Reproductive farming technology in Japanese eel and chub mackerel
Hiroshi Miyanishi, Naoki Nagano
Department of Marine Biology and Environmental Sciences, Faculty of Agriculture, University of Miyazaki, Miyazaki, 889-2192, Japan
Keywords:
Japanese eel
Chub mackerel
Full-lifecycle aquaculture
Hormonal maturation
Saccus vasculosus
A B S T R A C T
Japanese eel (Anguilla japonica) and chub mackerel (Scomber japonicus) are commercially valuable species in Asian aquaculture. The reduction in eel and chub mackerel resources has been a serious problem in recent years that should be addressed by reducing the catch of their natural populations to halt their decline and reach the Sustainable Development Goals. Furthermore, securing sufficient food supply by aquaculture is an important step in addressing the increasing demand for fish products in recent years. Traditionally, juveniles of the Japanese eel and chub mackerel have been captured to be raised in aquaculture. Owing to the extensive research on these species, new technologies have been developed for full-lifecycle aquaculture of Japanese eel in 2011 and chub mackerel in 2014. These technologies are expected to stop the decline of natural resources and provide a stable food supply. Recently, seed production of these species has increased owing to the development of successful broodstock management and larval rearing techniques. Fundamental information on oocyte maturation and ovulation and its application for artificial induction of sexual maturation is needed to produce good quality seeds of the Japanese eel and chub mackerel. Here, hormonal mechanisms and previously and newly developed methods for artificial seed production have been described.
1.Introduction
The farmed production of fish has increased about fourfold over the past 20 years (Naylor et al., 2021). However, reduction in fish populations has been a serious problem in recent years and other concerns,such as increasing water temperature caused by climate change, may have significantly affected the natural resources of not only freshwater but also seawater fishes. Under the scenario assuming no climate-change mitigation policy is implemented at +4.5 ℃ global warming and in the absence of dispersal, at least half of the geographic range will be threatened by projected climate extremes for 63% (±7%) of the freshwater fish species (Barbarossa et al., 2021). The conservation of natural fish resources and the improvement of fish protein productivity in aquaculture are important issues that need addressing to achieve Sustainable Development Goals (SDGs; Transforming our World, 2015). In response to this situation, the full-lifecycle aquaculture technology is required as a way to protect declining natural resources and develop sustainable fish aquaculture. Here, we focus on the aquaculture of Japanese eel (Anguilla japonica) and chub mackerel (Scomber japonicus),two economically important species in Japanese food culture. The Japanese eel is one of the most valuable eel species for the fisheries and aquaculture industry. However, in recent years, the catch of Japanese eel larvae, which are essential for aquaculture, has declined to about one- fiftieth of the amount caught in the 1960s (Jacoby and Gollock,2014). The species is designated endangered by the UCN Red List(Jacoby and Gollock, 2014) and requires prompt protection of its resources. The chub mackerel is a commercially important species widely distributed in warm and temperate coastal waters of the Indo-Pacific oceans (Fig. 1). The catch rate of wild chub mackerel in Japan has declined since the 1970s, stabilizing at a low level in the early 2000s(Yatsu et al., 2005; Yatsu, 2019). Recently, the catch has increased,although it remains at low levels, boosting the interest in cultured chub mackerel. The aquaculture of chub mackerel is well established in southwestern Japan, but it relies on wild-caught juveniles. Recent developments of successful broodstock management and larval rearing techniques have increased the seed production of this species.

Fig. 1.Three-year-old chub mackerel (Scomber japonicus).
The results of extensive research have led to the establishment of technologies for the full-lifecycle aquaculture of Japanese eels in 2011(Kagawa et al., 2013; Tanaka, 2011) and chub mackerel in 2017(Nagano, 2017).
