Impact of ovariectomy and estradiol-17β (E2) replacement on the brain steroid levels of the Indian stinging cat fish Heteropneustes fossilis
Surbhi Mishr, Rdh Chube
aZoology and Fisheries Department, Dolphin (PG) College of Science and Agriculture, Chunni Kalan, Fatehgarh Sahib, Punjab, 140406, India
bDepartment of Zoology, Institute of Science, Banaras Hindu University, Varanasi, 221005, India
Keywords:
Cat fish
Neurosteroids
Ovariectomy
Estrogen replacement
Seasonal variations
A B S T R A C T
In the present study, effects of ovariectomy (Ovx) and estradiol-17β (E2) replacement on the brain steroid levels were investigated in the resting and prespawning phases of the female cat fish Heteropneustes fossilis. The study showed that Ovx resulted in significant decreases in plasma levels of estradiol-17β (E2) and its precursors, and the effect varied with the reproductive stage and duration. Our study showed that E2 supplementation reversed the effect of Ovx in 3-week Ovx fish group, either restored or increased according to the dose. In contrast, E2 levels increased in the brain after Ovx in the resting stage and produced a biphasic effect of significant decreases on week 1 and 2, and significant increases on week 3, 4 and 5 in the pre-spawning stage. The brain E2 levels significantly inhibited in E2 supplementation groups. The testosterone (T) levels showed biphasic effects, an initial decrease (up to 3 weeks Ovx) and an increase later (week 4, 5) in the resting stage. In the pre-spawning stage, the T levels increased significantly after Ovx. The E2 supplementation significantly inhibited the T levels,more severely in the low dose group in the resting and pre-spawning stages. Brain 17-hydroxyprogesterone(17-OHP4) levels increased (week 1, 2, 3) but decreased on week 4 and 5 of Ovx in the resting stage and increased throughout Ovx in the pre-spawning stage except in the 5th week (though compared to other steroids, level was very low; almost negligible). The E2 replacement significantly decreased the steroid levels further down compared to the Ovx control group. Brain progesterone (P4) and pregnenolone (P5) levels decreased initially(week 1 and 2 Ovx) and recovered later. The E2 replacement significantly decreased P4 levels in the Ovx fish in the pre-spawning stage and in the lower dose group in the resting stage. The P5 levels remained inhibited after the E2 supplementation in the resting stage but unchanged or increased it in the pre-spawning stage. Brain cortisol (F) levels were inhibited initially (1 and 2 weeks and later increased after OVX in the resting stage. In the pre-spawning stage, the F levels increased in the week 1, 2 and 3 of Ovx and decreased in the 4th and 5th weeks.The E2 supplementation inhibited the cortisol (F) levels in both phases. The results show that ovarian steroids in fluence neurosteroidogenesis in a reproductive stage-dependent manner.
1.Introduction
Brain is considered a source of steroids, in addition to the classical steroidogenic tissues. Steroids serve many important physiological functions in the brain like development, behaviour, cognition, neuromodulation, neuroplasticity, and neuroin flammation (Chaube & Mishra,2012; Fontaine et al., 2020; Mishra & Chaube, 2022; Rajakumar &Senthilkumaran, 2020). It has been reported that in addition to the peripheral steroids that enter the brain through circulation, steroids are alsode novosynthesized in the brain from cholesterol or from circulating different inactive precursors (Chaube & Mishra, 2012; Corpechot et al.,1981; Do Rego et al., 2009; Fontaine et al., 2020; Mishra & Chaube,2022; Taves et al., 2011) in the nervous system and have a wide variety of functions (Rupprecht & Holsboer, 1999; Okubo et al., 2019). Neurosteroids exert actions not only through classical nuclear steroid hormone receptors but also through plasma membrane receptors (Nadal et al., 2001; Okubo et al., 2019). The brain contains receptors for all five classes of steroid hormones, progestin, estrogen, androgen, glucocorticoids and mineralocorticoids, and they resemble those found in the non-neural target tissues (McEwen et al., 1982). Furthermore, steroids persist in the brain, but not in the circulation, long after gonadectomy or adrenalectomy (Caruso et al., 2010; Croft et al., 2008; Higo et al., 2011).
Previous research has revealed that E2plays critical roles in organizing and activating sexual differentiation, neural growth, differentiation and sex change in fish (Devlin & Nagahama, 2002; Chaube &Mishra, 2012). In teleosts, as in higher vertebrates, gonadal steroids exert feedback actions on the brain-pituitary (B–P) axis to modulate gonadotropin secretion. The steroid-binding cells are located largely in the ventral telencephalon and hypothalamus of the brain and in gonadotropes of the pituitary (Fontaine et al., 2020). Earlier in our laboratory, we used ovariectomy and E2replacement to study steroid-monoamine interaction at the level of rate limiting tyrosine hydroxylation, vasotocin secretion, Kisspeptin and feedback regulation of luteinizing hormone (LH) secretion in the cat fishH. fossilis(Chaube et al., 2020; Chaube & Joy, 2002; Mishra & Chaube, 2022; Senthilkumaran & Joy, 1994, 1995; Singh & Joy, 2009). Previous studies in mammals and other group of vertebrates have shown that ovariectomy decreased allopregnanolone (a neuroactive steroid), synthesized mainly in gonads, adrenal cortex and central nervous system (CNS), levels in serum and brain cortex, hypothalamus, hippocampus and pituitary(Genazzani et al., 2000), implying that gonadal steroids can modify neurosteroid synthesis, metabolism and actions.
Gonads and interrenals are, respectively, the primary sources of sex steroids and corticosteroids in teleosts (Rousseau et al., 2021). Surgical or chemical ablations of these peripheral glands result in a fall in the circulating titres of the steroids and elicit negative feedback effects on the brain. Therefore, it is interesting to know the behaviour of brain steroids; whether the levels decrease, increase or unchanged, which will provide valuable inputs on dynamics of neurosteroid synthesis and consequently feedback effects on brain functions. In the present study,our objective was to investigate effects of ovariectomy and E2replacement on steroid hormone (P5, P4, 17-OHP4, testosterone (T)), E2and cortisol (F) levels in brain and plasma female cat fishH. fossilis.
2.Material and methods
2.1.Animal procurement and acclimation
The research work was conducted according to the guidelines of The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Reg. No.1802/GO/RE/S/15/CPCSEA 25/2/2015),Department of Zoology, Banaras Hindu University. All care was taken to prevent cruelty of any kind. Female cat fishHeteropneustes fossilisweighing (40–50 g) were obtained from a local fish market during previtellogenic stage (resting, month: December, Length-21.5 ±2.2 cm)and late-vitellogenic (prespawning, month: May, Lenrth-25.6 ±3.12 cm) stages. These fishes were acclimatized in flow-through aquarium tanks (cement tanks of 0.35 × 0.35 × 0.45 m3of 55 L capacity with 30 L water filling) under ambient photoperiod and temperature (resting stage- 11.0 L: and 14.0 D, 19 ± 2 ℃; pre-spawning stage: 13.0L: 11.0D,29 ± 2 ℃) for two weeks to overcome stress due to transportation and were fed daily with minced goat liverad libitum.
