis
Data were tested for normality and homogeneity using Kolmogorov-Smirnov’s test and Levene’s test,respectively(Yu et al.,2021).One-way ANOVA (analysis of variance) with LSD (least significant differences)tests were used to determine the effects of N treatments on soil CH4flux,NH4+-N,NO3--N,soil temperature and moisture.Linear regression models were applied to probe the relationships between CH4flux and measured soil variables. All statistical analyses were conducted with SPSS v. 20.0 software (IBM Co., Armonk, USA) and significant differences were determined at P <0.05.All figures were drawn using OriginPro v.2019b software(Origin Lab,Northampton, USA).
3. Results
3.1. Soil environmental conditions
Soil temperature and moisture at a 5-cm depth showed obvious seasonal variations in the N0 plots for the two forests during the observation period(Fig.1a and b).At the PTMR site,the mean soil temperature in the N0 plots was 20.0°C,ranging from 11.3°C in January to 24.3°C in June.The mean soil moisture in the N0 plots was 27.3%,ranging from 20.0%in March to 35.5%in August.At the STMR site,the soil temperature in the N0 plots varied between 14.9°C and 24.4°C,with a mean value of 20.7°C.The seasonality of soil moisture was similar to the PTMR site,ranging from 19.6%in March to 39.5%in August,with a mean value of 28.4%.Soil temperature and moisture had no significant variations among N treatments in the two forests by one-way ANOVA (Table 2). Compared with the control,N additions tended to increase the concentrations of soil NH4+-N and NO3--N in both forests,but the effect was not significant(all P>0.05;Fig.2).
3.2. Soil CH4 fluxes
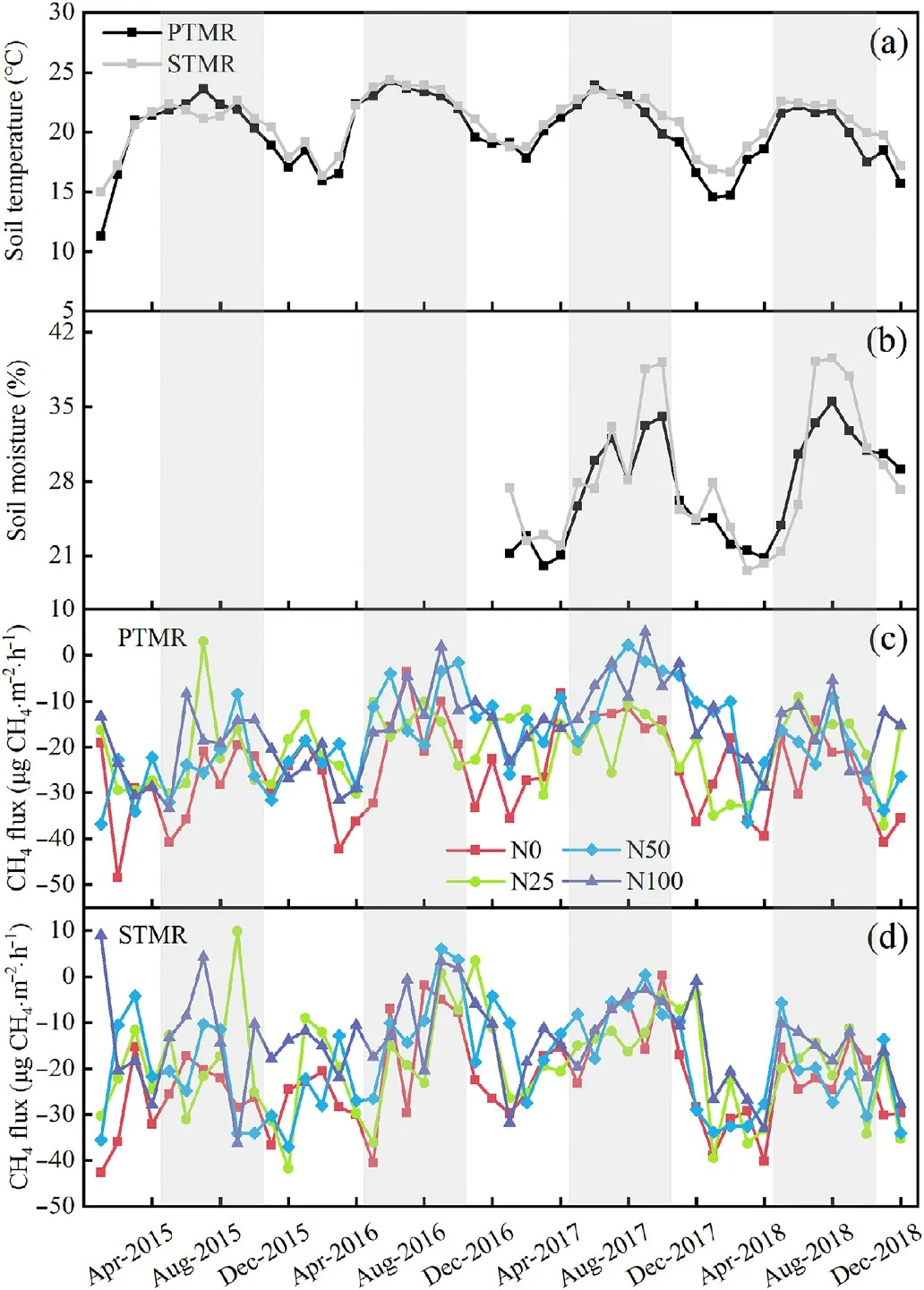