2.Current status of mackerel farming in Japan
Farming of the Japanese eel has been practiced for many years,however, the eel is produced from natural seedlings that are harvested,which accounts for more than 99% of the eel production. In contrast,mackerel resources in Japan depend on capture by fisheries, although recently the aquaculture of chub mackerel has been gradually expanding. Cultured chub mackerel has a better and more stable fat content throughout the year compared to the products obtained from natural populations, it is always fresh, and it can be shipped at any time of the year. Since around 2000, chub mackerel in Japan has been cultivated by several aquaculture companies in Oita, Nagasaki, Miyazaki, and other prefectures. Due to the recent increase in demand for mackerel and the decrease in its catch, the number of mackerel farms in the last five years has increased and expanded into other prefectures, including Kagoshima, Saga, Tottori, Fukui, and Hyogo, producing more than 1 million fish per year (Nagano, 2019). Most of the seedlings for cultured chub mackerel currently in circulation are caught from natural populations by purse seine or set net fishing. These fishing methods capture seedlings that are often weighing 100 g–300 g, while the number of seedlings is dependent on the number of resources. Artificial seedlings of chub mackerel are produced in Kochi, Tottori, Saga, Oita, Wakayama, and Fukui prefectures. The actual number of produced artificial seedlings is unknown, but their number has been increasing in each region with the growing demand for producers. Considering that most chub mackerel farming still depends on natural seedlings, the rapid development of techniques for the production of artificial seedlings is desired.
3.Induced maturation and spawning in chub mackerel
Production of artificial seedlings is usually carried out between April and June when the water temperature rises from 18 ℃ to 22 ℃ in Karatsu, Saga Prefecture, Japan (Nagano, 2017). Generally, two-to three-year-old broodstock has been used for seed production in Karatsu, although this species reaches maturity at one year of age (Ishibashi et al., 2007). The embryo-forming eggs can be obtained from one-year-old fish that have been induced to spawn by the administration of human chorionic gonadotropin (hCG). Parent fish used for mating are either naturally caught or cultured from artificial seedlings (Murata et al., 2005; Shiraishi et al., 2005). These fish are then transferred to a land tank before the spawning season and cultured until egg collection.When assessing the ripeness of the eggs, the fish is anesthetized and a part of the ovarian tissue is collected using a cannula to examine the condition of the gonads (Fig. 2A). Then, females with eggs 600 μm in diameter or larger and completed egg yolk formation are selected and administered with hCG or gonadotropin-releasing hormone (GnRH) by intramuscular injection to promote final maturation of the eggs(Fig. 2B). Males are selected among individuals that are ejaculating under abdominal compression (Fig. 2C). It has been reported that individuals in rearing tanks develop mature ovaries and testes but do not spawn because gonadotropins are not released (Shiraishi et al., 2005,2008). Therefore, hCG and GnRH are administered at concentrations of 500 IU/kg body weight (BW) and 400 mg/kg BW, respectively. Ovulation occurs 33 h after administration of hCG and 36 h after administration of GnRH at 19 ℃ (Shiraishi et al., 2005, 2008). Fertilized eggs are obtained by natural spawning or artificial insemination (Fig. 3A).Fertilized eggs have an egg diameter of 0.9–1.0 mm, and an average of about 50,000 eggs can be obtained per female. The larvae open their mouths two days after hatching and are fed docosahexaenoic acid(DHA)-enriched rotifers (Fig. 3B). Approximately one week after hatching, the larvae are fed DHA-enrichedArtemiaand frozen copepods,which are replaced with compound feed about two weeks after hatching.After the compound feed is introduced, the larvae grow rapidly and reach a length of 70–80 mm at 40–50 days of age. One of the problems in raising larvae is the control of cannibalism (Fig. 4), which reduces the yield by 10–40%. The artificial culturing of seedlings in chub mackerel farming control spawning time and yield, and also eliminate the infestation of the seedlings byAnisakis, a parasitic nematode that causes food poisoning in humans. In a land tank, artificial seedlings are fed rotifers,Artemia, and finally compound feed until their transfer to a sea cage,therefore eliminating the infection route ofAnisakis. The probability of anisakis infestation on mackerel is even lower in land-based aquaculture systems.