2.2.Chemicals and reagents
Estradiol -17β (E2), 3-amino benzoic acid ethyl ester (MS222),propylene glycol were purchased from Sigma Chemical Company, St.Louis, USA. Estradiol-17β (FR E-2000), Testosterone (AA E-1300), 17-OH Progesterone (FR E-2800), Pregnenolone (FR E-2700), Progesterone (FR E-2500) and Cortisol (MS E-5000) ELISA kits were purchased from Labor Diagnostika Nord GmbH & Co.KG, Germany. Degassed and filtered nanopure water (Barnstead International, Dubuque, and IO,USA) and HPLC grade methanol and Diethyl ether (SRL), Mumbai, India was used in the ELISA assays.
2.3.Experimental design
2.3.1.Ovariectomy
Acclimatized 120 fish were ovariectomized or sham ovariectomized in resting (between 10 and 15 day of December) and prespawning(10–15 day of May) stages. The fish were anaesthetized by spraying MS222 (100 mg/250 mL distilled water) over the gills. The details of Ovx and E2 replacement were described previously (Chaube et al., 2019 for details). In each group (5–10 fishes) were kept. Further, fish were treated with benzathine penicillin (16000 IU/l) for 3–5 days to prevent any kind of skin infection. The details were described earlier (Senthilkumaran & Joy, 1994). The operated fish were maintained for 1–5 weeks. Mortality was negligible (<4%; N =2–4 of total). The peritoneal cavity of the fish was checked at the time of sampling to check any regrowth of the ovary (<4%; N =2–4 of total) in ovariectomized fish.Tissues from completely ovariectomized fish (N =5 from each group)were used for steroid hormone assay.
2.3.2.Estradiol - 17β treatment
In order to perform estradiol-17β replacement experiment, 3- week ovariectomized fish in both resting and pre-spawning stages were selected and intraperitoneally (i.p) injected with E2in dosages of 0.5 or 1.0 μg/g body weight (BW), as a single dose (5 fish each). Five ovariectomized and sham ovariectomized fish were given an equal volume of vehicle propylene glycol (0.1 mL; PG) as controls. After 24 h, all fishes were sampled (10.30 a.m.–11.30 a.m.). In heparinised vials blood was collected from a caudal vein and were kept at 4 ℃ for separation of plasma. For plasma extraction, it was centrifuged at 2800 g for 15 min(4 ℃) and plasma was collected in separate tubes and stored at - 70 ℃ until processed for further assay. After this same group of fishes were sacrificed by decapitation and brains along with pituitaries were removed for steroid extraction and estimation.
2.3.3.Steroid hormone extraction and estimation
The brain tissues were homogenized separately or group-wise in 4 vol of cold PBS (0.02M, phosphate buffer saline, pH 7.4) with an ultrasonic homogenizer (XL-2000 Microson, Misomix, USA) at 0 ℃ for 5–10 s. The extraction procedure was as described in (Chaube et al.,2020). Both tissue samples and plasma were evaporated, dried under nitrogen until processed for estimation of steroid hormones using the ELISA kits according to the manufacturer’s protocol. Readings were taken at 450 nm using a Multiscan microplate reader (Thermo Electron Corporation, USA) ELISA reader. These protocols are already standardized in our laboratory by using specific ELISA kits (Chaube & Mishra, 2012; Mishra & Chaube, 2021, 2022).
2.3.4.Hormone assay (P5, P4,17-OH P4, T, E2, and F)assay
P5, P4,17-OH P4,was assayed by an ELISA kit according to the manufacturer’s protocol (Mishra & Chaube, 2021). For assay of T and E2 ELISA Kit and standardized procedure was followed as per instructions provided in (Chaube et al., 2020) and Cortisol (F) was assayed by an ELISA kit, according to the manufacturer’s protocol (Singh & Chaube,2019). The response–concentration curves for P5, P4, 17-OH-P4, T, E2,and F were linear over 0.1–25.6 ng/mL,0.1–60 ng/mL, 0.1–20 ng/mL,0.1–20 ng/mL, 20–3200 pg/mL and 0.5–60 ng/mL ranges, respectively in the assay. The sensitivity was 0.05 ng/mL, 0.1 ng/mL, 0.1 ng/mL,0.022 ng/mL, 10 pg/mL and 0.4 μg/mL, respectively. Percentage recovery was; P5(6.30 ng/mL added) 98 ±2% (n =5), P4(15.4 ng/mL added) 97 ± 2% (n =5), 17-OH-P4(16.4 ng/mL) 91.8 ± 1.6% (n =5),testosterone (1.1 ng/mL added) 94 ±3% (n =5), E2(77.417 pg/mL added) 98.2 ± 1% (n =5) and cortisol (15.3 μg/dL added) 93.8 ± 1.6%(n =5). The coefficients of inter and intra-assay variations were 10.2%,11.2% for P5, 10.4%, 11.4% for P4,5.2%, 7.3% for 17-OH-P4,7.7%, 7.3% for testosterone, 9.2%, 9.9% for E2and 5.6%, 5.76% for cortisol.
在银行信贷业务贷后管理阶段,除却为客户提供高质量服务和日常规范维护外,借助区块链数据记录的不可逆性及不可篡改性,银行可对贷款相关资料的合规性及时做出有效监督及核查,以应对突发问题。例如,通过区块链节点企业高透明的交易流转数据真实反映企业的贷款实际用途是否合规;或可通过节点企业的上下游企业的可溯源交易数据反映企业的生产经营状况;或利用区块链技术监测企业的资金筹集情况,对企业的异常行为作为有针对性的措施以减少贷款损失。
2.4.Statistical analysis
All data were first tested for normality and homoscedasticity and were expressed as mean ±SEM. Statistical significance was analyzed by one-way analysis of variance (ANOVA) at (P <0.001), followed by Newman–Keuls’ Post hoc test (P <0.05).
3.Results
3.1.Effects of ovariectomy on plasma steroid (P5, P4, 17-OH-P4, T, E2 and F) levels during the resting phase
In the present study, we showed that Ovx resulted in significant and differential changes in plasma steroid levels. Plasma P5, P4, 17-OH-P4, T,E2and F levels were significantly decreased in the resting stage in duration-dependent manner compared to initial and sham control groups (Table 1; One-way ANOVA,P <0.001; FP5=6452.7, FP4=38126.7, F17–OHP4=31381.6, FT=85796.59, FE2=5662.99, FF=19223.8).