Fig. 1. Monthly dynamics of soil temperature (a) and soil moisture (b) under the N0 treatment in the primary tropical montane rainforest (PTMR) and secondary tropical montane rainforest (STMR) within the Jianfengling National Natural Reserve. Monthly dynamics of soil CH4 fluxes under four nitrogen (N)treatments in the PTMR(c)and STMR(d)during the study period.Shaded area denotes the wet seasons (May to October). From January 2015 to December 2016,soil moisture was not recorded.N0,N25,N50,and N100 represent 0,25,50, and 100 kg N⋅ha-1⋅year-1 N addition treatments, respectively.

Table 2 Mean values (± standard errors) of soil temperature and moisture for each treatment at the PTMR and STMR sites during the study period. There was no treatment effect on soil temperature and soil moisture in either forest during the study period.
Fluxes of CH4showed clear seasonal variation in both forest types,with relatively high rates of soil CH4uptake during the dry season and low rates of soil CH4uptake during the wet season for all treatment plots at the PTMR(Fig.1c)and STMR sites(Fig.1d).Both forest soils were net sinks of atmospheric CH4regardless of the N treatments. At the PTMR site, CH4fluxes in the N0 plots ranged from -48.51 to -3.72 μg CH4⋅m-2⋅h-1, with a mean of -25.11 μg CH4⋅m-2⋅h-1(-2.20 kg CH4⋅ha-1⋅year-1)(Fig.1c;Table 3).At the STMR site,CH4fluxes in the N0 plots had a minimum rate of-42.61 and a maximum rate of 0.24 μg CH4⋅m-2⋅h-1, with a mean of -22.55 μg CH4⋅m-2⋅h-1(-1.98 kg CH4⋅ha-1⋅year-1)(Fig.1d;Table 3).The overall net CH4uptake in the N0 plots was a little higher at the PTMR site than at the STMR site.

Fig. 2. Soil NH4+-N(a)and NO3--N(b)concentrations under different N treatments in the PTMR and STMR.No significant variations were found for soil NH4+-N and NO3--N among the four N treatments by one-way ANOVA.Error bars represent standard errors of the means.Data were collected in June and December of 2016,2017,and 2018.