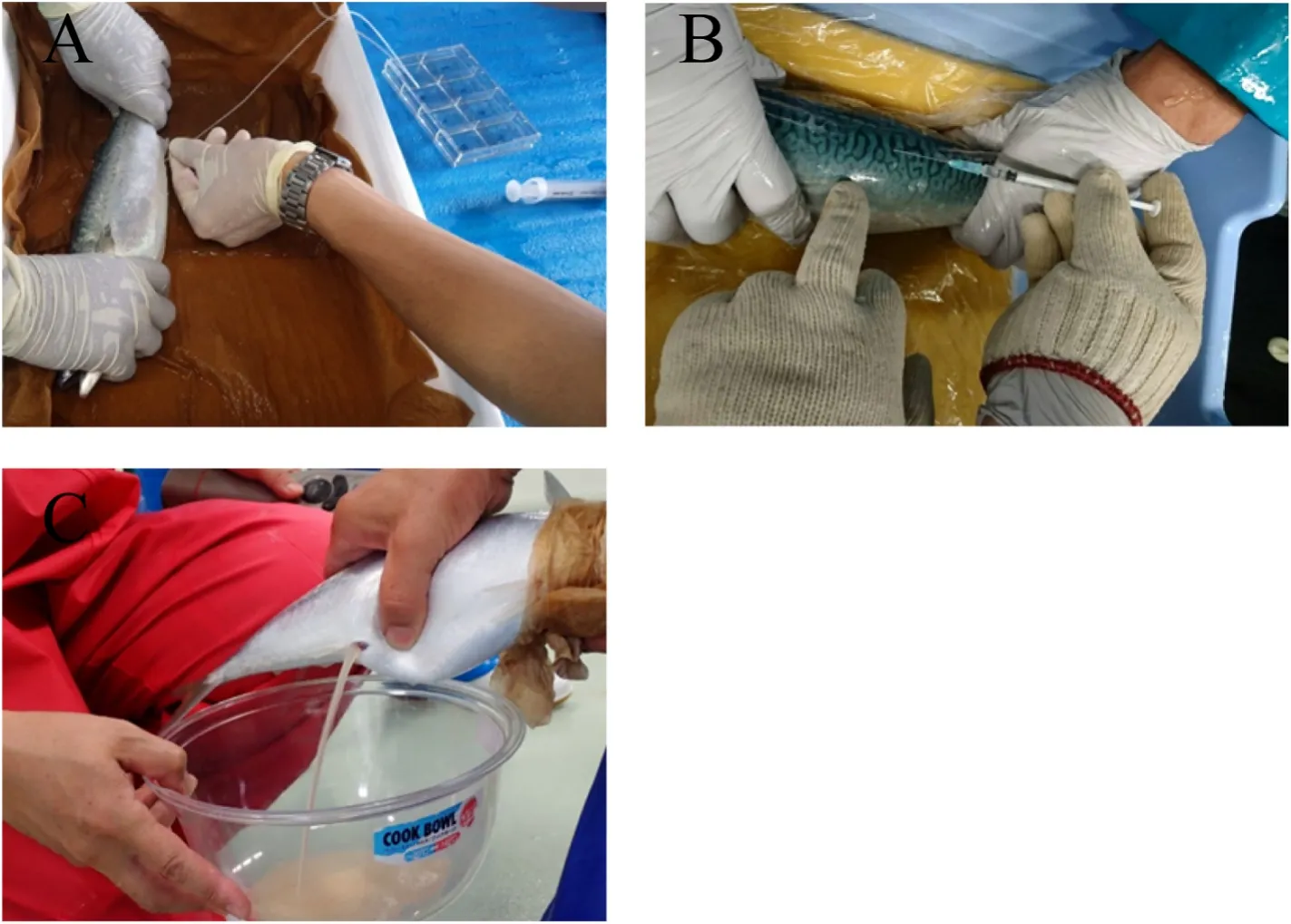
Fig. 2.Egg collection in chub mackerelA part of the ovarian tissue is collected using a cannula to assess the condition of the gonads after the fish is anesthetized (A). Human chorionic gonadotropin (hCG) or gonadotropin-releasing hormone(GnRH) was administrated by intramuscular injection to promote final maturation of the eggs (B). Mature mackerel eggs are squeezed out (C).
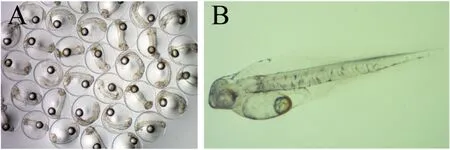
Fig. 3.Chub mackerel fertilized eggs (A) and larva 2 days after hatching (B).

Fig. 4.Cannibalism of chub mackerel larva.
Recent reports suggested that kisspeptins, leptins, and gonadotropininhibitory hormone homolog LPXRFamide play important roles in the reproduction of chub mackerel. Kisspeptins increased the expression of gonadotropins (GTHs) (Ohga et al., 2018). Leptins and LPXRFamide promote GTHs secretion in chub mackerel (Ohga & Matsuyama, 2021a and 2021b; Ohga et al., 2021). Based on these results, it is important to further elucidate the maturation mechanism in mackerel. Results of these studies will lead to the development of more efficient full-lifecycle aquaculture technologies.