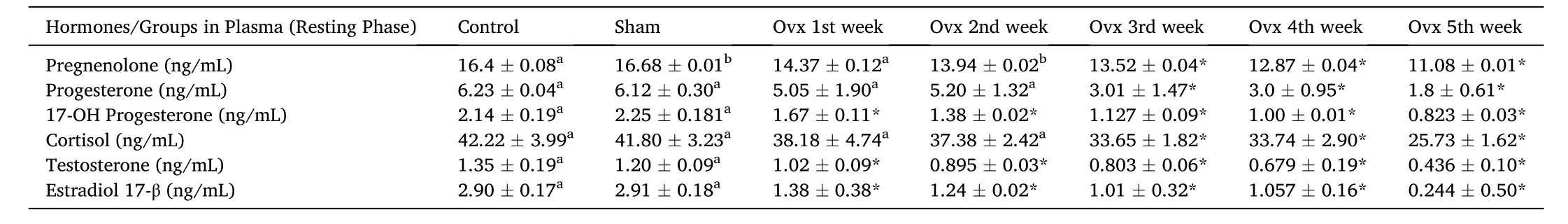
Table 1Effects of ovariectomy on plasma pregnenolone, progesterone, 17-hydroxy-progesterone, cortisol, testosterone and estradiol-17β concentrations in the cat fish Heteropnuestes fossilis during the resting phase. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test (P <0.05). Groups with same letters are not significantly different. Asterisks indicate significant variations with control and sham groups.
3.2.Effects of ovariectomy on plasma steroid (P5, P4, 17-OH-P4, T, E2 and F) levels during pre-spawning stage
In the present study, we showed that Ovx resulted in significant and differential changes in plasma steroid levels. Plasma P5, P4, 17-OH-P4,T, E2 and F levels were significantly decreased in the pre-spawning stage in duration-dependent manner compared to initial and sham control groups (Table 2; One-way ANOVA,P <0.001; FP5=376.9, FP4=812.6,F17–OHP4=381.2, FT=796.5, FE2=652.99, FF=192.3). The effect of OVX was more significant in the pre-spawning stage in comparison to the resting phase.
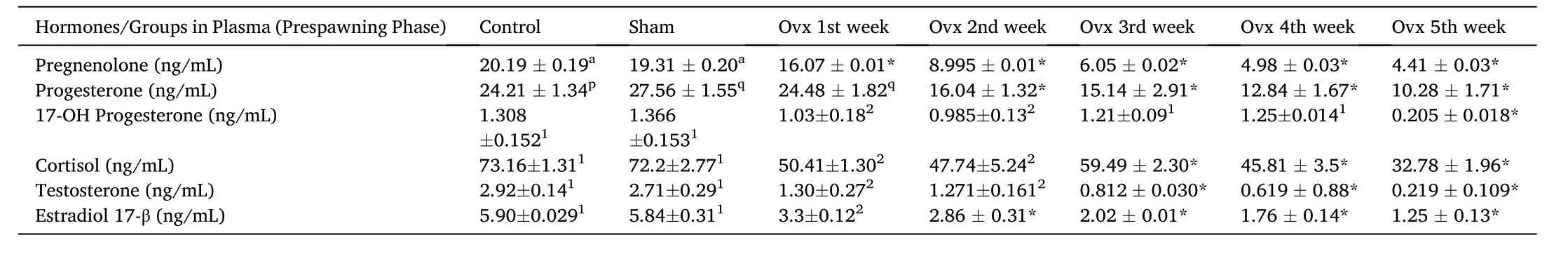
Table 2Effects of ovariectomy on plasma pregnenolone, progesterone, 17-hydroxy-progesterone, cortisol, testosterone and estradiol-17β concentrations in the cat fish Heteropnuestes fossilis during prespawning phase. Data were expressed as mean ± SEM (n =5) and analyzed by one-way ANOVA, followed by Newman-Keuls’ test (P <0.05). Groups with same letters or numbers are not significantly different and those with different letters or numbers are significantly different. Asterisks indicate significant variations with control and sham groups.
3.3.Effects of estradiol-17β supplementation on 3 week ovariectomized plasma steroid (P5, P4, 17-OH-P4, T, E2 and F) levels during the resting phase
In the present study, we showed that E2supplementation in 3-week ovariectomized fish resulted in an overall significant change in plasma P5, P4, 17-OH-P4, T, E2 and F levels (Table 3; one-way ANOVA,P <0.001; FP5=212.2, FP4=170.1, F17–OHP4=306.5, FT=442.64, FE2=375.4, FF=423.7). It showed a biphasic effect, low E2 (0.5 μg/g BW)increased and high E2 (1.0 μg/g BW) decreased hormone levels significantly in the prespawning stage. The E2treatment increased signi ficantly the P5, P4, 17-OH-P4, T, E2and F levels in the sham groups and in ovariectomized group, it modulated the effect.
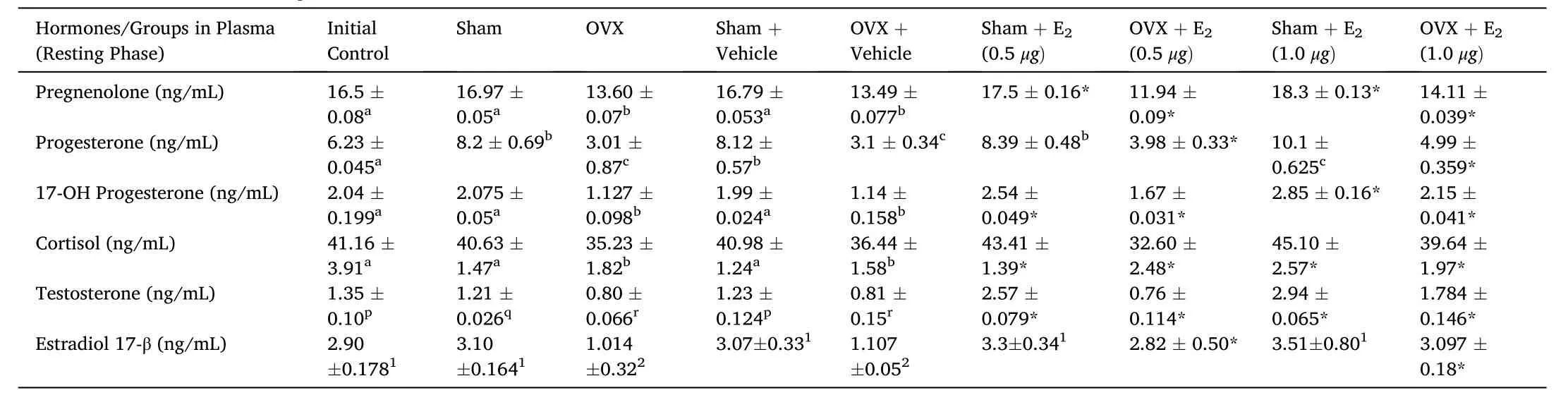
Table 3Effects of E2 replacement in 3-week ovariectomized fish on plasma pregnenolone, progesterone, 17-hydroxy-progesterone, cortisol, testosterone and estradiol-17β concentrations in the cat fish Heteropnuestes fossilis during the resting phase. Data were expressed as mean ±SEM (n =5) and analyzed by one-way ANOVA, followed by Newman-Keuls’ test (P <0.05). Groups with same letters or numbers are not significantly different and those with different letters or numbers are significantly different. Asterisks indicate significant variations with control and sham groups.