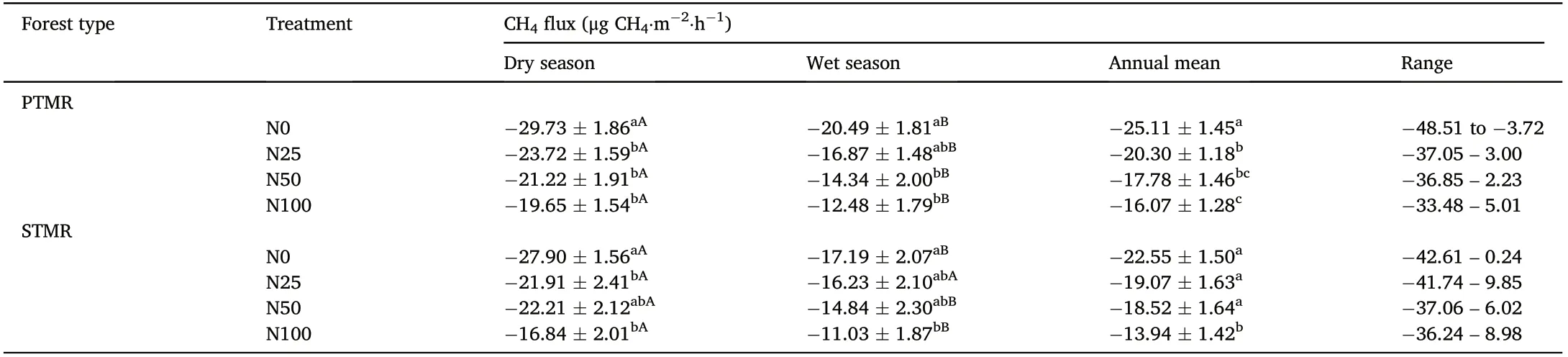
Table 3 Effects of N treatments on the mean soil CH4 fluxes from January 2015 to December 2018 at the PTMR and STMR sites,China.
Our data showed that the impacts of N treatments on soil CH4uptake varied depending on forest type and N addition level (Table 3). At the PTMR site, the mean annual CH4uptake in the N25, N50, and N100 treatments was significantly reduced by 19%, 29%, and 36% (with a mean of 28%), respectively, when compared with the N0 treatment.Under the same level of N addition,the mean annual CH4uptake at the STMR site was reduced by 15%, 18%, and 38% (with a mean of 24%),respectively,relative to N0 treatment.Notably,with the exception of the N100 treatment, the other N treatments had insignificant effects on the mean annual CH4uptake at the STMR site (Table 3). Nitrogen addition also showed an inhibitory effect on soil CH4uptake in both the dry and wet seasons at both forest sites(Table 3).
3.3. Relationship between soil CH4 flux and related environmental variables
The relationships between soil CH4flux and soil temperature were characterized with a simple linear regression model at both forest sites(Fig. 3a and c). A weak but significant relationship was found between soil CH4flux and soil moisture at both forest sites (Fig. 3b and d). Soil CH4flux was positively related to soil NH4+-N concentration (i.e., a negative relationship between CH4uptake rates and NH4+-N concentration) at both forest sites (all P <0.05; Fig. 4a and c). At the PTMR site, soil CH4flux was positively related to the soil NO3--N concentration(P <0.05;Fig.4b),but no such relationship was found at the STMR site(P >0.05;Fig.4d).
4. Discussion
4.1. Annual CH4 fluxes
This study revealed that tropical montane rainforest soil can act as an atmospheric CH4sink.The mean annual soil CH4uptake in the N0 plots of the PTMR and STMR sites was-2.20 and-1.98 kg CH4⋅ha-1⋅year-1,respectively, which was very close to the flux observed by Zhao et al.(2019) in an adjacent lowland tropical forest in Diaoluoshan, Hainan(-2.67 kg CH4⋅ha-1⋅year-1). Our values were comparable with those reported from other tropical forest ecosystems in southern China(ranging from-5.47 to -0.67 kg CH4⋅ha-1⋅year-1;Table 4). According to the review by Liu et al. (2019) dealing with CH4flux from global tropical forest soils, the annual average CH4flux ranges from -40.5 to 0.07 kg CH4⋅ha-1⋅year-1, and the average uptake rate is -4.08 kg CH4⋅ha-1⋅year-1.The rates of CH4uptake in this study were also within this reported range, but clearly below the average value reported for tropical forest soils.