4.Hormonal maturation and natural maturation in Japanese eel
The progress of the induced maturation has been reviewed previously (Ohta et al., 1997; Liao & Chang, 2001; Kagawa, 2003; Tanaka,2003; Palstra & van denThillart, 2009; Ijiri et al., 2011; Kagawa et al.,2013; Okamura et al., 2014). After two years of rearing, the eels are treated with the salmon pituitary extract (SPE) once a week to induce sexual maturation, and then 17α, 20β-dihydroxy-4-pregnen-3-one(DHP) was administered to induce final maturation and ovulation(Tachiki et al., 1997; Kagawa, 2003). In males, human chorionic gonadotropin (hCG) has been used to induce spermatogenesis (Miura et al., 1991). The SPE contains large amounts of GTHs,follicle-stimulating hormone (FSH), and luteinizing hormone (LH)(Yamamoto & Yamauchi, 1974; Yamauchi & Yamamoto, 1982), as well as other pituitary-produced hormones (such as growth hormone), the effects of which are not well understood. Kazeto et al., (2008) established a method to produce recombinant eel FSH (reFSH) and LH (reLH).In addition, the improved recombinant eel GTHs using a transient expression system of mammalian cells with an O-glycosylation site,which is present in mammalian glycoproteins, extends the half-life of the recombinant GTH and increases its maturation-inducing ability (Kazeto et al., 2019). In vitro, the FSH receptor is activated by both reFSH and reLH, whereas the LH receptor is activated only by reLH (Suzuki et al.,2020). Thus, reFSH and reLH may improve the efficiency of the maturation-induced process and further the development.
Much of the life history of Japanese eel remains unknown, which along with the fact that their spawning grounds are located in the southern part of the Western Mariana Ridge (Tsukamoto et al., 2011),hinders the collection of mature parent fish in the wild. In addition,Japanese eel does not reach sexual maturity in captivity. This may be due to the lack of external factors that are normally present under natural conditions and stimulate endocrine factors in the body. The early maturation of Japanese eel progresses when they are kept under short-day conditions. Maturation in bony fishes is generally controlled by a cascade of hormones secreted from the hypothalamic-pituitary-gonadal (HPG) axis (Mylonas et al., 2010; Zohar et al., 2010). First, environmental factors such as photoperiod and water temperature are perceived and converted into hormonal signaling in the body, which in turn regulates the synthesis and secretion of GnRH in the hypothalamus. Next, GnRH acts on the pituitary gland to promote the synthesis and secretion of the gonadotropic hormones, FSH, and LH. In addition, gonadotrophs act on the gonads to promote the synthesis of various steroid hormones, leading to maturation and finally ovulation or fertilization (Nagahama, 1987). The sensitivity of fish to external stimuli and the factors that promote maturation are not well understood.Nakane et al. (2013) suggested that the saccus vasculosus (SV) is the seasonal sensor in the brain. The experiments on masu salmon (Oncorhynchus masou) revealed that the SV in the brain expresses photoreceptor function, when isolated the SV shows a photoperiodic response,and the removal of the SV prevents sexual maturation even under short-day conditions (Nakane et al., 2013). The SV, a red organ with well-developed capillaries, is only found in fish and located posterior to the pituitary gland (Altner and Zimmermann, 1972), although SV is absent in some fish (eg, zebra fish and medaka). Recent studies have elucidated the signaling pathways that regulate photoperiodism in mammals and birds. In both groups, the mediobasal hypothalamus(MBH) and ependymal cells of the third ventricle within the medial basal hypothalamus play an important role in the control of photoperiodism(Nakao et al., 2008; Ono et al., 2008). In birds, light is received by intracerebral