3.4.Effects of estradiol-17β supplementation on 3 week ovariectomized plasma steroid (P5, P4, 17-OH-P4, T, E2 and F) levels during pre-spawning stage
In the present study, we showed that E2replacement in 3-week ovariectomized fish resulted in an overall significant change in plasma P5, P4, 17-OH-P4, T, E2and F levels (Table 4; One-way ANOVA,P <0.001; FP5=412.2, FP4=170.1, F17–OHP4=306.5, FT=442.64, FE2=375.4, FF=423.7). It showed a differential effect, low E2 (0.5 μg/g BW)and high E2 (1.0 μg/g BW) decreased hormone levels significantly. In the sham groups, E2treatment increased significantly P5, P4, 17-OH-P4,T, E2and F levels and in ovariectomized group, it varied differentially.The E2replacement reversed the effect of Ovx and increased P5, P4, 17-OH-P4, T, E2and F levels dose-dependently. The E2treatment increased the P5, P4, 17-OH-P4, T, E2and F levels in the sham control groups.However, the T levels remained low in both low and high dose E2groups compared to the Ovx-vehicle group. In the sham control groups, only the high dose increased the T levels. In the sham control groups, the E2treatment increased the plasma E2level. There was no significant variation in the plasma cortisol level after E2 treatment, but ovariectomy decreased cortisol level.
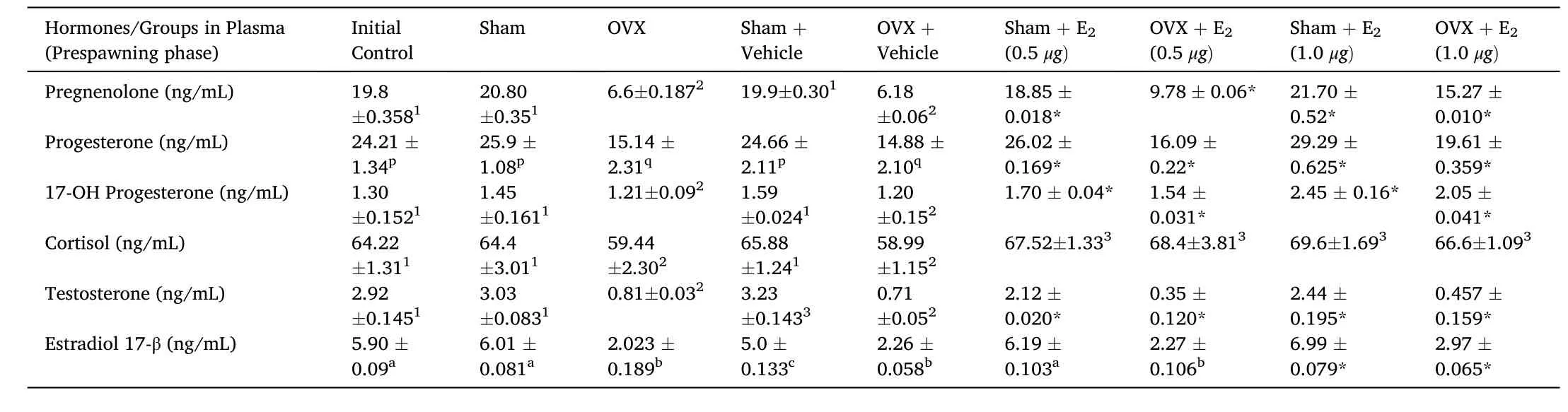
Table 4Effects of E2 replacement in 3-week ovariectomized fish on plasma pregnenolone, progesterone, 17-hydroxy-progesterone, cortisol, testosterone and estradiol-17β concentrations in the cat fish Heteropnuestes fossilis during prespawning phase of reproductive cycle. Data were expressed as mean ±SEM (n =5) and analyzed by oneway ANOVA, followed by Newman-Keuls’ test (P <0.05). Different letters or numbers show significant difference from control groups. Groups with same letters or numbers are not significantly different and those with different letters or numbers are significantly different. Asterisks indicate significant variations with control and sham groups.
3.5.Effects of Ovx and E2-replacement on brain steroid (P5, P4, 17-OHP4, T, E2 and F) levels
We observed significant differential variations in brain steroid hormones (P5, P4, 17-OH-P4, T, E2and F) levels after ovariectomy and E2supplementation during resting and pre-spawning stages. In comparison to other brain steroid hormone levels (P5, P4, T, E2and F), 17-OH-P4level was lowest in all groups in both ovariectomized and E2-replacement groups under control and experimental conditions (P >0.1).
P5levels: Brain P5levels were significantly high in the resting phase than in the pre-spawning stage in the initial and sham control groups.Ovx produced significant variations in brain P5levels (Fig. 1A; one way ANOVA,P <0.001; F =602.90). The P5levels decreased significantly(~50%) in the first and second week of Ovx, and the inhibition remained significant but modest at later duration of Ovx in the resting phase.However, in the pre-spawning stage, an opposite trend was noticed(Fig. 1B; One-way ANOVA,P <0.001; F =260.2), the P5levels were increased up to the fourth week of Ovx compared to the sham control and decreased in the 5th week. The E2replacement in 3-week ovariectomized fish resulted in overall significant changes in the P5levels(Fig. 1C; One-way ANOVA,P <0.001; F =506.4). Both low (0.5 μg/g BW) and high (1.0 μg/g BW) doses inhibited the P5levels in the resting phase; the low dose being more than the high dose compared to the Ovxvehicle group. The high E2dose increased the level in the pre-spawning stage. However, it reversed the effect in the ovariectomized group. The E2treatment in sham control fish decreased the P5levels dosedependently in the resting phase but increased it in the pre-spawning stage (Fig. 1D; one way ANOVA,P <0.001; F =603.21).
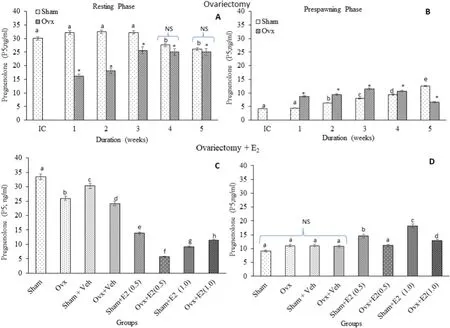
Fig. 1.Effects of ovariectomy (A, B) and E2 replacement in 3-week ovariectomized fish (C, D) on brain pregnenolone (P5) concentrations in the cat fish Heteropnuestes fossilis during the resting and prespawning phases. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test(P <0.05). Different letters or numbers show significant difference from control groups. IC-Initial Control, Ox-Ovariectomy, S +V-Sham +Vehicle, Ox +VOvariectomy +Vehicle, S +E2-Sham +Estradiol-17β, Ox +E2-Ovariectomy +Estradiol-17β.