Fig. 3. Relationships between soil CH4 fluxes and soil temperature and moisture in the PTMR (a, b) and STMR (c, d).

Fig. 4. Relationships between soil CH4 fluxes and concentrations of soil NH4+-N and NO3--N in the PTMR (a, b) and STMR (c, d).

Table 4 Studies on the soil CH4 fluxes from topical forests in southern China.
Reported data on soil CH4fluxes in tropical forests show a considerable variation in the literatures. Methane net fluxes are the balance of consumption and production of CH4by both methanotrophs and methanogens (Aronson and Helliker, 2010), which are influenced by some factors including vegetation,climate,soil properties,N availability and microbial activity (Rowlings et al., 2012; Luo et al., 2013; Li et al.,2021).In our observations,soil CH4uptake in the N0 treatment tended to be higher at the PTMR site than at the STMR site(Table 3).The difference in forest succession stages and soil properties could be responsible for the differences in soil CH4flux between the two forest sites. Previous research has indicated that soil CH4uptake rates from primary or old-growth forests are similar to, or greater than, those of secondary or young-growth forests (Hudgens and Yavitt, 1997; Verchot et al., 2000;Zhang et al.,2008).As noted,at our PTMR site,the trees were more than 300 years old,while at the STMR site,the trees were mainly young trees,less than 50 years old (Table 1). In our experiment, the measured soil NH4+-N and NO3--N concentrations in the N0 plots were greater at the STMR site when compared to the PTMR site (Fig. 2). Greater soil N-species contents have been shown to have an inhibitory effect on soil CH4uptake(Wang and Ineson,2003;Zhang et al.,2012).Moreover,the relatively high soil aeration capacity at the PTMR site may facilitate the diffusion of O2and CH4into the soil(Table 1),leading to increased CH4oxidation activity(Werner et al.,2007;Barrena et al.,2013).
4.2. Effects of soil temperature and moisture on CH4 fluxes
Soil CH4uptake rate in the two forests was negatively correlated with soil temperature, indicating that net CH4uptake is lower for higher soil temperatures. This result is consistent with previous findings (Jones et al.,2016;Yu et al.,2021).The decrease in net soil CH4uptake caused by the increase in temperature may be related to two factors:the increase in soil CH4production and the decrease in soil CH4oxidation(Le Mer and Roger,2001;Luo et al.,2013).Relatively high temperatures can not only increase the availability of methanogenic substrates (Teh et al., 2005),but also increase the activity of methanogens(Koh et al.,2009),resulting in more CH4production (Feng et al., 2021). Higher temperature may accelerate soil N mineralization(Karbin et al.,2015),resulting in higher NH4+content, which may inhibit soil CH4oxidation (Schnell and King,1994). Some studies have found that when the temperature exceeds a critical temperature value, soil CH4oxidation would become relatively independent, and may even weaken as the temperature rises (Castro et al.,1995;Fang et al.,2010).For example,in a subtropical forest,Wang et al. (2014) indicated that the ability of soil methanotrophs to oxidize CH4will decrease when soil temperature is higher than 15°C. In a tropical rainforest, Fang et al. (2010) found that when the soil temperature was higher than 15.67°C, the ability of forest soil to oxidize CH4gradually decreased.We deduced that the soil temperature may also have a negative effect on CH4oxidation activity in this study because most of the soil temperatures at our study sites were over 15°C (Fig.3a and c).