photoreceptors in the paraventricular organ of the deep brain, and daylength information is transmitted to the pituitary ramus via neurotransmission (Nakane et al., 2010). Thyroid hormones (THs)and their axis are involved in the sensing of circadian and seasonal cycles (Deal & Volkoff, 2020). In quail (Coturnix japonica), the MBH which includes the nucleus hypothalamus posterior medialis, induced the thyroid-stimulating hormone (TSH) under long-day conditions, which binds to thyroid-stimulating hormone receptors in ependymal cells of the third ventricle and triggers the expression of thyroid hormone converting enzyme 2 (DIO2). DIO2 converts thyroxine (T4) to locally active triiodothyronine (T3), and T3 causes a morphological change in the glial cells surrounding the nerve endings of GnRH neurons in the median ridge, resulting in the release of GnRH into the pituitary gland. After GnRH release, the hypothalamus-gonadal axis is activated and the pituitary gland secretes a gonadotropic hormone, leading to gonad development (Yoshimura et al., 2003). In contrast, in mammals, daylength information received by the retina passes through the suprachiasmatic nucleus and pineal gland, and the photoperiod is controlled by the rhythm of melatonin secretion. In the case of long-day stimulation,when the secretion time of melatonin is shortened, TSH is produced in the pituitary ramus and acts on the ependymal cells of the third ventricle to induce the expression of DIO2. This results in the development of gonads in long-day breeding animals (Ono et al., 2008; Yasuo et al.,2009). TH circadian cycles have been shown in Atlantic salmon (Salmo salar) (Ebbesson et al., 2008). In rainbow trout, the TH rhythms appear to be sex-specific—TH levels increase during the day and decrease at night in males, but increase at night and decrease in the morning in females (Ganzha & Pavlov, 2019). However, the MBH, which is thought to be the photoperiodic center in mammals and birds, is not anatomically present in fishes, and it has been unclear which organ serves as the photoperiodic center. The retina and pineal gland are commonly known to be photoreceptor organs in fish and have been the subject of numerous studies (Ekström & Meissl, 1997; Stenkamp, 2011). In visual and non-visual opsin families, the expression of deep-sea rhodopsin(DSO) increased in the retina, and DSO and short-wavelength-sensitive 2(SWS2) also increased in the brain after sexual maturation, suggesting that DSO and SWS2 are closely related to sexual maturation in eel (Byun et al., 2020). The injection of hCG and a new moon exposure led to the higher eye and plasma melatonin levels, correlated with nocturnal behavioral response, including testis development and spawning (Hyeon et al., 2021). These findings have the potential to connect the ecological and physiological aspects of eels, but whether they are involved in sexual maturation remains to be verified.
In eels, similar to trout salmon, the SV is located in the brain(Fig. 5A). We have developed a method for SV removal in eels (Fig. 5B and C). Immature female eels (431 ±55 g) used in this study at the previtellogenic stage were produced by treating glass eels with estradiol-17β (Ijiri et al., 1995). A hole is made in the maxilla just below the SV by a dental drill to expose the SV, and the SV is then completely removed with an electrosurgical scalpel. Eels with removed SV stop accumulating oil globules in their ovaries, which typically proceeds under short-day conditions, compared to the untreated and sham-operated groups(Fig. 5D, E, F, and G; Miyanishi et al., unpublished results). This suggests that the SV acts as a seasonal sensor and has an important role in the early maturation of eels. Therefore, elucidating the role of SV in eel maturation may enable us to promote the maturation of the Japanese eel by manipulating the light environment in the future.