P4levels: Brain P4levels were significantly high in the resting phase in the initial and sham control groups. Ovx produced an overall significant variation in the P4levels (Fig. 2A; One-way ANOVA,P <0.001; F=498.16). In the resting phase, the P4level decreased initially (1st and 2 nd weeks) but increased over time (3rd, 4th and 5th week of Ovx)compared to the sham groups. In the pre-spawning phase, there was a significant increase in P4level from the first to fourth week of Ovx and a drastic decrease in the 5th week (Fig. 2B; one-way ANOVA,P <0.001; F=985.12). The E2treatment resulted in overall significant changes in the P4levels (Fig. 2C; One-way ANOVA,P <0.001; F =432.51). E2produced a significant decrease in the P4levels in the Ovx fish in both resting (Fig. 2C; One-way ANOVA,P <0.001; F =432.51) and prespawning phases (Fig. 2D; One-way ANOVA,P <0.001; F =432.51). In the sham groups, E2produced a decrease in the prespawning phase and a decrease (low dose group) and an increase (high dose group) in the resting phase.
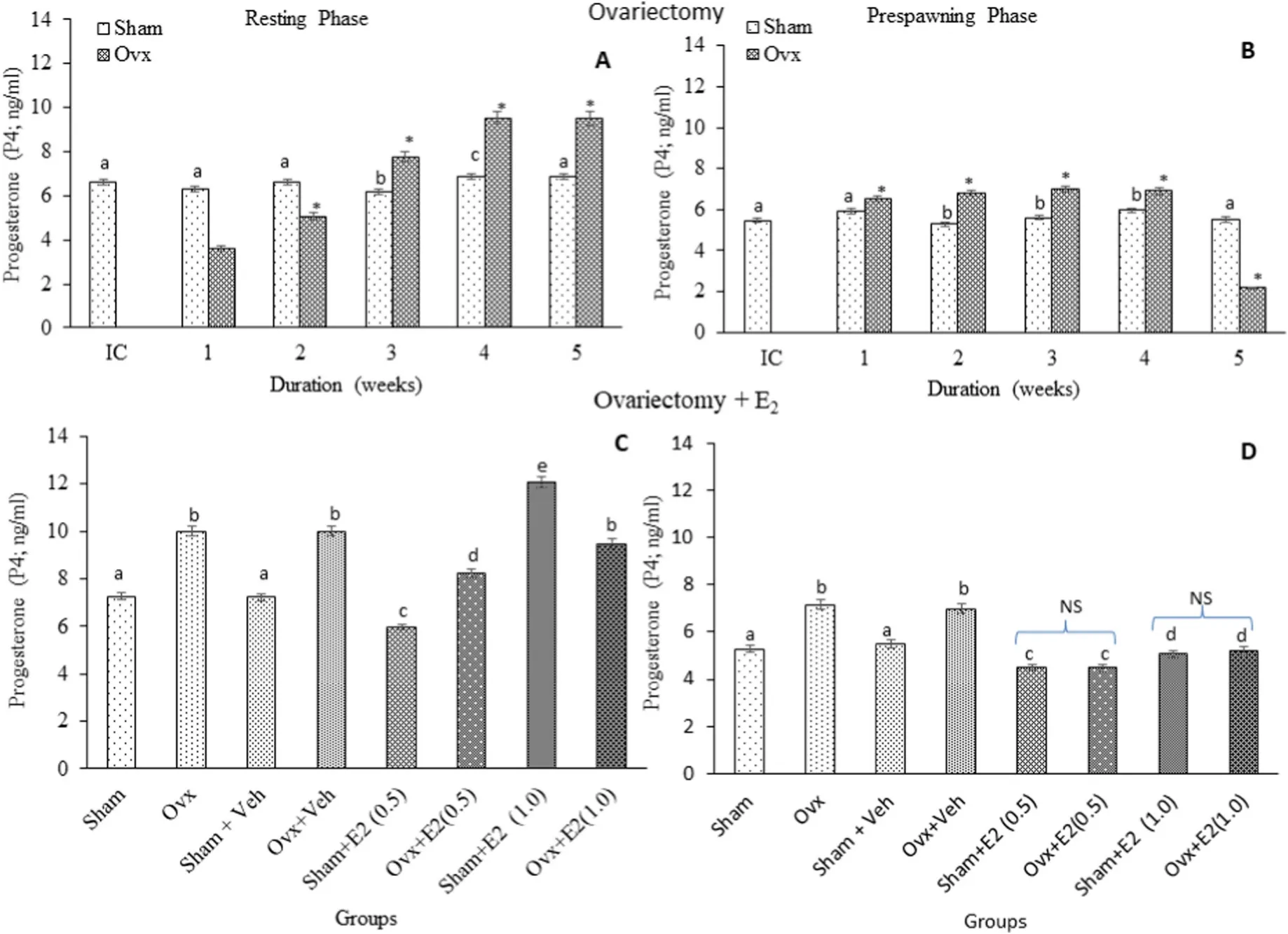
Fig. 2.Effects of ovariectomy (A, B) and E2 replacement in 3-week ovariectomized fish (C, D) on brain progesterone (P4) concentrations in the cat fish Heteropnuestes fossilis during the resting and prespawning phases. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test(P <0.05). Different letters or numbers show significant difference from control groups. IC-Initial Control, Ox-Ovariectomy, S +V-Sham +Vehicle, Ox +VOvariectomy +Vehicle, S +E2-Sham +Estradiol-17β, Ox +E2-Ovariectomy +Estradiol-17β.
T levels: Brain T levels were significantly higher in the resting phase in the initial and sham control groups. Ovx produced an overall significant effect on the T levels (Fig. 3A; One-way ANOVA,P <0.001; F =834.1). In the resting phase, the T levels decreased significantly in the 1st, 2nd and 3rd weeks of Ovx with the inhibition being decreased over the time and in the 4th and 5th weeks, the levels were significantly higher than the respective sham control groups. In the pre-spawning stage, the T levels increased significantly over the time after Ovx compared to the sham controls (Fig. 3B; One-way ANOVA,P <0.001; F=462.3). The E2treatment resulted in overall significant changes in the T levels (Fig. 3C; One-way ANOVA,P <0.001; Fseason=245.1). In the resting phase, the E2supplementation inhibited significantly the T levels; more severely in the low dose group. In the pre-spawning stage,the E2treatment also decreased the T levels, more intensely in the low dose group (Fig. 3D; One way ANOVA,P <0.001; F =602.7). In the sham groups, the low E2dose inhibited the T levels while the high E2dose inhibited it in the resting stage only.
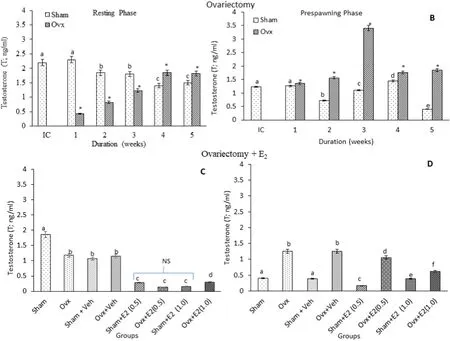
Fig. 3.Effects of ovariectomy (A, B) and E2 replacement in 3-week ovariectomized fish (C, D) on brain testosterone (T) concentrations in the cat fish Heteropnuestes fossilis during the resting and prespawning phases. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test(P <0.05). Different letters or numbers show significant difference from control groups. IC-Initial Control, Ox-Ovariectomy, S +V-Sham +Vehicle, Ox +VOvariectomy +Vehicle, S +E2-Sham +Estradiol-17β, Ox +E2-Ovariectomy +Estradiol-17β.