Soil moisture has been reported to play a vital role in regulating soil CH4flux.Soil moisture can control CH4flux by adjusting the gas diffusion of the soil for both atmospheric O2and CH4(Kiese et al.,2003;Liu et al.,2019).An increase in soil moisture can reduce net soil CH4uptake,as the increased soil moisture can reduce the movement of atmospheric O2into the soil,which may result in a decrease in CH4oxidation and an increase in methanogens (Teh et al., 2005; Rowlings et al., 2012). Higher soil moisture can also restrict CH4diffusion into the soil, resulting in substrate limitation for methanotrophs in the soil profile. In our study, we also found that soil CH4uptake rates in these two forests were negatively related with soil moisture (Fig. 3b and d), which agrees with previous studies from a tropical rainforest (Yan et al., 2008), a subtropical plantation forest (Wang et al., 2014) and a temperate mixed forest (Geng et al.,2017).Notably,the response of soil CH4uptake to soil moisture at our study sites was less pronounced than in the aforementioned studies.The influence of soil moisture on soil CH4uptake was also reflected by the seasonal dynamics of CH4fluxes,showing that the CH4uptake rates during the wet season were lower than during the dry season(Fig.1c and d).Similar findings have also been reported by Yan et al.(2008)and Yu et al. (2021). In addition, because the addition of N had no significant effect on soil moisture and temperature in the two forests(Table 2), we speculated that soil moisture and temperature may not be the key reasons for the inhibition of soil CH4uptake under different N addition treatment conditions in this study. Overall, our results suggested that the soil CH4sink strength may be declining at our study sites in the context of global climate warming and increased precipitation.
4.3. Effects of N addition on CH4 fluxes
Our data indicated that soil CH4flux was affected by NH4NO3fertilization. The soil CH4uptake in N-fertilized plots was always lower than that of the N0 plots at both forest sites(Fig.1c and d).The inhibitory impacts of N addition on CH4uptake in well-drained forest soils have been widely observed (Kim et al., 2012; Yang et al., 2017b; Li et al.,2021).Generally,the addition of N will increase the contents of soil NH4+and NO3-, thereby inhibiting the uptake of CH4by the soil (Wang and Ineson, 2003; Zhang et al., 2012). This pattern can be attributed to the following potential mechanisms:osmotic stress due to the high contents of NH4+and/or NO3-, toxic inhibition to methanotrophs by nitrite and hydroxylamine produced via NH4+oxidation,and the competition of NH4+with CH4at the reaction site for the monooxygenase enzyme of methanotrophic bacteria(Schnell and King,1994;Whalen,2000;Bodelier and Laanbroek, 2004; Geng et al., 2017). At both sites, we observed the lowest soil CH4uptake in the plots(N100 treatment)with the highest soil NH4+-N concentrations,and the highest soil CH4uptake in the plots(N0 treatment) with the lowest soil NH4+-N concentrations, although N addition did not significantly increase the soil NH4+-N concentration at these two forest sites. The significant and negative correlation between soil CH4uptake rate and NH4+-N concentration at the two sites (Fig. 4a and c) illustrated that NH4+-N availability can suppress the soil CH4uptake through the mechanisms mentioned above.
In contrast,the relationship between soil CH4uptake rates and NO3--N concentration in these two forests was inconsistent. The possible cause may be that the soil available NO3--N at the SMTR site did not meet the toxicity threshold of CH4-oxidizing microbes (Wang and Ineson, 2003).In addition, Bodelier and Laanbroek (2004) demonstrated that NO3--N exerted an inhibitive effect on soil CH4uptake only at very high concentrations,which may relate to a greater osmotic effect limiting methanotroph activity.
Numerous studies have shown that the performance of N addition on cumulative CH4flux depended on the level of N addition (Geng et al.,2017; Yang et al., 2017b; Deng et al., 2020). In this study, low to high levels of N addition decreased the four-year mean annual CH4uptake by 15%–38%. Compared with low levels, high levels of N addition had a stronger inhibitory effect on soil CH4uptake at both forest sites. Our finding here is consistent with many previous studies. For example,Zhang et al.(2008)showed for a tropical mature forest that the rates of CH4uptake decreased by 6%, 14%, and 32% when compared to the control for 50,100,and 150 kg N⋅ha-1⋅year-1plots,respectively.Studies undertaken by Yang et al. (2017b) in a temperate deciduous forest showed that the annual mean rates of CH4uptake were decreased by 20%and 41%in the Low-N (50 kg N⋅ha-1⋅year-1) and High-N plots(150 kg N⋅ha-1⋅year-1), respectively, when compared to the control plots (0 kg N⋅ha-1⋅year-1). There are two potential explanations that could help to understand why high levels N addition exerted stronger inhibitory impacts on soil CH4uptake.First,high levels of N addition could promote N to reach deeper soils and affect the CH4oxidation activity there, so the impact was stronger (Yang et al., 2017b; Zhao et al., 2019). Second,compared with low level N addition,high level N addition could produce a lower soil pH,which has been proved to be unfavorable to the soil CH4uptake (Benstead and King, 2001; Li et al., 2021). As expected, a study conducted at the same sites in the Jianfengling Reserve showed that as the level of N addition increased, the pH values of both the PTMR and STMR sites dropped rapidly (Tang et al., 2018). In summary, further investigations are necessary to confirm these possible mechanisms and to explore whether there is a critical threshold of N addition that can control the inhibitory impact of N addition on soil CH4uptake.