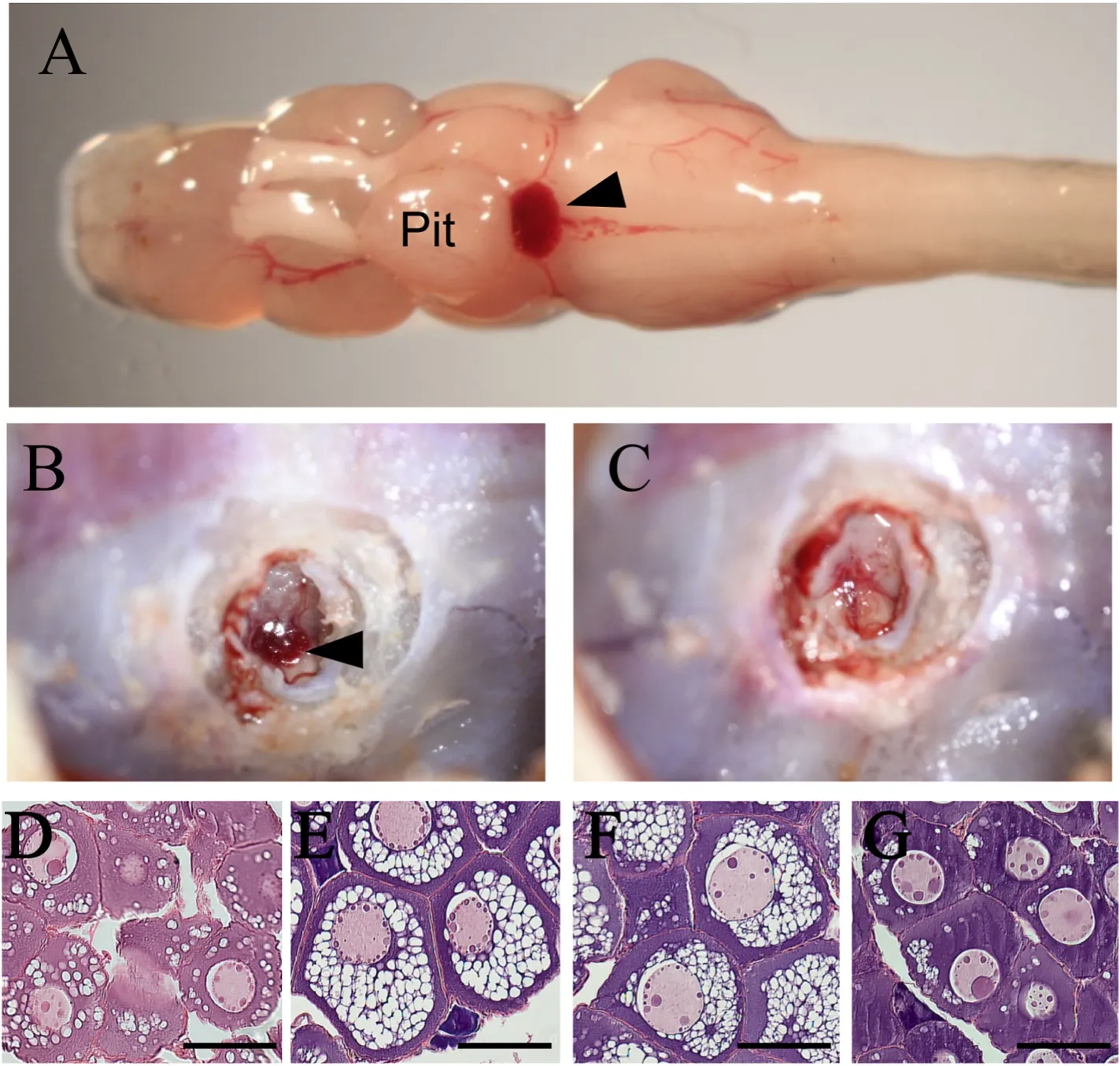
Fig. 5.Removal of the saccus vasculosus in Japanese eelSaccus vasculosus (SV) is located posterior to the pituitary (pit) in dorsal area of the brain (A). Arrowheads indicate SV. The SV is observed by drilling through the maxilla (B). After SV removal, the hole is temporarily sealed with dental cement and recovered approximately one week later (C). Histological ovarian observations of initial group (August) (D),intact group (February) (E), sham control group(February) (F), and SV-ectomy group (February) (G).Scale bar, 100 μm.
5.Conclusions
The techniques of induced reproduction of Japanese eel and chub mackerel have been improved significantly. Full-lifecycle aquaculture of chub mackerel is reaching a practical level, but that of Japanese eel is still far from a commercial level. The full-life cycle aquaculture technology in eel and chub mackerel have still a problem in the cost aspect.Many difficult unsolved problems still remain before the success of mass production. However, accumulated evidence in various research fields in Japanese eel and chub mackerel will resolve this issue in the near future. When these techniques are improved at a commercial level,replacement of part of wild-caught juveniles is achived. This can help to reduce the catching natural resources, which will contribute to the conservation of eel and mackerel species worldwide and to achieve SDGs.
Author contributions
HM and NN both designed, wrote, and approved the final version of the manuscript.
Funding
The authors acknowledge funding from Takahashi Industrial and Economic Research Foundation, Japan.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential con flict of interest.
Acknowledgments
We acknowledge former and current researchers adding to the field.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Understanding the impact of stress on teleostean reproduction
- Control of gonadal maturation and sex in grouper
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