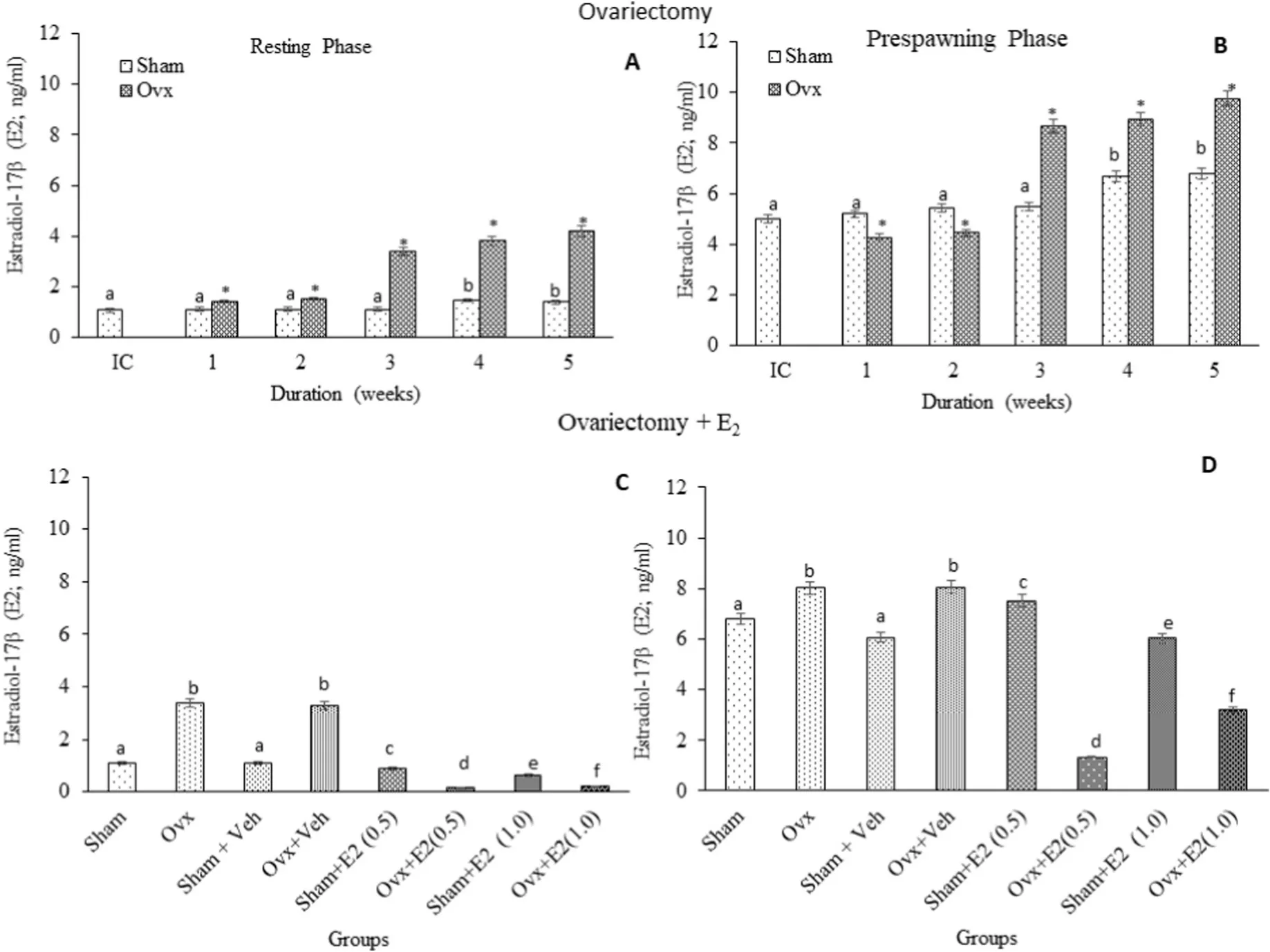
Fig. 4.Effects of ovariectomy (A, B) and E2 replacement in 3-week ovariectomized fish (C, D) on brain estradiol17β (E2) concentrations in the cat fish Heteropnuestes fossilis during the resting and prespawning phases. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test(P <0.05). Different letters or numbers show significant difference from control groups. IC-Initial Control, Ox-Ovariectomy, S +V-Sham +Vehicle, Ox +VOvariectomy +Vehicle, S +E2-Sham +Estradiol-17β, Ox +E2-Ovariectomy +Estradiol-17β.
Cortisol: Brain F levels are higher in the resting phase in the initial and sham control groups. Ovx caused an overall significant variation in the F levels (Fig. 5A; One-way ANOVA, (P <0.001; F =528.8)). In the resting phase, Ovx inhibited the F levels on week 1 and 2, which later increased with the time of Ovx. In the pre-spawning stage, the F levels increased in the 1st, 2nd and 3rd weeks and decreased in the 4th and 5th weeks of Ovx compared to the sham groups (Fig. 5B; One-way ANOVA,P <0.001; F =162.4). The E2replacement resulted in overall significant changes in the F levels (Fig. 5C; One-way ANOVA,P <0.001; Fs=623.76). Both low and high doses of E2decreased the F levels in both phases. Similarly, in the sham groups, both E2doses decreased the F levels and the effect was more pronounced in the pre-spawning stage(Fig. 5D; One-way ANOVA,P <0.001; Fs=808.06).