The inhibitory impact of N addition on soil CH4uptake at the STMR site was less than at the PTMR site, which was probably due to the difference in soil N content between the two forests(Fig.2).In three tropical forests, Zhang et al. (2008) discovered that the response of soil CH4uptake to N addition directly depends on the soil N status.As illustrated by Aronson and Helliker (2010), the historical N status of soils plays a crucial role in predicting the response of soil CH4flux to N addition or N deposition. It is remarkable that N additions can also change the litter layer, soil pH, and Al3+content, which were reported to play an important role in controlling the CH4exchange between the soil and atmosphere (Nanba and King, 2000; Benstead and King, 2001; Zhang et al., 2008; Fan et al., 2020). Therefore, more auxiliary data (e.g., soil pH,Al3+content)are needed to help us better resolve the mechanism of the impact of N additions on soil CH4flux.
However,the above discussion was mainly focused on the impacts of major environmental factors,while the microbial mechanisms of soil CH4flux response to N additions have not yet been addressed.Li et al.(2021)recently showed that the addition of N could change the diversity,abundance, and community structure of methanogens and methanotrophs,thereby altering net soil CH4fluxes.In the Jianfengling National Natural Reserve,the responses of soil methanogenic and methanotrophic communities to N additions should be the focus of future studies.
5. Conclusions
Soil CH4flux at both forest sites showed clear seasonal variations and patterns,with higher uptake rates often observed during the dry season and lower uptake rates during the wet season.Our findings suggest that the tropical montane rainforest soil of southern China acts as a substantial sink for atmospheric CH4. Nitrogen addition had an inhibitive effect on soil CH4uptake at both forest sites. For the PTMR site, all N additions (N25, N50, and N100 treatment) significantly inhibited soil CH4uptake. In contrast, at the STMR site, only the highest N addition(N100 treatment) significantly inhibited soil CH4uptake. Forest types and their corresponding soil properties played an important role in tropical soil CH4uptake and its response to N addition. Overall, N addition impacted the soil CH4flux,which implies that tropical rainforest soils can be sensitive to N deposition. To better understand the biogeochemical processes and mechanisms of the impacts of N deposition on soil CH4flux dynamics,further detailed investigations are needed.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors’contributions
CP and HC conceived the idea and designed the study. FW, CW, ZL,HW,DC and YL collected the data.FW,HL and SL processed the data and analyzed the results. FW, CP and WL contributed to the manuscript writing and editing.All authors gave final approval for publication.
Funding
The work was funded by the National Key R&D Program of China(No. 2016YFC0500203) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Many thanks are due to others for participation or helps in the data collection.
Abbreviations
- Forest Ecosystems的其它文章
- Vegetation structure and edaphic factors in veredas reflect different conservation status in these threatened areas
- Black locust coppice stands homogenize soil diazotrophic communities by reducing soil net nitrogen mineralization
- Importance of Quercus spp. for diversity and biomass of vascular epiphytes in a managed pine-oak forest in Southern Mexico
- Novel evidence from Taxus fuana forests for niche-neutral process assembling community
- Using machine learning algorithms to estimate stand volume growth of Larix and Quercus forests based on national-scale Forest Inventory data in China
- Effects of fertilization on the growth dominance of Inland Northwest forests of the United States