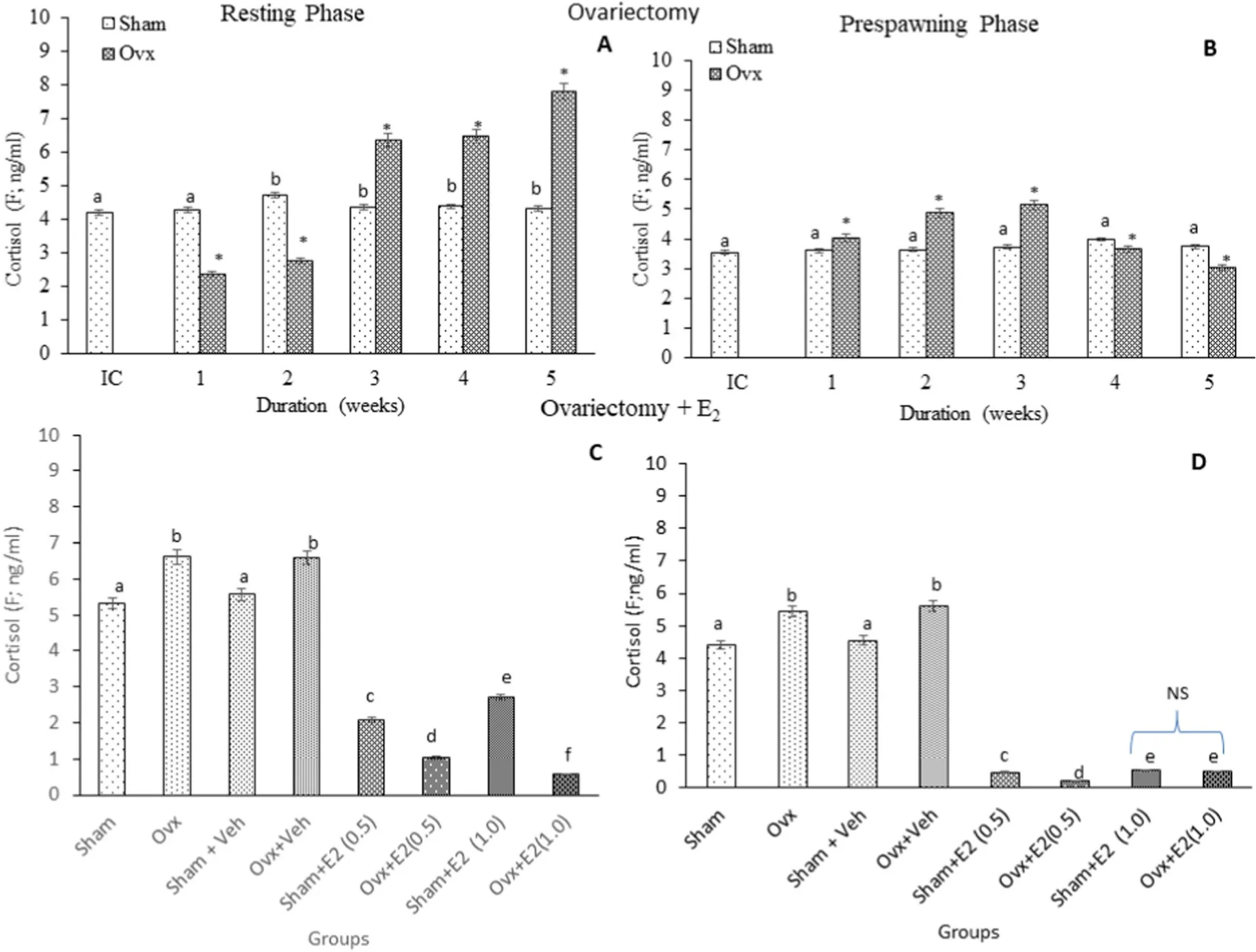
Fig. 5.Effects of ovariectomy (A, B) and E2 replacement in 3-week ovariectomized fish (C, D) on brain cortisol (F) concentrations in the cat fish Heteropnuestes fossilis during the resting and prespawning phases. Data were expressed as mean ± SEM (n =5) and analyzed by One-way ANOVA, followed by Newman-Keuls’ test (P <0.05). Different letters or numbers show significant difference from control groups. IC-Initial Control, Ox-Ovariectomy, S +V-Sham +Vehicle, Ox +V-Ovariectomy+Vehicle, S +E2-Sham +Estradiol-17β, Ox +E2-Ovariectomy +Estradiol-17β.
4.Discussion
4.1.Seasonal variations
The present study showed that plasma and brain steroids of initial and sham control groups elicited significant changes between the two reproductive stages (resting and pre-spawning stages). Plasma steroids(P5, P4, T, E2and F) were higher in the pre-spawning stage except 17-OHP4level, which was higher only in the resting stage. On the other hand, brain P5, P4, T and F levels were higher in the resting stage and 17-OHP4and E2levels were higher in the pre-spawning stage. It is likely that the brain may contribute to the plasma pool of steroids especially in the gonad resting stage when ovarian steroidogenesis is at the basal level. E2is the principal functional estrogen in the cat fish (Lamba et al.,1983; Senthilkumaran & Joy, 1994; Mishra & Joy, 2006) and its high level in the brain and plasma is associated with reproductive functions.Interrenal (teleost adrenocortical homolog) is the principal source of corticosteroids and cortisol is the major glucocorticoid and stress hormone in teleost (Mommsen et al., 1999). It shows seasonal changes with the level low in the resting stage, increasing to peak levels in the spawning stage in the head kidney (Kumar & Joy, 2017) and plasma(Lamba et al., 1983). The present data show that cortisol is also secreted in the brain thus contributing to the plasma pool. The high level of plasma 17-OHP4in the gonad resting (quiescent) phase can be attributed to its secretion from the brain and the interrenal gland. The functional role of the precursor (metabolite) steroids in the blood plasma is not known and these may be used for uptake to tissues like brain, liver, etc.,for steroid conversions and metabolic regulations.
4.2.Ovariectomy and E2 effects on plasma steroids
Ovx is a widely used classical endocrine approach to study the feedback relation between brain-pituitary axis and the ovary. In such studies, only estrogens were measured to monitor Ovx effects. The Ovx effects varied with the reproductive phase and duration. All steroids but P4and F decreased after Ovx from the first week onwards and P4and F showed significant changes towards the later part of the operation,indicating that the ovary constitutes a major source of plasma steroids.The recovery/reversal of the plasma steroids after E2administration showed varied responses according to the dose used and reproductive phase. High E2dose (1.0 μg/g BW) was more effective (P5, P4and 17-OHP4) than the low dose (0.5 μg/g BW), and the stimulatory effect was higher in the resting phase (T and E2). The F level responded differentially to the E2doses; the low E2dose inhibited it in the resting phase. In the sham groups, E2exerted a stimulatory effect, higher for P5, P4and E2in the prespawning phase and for 17-OHP4and T in the resting phase. E2exerted an inhibitory effect on the T levels in the prespawning phase.The F levels in the sham groups were stimulated by E2in the prespawning phase. The results suggest that E2has an important role in maintaining the circulating titer of steroids. However, the mechanism by which E2alters these steroids in intact and Ovx fish is unknown. It is assumed that E2may act at specific enzyme sites of steroidogenesis in the ovary, brain and interrenal gland to alter the steroid milieu, according to the season.
4.3.Ovariectomy and E2 effects on brain steroids
Brain steroids responded differentially to Ovx and/or E2replacement depending on the reproductive stage and duration. Estrogens, progestogens, androgens and corticosteroids of peripheral origin in the circulation are able to modulate brain functions through specific receptors (Baulieu et al., 2007; Lambert et al., 1995; Majewska et al.,1986; Mellon, 2007; Paul & Purdy, 1992). In mammals, receptors of gonadal steroids have been identified in several brain areas: amygdala,hippocampus, cortex, basal forebrain, cerebellum, locus coeruleus,midbrain raphe nuclei, glial cells, pituitary gland, hypothalamus and central gray matter (Genazzani et al., 1996; Speroff et al., 1995). It is hypothesized that blood-borne steroids are taken up in the brain and may act as precursors for the synthesis of active neurosteroids (Diotel,Do Rego, et al., 2011). Diotel, Servili, et al. (2011) have investigated the steroidogenic pathways and identified the concerned enzymes in the brain of zebra fish. In the cat fish brain, 3β-HSD and P450 aromatase have been characterized, which showed seasonal variations (Mishra &Chaube, 2017; Chaube et al., 2015). Brain steroids reported in the catfish showed seasonal and dimorphic variations (Chaube & Mishra, 2012;Mishra & Chaube, 2022). In the present study, Ovx decreased the P5levels while the P4levels were stimulated in the resting phase, suggesting the conversion of P5to P4by 3β-HSD. Unlike the resting stage,the P5level was very low in the pre-spawning stage, which may be due to the high turnover of P5to its products like P4or 17-OHP5. In this stage,Ovx produced a stimulatory effect up to 4th week and an inhibitory effect in the 5th week. Ovx stimulated brain P4levels in a duration-dependent manner in the resting phase, and in a biphasic manner with a modest stimulation up to the 4th week and a significant inhibition in the 5th week in the prespawning phase. Progesterone (P4),a neurosteroid hormone, and a progesterone receptor (Pgr) has been detected in peripheral and central glial cells (Baulieu et al., 2007;Schumacher et al., 2000). In zebra fish, a single nuclear Pgr locus and a unique full - length Pgr transcript were characterized, and can be activated by various progestins like P4, 17-OH-P4, dihydro-progesterone and 4-pregnen-17, 20β –diol-3-one (teleost MIH) (Chen et al., 2010; Hanna et al., 2010). Pgr has wide distribution in the brain of zebra fish, suggesting reproductive, social and central actions, including neurogenesis,neuronal plasticity and/or neuroprotection. A wide expression of Pgr was also documented in an African cichlid,Astatotilapia burtoni, and implied reproductive and non-reproductive functions (Munchrath &Hofmann, 2010).
The brain 17-OHP4level was detected very low in the initial control,sham control and Ovx fish, implying that the steroid production is transient. The steroid is synthesized by P450c17, which was characterized in the brain of fathead minnow (Pimephales promelas) by Halm et al.(2003). The authors reported that the enzyme expression was very low in the brain than in gonads. This could be a reason for the high level of P4. Alternatively, P4may be converted into allopregnanolone (AP) via A-ring reduction (Celotti et al., 1992; Do Rego et al., 2009), a neuroactive steroid implicated in GABA neurotransmission, inhibition of toll-like receptors or suppression of adrenal stress axis. Ovx inhibited significantly AP levels in blood and brain (Bernardi et al., 1998; Genazzani et al., 2000; Ichikawa et al., 1974). AP can accumulate in the brain after adrenalectomy and gonadectomy (Purdy, Morrow, Moore, &Paul, 1999; Corpechot et al., 1993; Cheney et al., 1995). Estrogen treatment restored AP levels or countered the aging-related AP decline in the brain (Genazzani et al., 2004). Whether the cat fish brain secretes AP is to be examined in future studies.
Ovx caused a biphasic effect on T levels; a significant inhibition up to 3 weeks and mild increases subsequently in the resting stage, and a stimulatory effect with the peak rise at the 3rd week in the pre-spawning stage. Ovx resulted in stimulation of E2levels on 3rd to 5th week in the resting phase.
In the pre-spawning stage, a biphasic effect with significant inhibition up to the 2nd week, followed by a significant elevation in the 3rd,4th and 5th week was obtained. The inhibition of T level in the resting phase with a simultaneous stimulation of E2may suggest the former’s conversion into E2. Gonadal steroids (T and E2) are directly responsible for seasonal modulation of P450 aromatase (cyp19a1b) in females since they show the most dramatic fluctuations of T and E2levels (Forlano &Bass, ). The authors reported that in OVX adult female plain fin midshipman, T- and E2treatments elevated P450 aromatase mRNA expression in brain areas, T affected in a level-dependent manner and E2decreased the expression at higher concentrations.
Ovx produced a biphasic effect on brain F level, an initial inhibition(1st and 2nd week), followed by stimulation (3rd, 4th and 5th week) in the resting phase, and an initial stimulation (1st to 3rd week), followed by inhibition (4th and 5th week) in the pre-spawning stage. Ovx was shown to decrease basal corticosterone levels in the rat (Kitay, 1963)and in rhesus monkey, Ovx significantly decreased both mean and peak basal cortisol levels (Smith & Norman, 1987). These effects of Ovx were attributed to the stimulatory effect of E2on the hypothalamo-hypophysial-adrenal (H–P-A) axis.
The E2replacement in Ovx or treatment in the sham fish produced varied effects on the brain steroid levels. The P5level was decreased in a dose-dependent manner in the resting stage, but the level was stimulated in the pre-spawning stage. The high dose showed a higher recovery or stimulation as the case may be. The P4levels behaved differently to the E2replacement or supplementation. In the Ovx groups, the E2treatment did not restore the steroid level in both seasons except in the high dose group in the resting phase in which the level was elevated above the control groups, but next to the Ovx group. In the sham group, the high E2dose was effective to stimulate the P4level in the resting phase. The brain 17-OHP4level was not affected by the E2treatment in the Ovx or sham groups. The brain T level remained low after the E2treatment in the Ovx or sham groups but the high E2dose registered a higher response in both seasons.
In all studies conducted till date in teleost fishes, E2treatment alone up regulates brain P450 aromatase activity and/or mRNA expression in adults (Pasmanik et al., 1988; Gelinas et al., 1998; Melo & Ramsdell,2001; Halm et al., 2002). In adult gold fish, T and E2up-regulated aromatase mRNA in forebrain and mid/hindbrain homogenates as compared to the gonadectomised control fish, and the controls showed a reduction in transcript levels when compared to intact (reproductive)fish (Gelinas et al., 1998). Thus, changes in the levels of T and E2after OVX and hormone replacement can be associated with the regulation of aromatase enzyme (brain type aromatase, cyp19a1b) in the cat fish. The mechanism by which E2modulates the synthesis of neurosteroids is still unclear. It is hypothesized that estrogens may act directly on the enzymes involved in the biosynthetic pathway of steroid hormones and neurosteroids are modulated by peripheral steroids by endocrine and autocrine/paracrine feedback loops that regulate its synthesis. A functional interaction between brain P4and E2is envisaged, which can be modified by peripheral steroids (Diotel, Servili, et al., 2011). The E2supplementation was ineffective to restore the F levels in the Ovx fish in either season. In the sham groups, the E2treatment inhibited F levels.Taken together, E2supplementation alone is not enough to restore the brain steroid levels and E2exerts inhibitory or stimulatory effects on brain steroidogenesis according to the season and dosage.
5.Conclusion
Ovx resulted in changes in brain steroid levels and the changes were dependent on the reproductive phase and duration. E2replacement in Ovx fish caused varied effects on the neurosteroid levels, which is again dependent on the season and treatment dose. Similarly, the E2treatment in intact fish elicited varied responses, different from the Ovx fish. Our results suggest that circulatory steroids of peripheral origin alter brain steroidogenesis and the mechanisms are to be investigated in future research work.
Declaration of competing interest
The authors declare no con flict of interest pertaining to the research report in this manuscript.
Acknowledgments
This work was partially supported by a University Grants Commission and Department of Science and Technology, New Delhi, India grants to RC. SM was a recipient of UGC-BSR Research Fellowships for meritorious students (RFSMS), Department of Zoology, Banaras Hindu University.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Understanding the impact of stress on teleostean reproduction
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
