Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
Nirmalendu Saha, Debaprasad Koner, Ritu Sharma
Biochemical Adaptation Laboratory, Department of Zoology, North-Eastern Hill University, Shillong, 793022, India
Keywords:
Sex hormones
Endocrine disruption
Gametogenesis
Gonadal development
Fish reproduction
HPG-Axis
A B S T R A C T
Vast stretches of open water bodies are gradually becoming hypoxic as a result of depletion of oxygen levels mainly due to various human anthropogenic activities. This problem of hypoxic stress on the fish population is likely to be exacerbated soon since the aquatic hypoxic environment is continuously spreading over vast areas worldwide. In recent years, various harmful effects of hypoxia to bony fishes have been reported, such as the restriction of energy-consuming metabolic processes, arrest of the growth of ovary and testes that are associated with endocrine disruption, loss of sperm and egg quality, inhibition of fertilization, hatching success, and also the reduction of larval survivability, thereby impairment of overall reproductive and developmental processes in fish. Disruption of the brain-pituitary-gonad axis, and certain enzymes related to steroidogenesis and vitellogenesis in fish have also been reported as the primary targets for an endocrine malfunction during hypoxia.Hypoxia-sensitive downregulation of key genes responsible for controlling sex hormones’ synthesis has been documented in certain bony fishes. Further, continuous exposure to hypoxia was reported to induce early expression of pro-apoptotic/tumor suppressor p53 genes, thereby causing immense cell death in hypoxic embryos. However, the cellular responses to long-term hypoxia exposure and the degree of reproductive impairments in bony fishes are still not adequate to figure out the actual underlying mechanisms. The present review intends to highlight the current knowledge about the detrimental impact of chronic/acute hypoxia at different stages of fish reproduction and the associated underlying molecular mechanisms.
1.Introduction
1.1.En route to aerobic respiration
The emergence of molecular oxygen (O2) in the atmosphere is considered a crucial factor for transforming the biochemical makeup of life and probably paved the way for the evolution of organismal diversification. From extensive biological and geological investigations, the vital role of atmospheric oxygen on biological evolution has been recognized (Jiang et al., 2012; Reinhard et al., 2016; Sessions et al.,2009). The atmosphere of the Earth existed in anoxic conditions during the Archean era (about 2.4–2.5 billion years ago), and the stromatolites/bio films of microorganisms, especially the cyanobacteria, were the primary life forms on the planet earth (Kump, 2008; Makishima, 2017).During the Paleoproterozoic era (approximately 2.0–2.4 billion years ago), the shallow ocean and the atmosphere of the Earth first experienced a rise in oxygen after the great oxidation event (GOE) and subsequently triggered the appearance of eukaryotes (Lyons et al., 2014;Zimorski et al., 2019). The Cambrian explosion of animal diversity,observed over 750 million years ago, was probably associated with the substantial rise in the level of atmospheric oxygen (Hsia et al., 2013;Lutz & Prentice, 2002). About 205 million years ago, the changes in animal body size and their metabolic activities that had occurred were mainly due to the elevation of atmospheric oxygen levels (Wheaton &Chandel, 2011). The GOE led to the extermination of most of the contemporary life forms, having no line of defense against reactive oxygen species (ROS), in a process that has been characterized as the“oxygen holocaust.” However, through the evolutionary process,oxygen-consuming organisms took advantage of more energy-efficient aerobic metabolism via aerobic respiration.
1.2.Normoxia v/s hypoxia
Aerobic organisms require molecular oxygen to regulate interdependent cell metabolism, growth, and survival. The oxygen demand of aerobes is ful filled through an effective system that distributes oxygen into the entire system and satis fies the metabolic requirements (Hsia et al., 2013). In mitochondria’s electron transport chain (ETC), the molecular oxygen acts as the final electron acceptor to produce adenosine triphosphate (ATP) through oxidative phosphorylation as the ultimate energy source, and the rate of oxidative phosphorylation depends on oxygen concentration in its physiological range bothin vivoandin vitroconditions (Wilson & Erecinska, 1985). Various physiological systems have been evolved in aerobic organisms to maintain oxygen homeostasis and ensure the ‘normoxia.’ In different physiological,pathological, and environmental conditions, organisms encounter insufficient oxygen availability, which causes ‘hypoxia’ (physiologically inadequate oxygen level). Hypoxia causes a collapse in bioenergetics due to the discrepancy in ATP production by oxidative phosphorylation and its utilization for energizing biochemical processes (Wheaton &Chandel, 2011). Depending on the respiratory response to hypoxia in animals, organisms that maintain their metabolic rates are known as‘oxyregulators,’ while those organisms that decrease energy expenditure during lower oxygen availability are known as ‘oxyconformers’ (Leiva et al., 2018). Most of the bony fishes are found to be oxyregulators(Svendsen et al., 2019; Ultsch et al., 1981), where during the progressive hypoxia, fishes try to maintain the oxygen consumption as long as the ambient partial pressure of oxygen does not become extremely low reaching the critical oxygen tension (pO2crit) (Tang, 1933), Generally it is not possible to sustain the aerobic metabolic below the level of pO2crit. The fish transform to anaerobic oxyconformers as their oxygen consumption declines with the decrease of ambient oxygen concentration. A few teleost species are known to be oxyconformers, including the marble swamp eel (Synbranchus marmoratus) (Grham & Baird, 1984),sturgeons (Acipenserspp.) (McKenzie et al., 2007), walking cat fish(Clarias batrachus) (Tripathi et al., 2013), and the plain fin midshipman(Porichthys notatus) (Craig et al., 2014; LeMoine et al., 2014). The depletion in the concentration of dissolved oxygen (DO) in water bodies causes substantial stress in fish and exerts adverse effects on numerous physiological processes like inhibition of growth rates, poor immune responses, and endocrine disruption (Abdel-Tawwab et al., 2019; Wu et al., 2003).
In this review, we assess the harmful effects of environmental hypoxia in fish reproduction, emphasizing gonadal development and maturation. We initially focused on reports from laboratory studies investigating reproductive impairments caused by hypoxia, followed by studies conducted in wild environments. Finally, we provide some future research dimensions that will aid in understanding the knowledge of the impacts of hypoxia on gonadal development and reproduction in bony fishes.
2.Prevalence of hypoxia in the aquatic habitat
Hypoxia means ‘low oxygen,’ and it happens only when the dissolved oxygen level reaches below 2–3 mg/L (Diaz & Rosenberg, 1995). An extreme form of hypoxia, i.e., when there is a complete lack of dissolved oxygen (0 mg/L), is de fined as anoxia. It is essential to establish the threshold level of oxygen, which will have lethal and sub-lethal effects on aquatic organisms. Simultaneously, the aquatic organisms will have to initiate different mechanisms to avoid catastrophic mortality due to hypoxia. In aquatic organisms, a broad range of oxygen thresholds starting from 0.28 mg/L to 4 mg/L have been suggested to be the major concern for hypoxia in different aquatic organisms (Paerl, 2006; Service,2004). Low dissolved oxygen environments occur in many aquatic bodies, varying in temporal frequency, persistence, and seasonality. The dissolved oxygen levels in littoral waters were recorded to change drastically over the last few decades, resulting in the widespread occurrence of hypoxia (Diaz & Rosenberg, 1995; Diaz, 2001). In several coastal sites, a continuous increase of hypoxic environment has been reported, leading to an exponential growth rate of 5.54% per year(Vaquer-Sunyer & Duarte, 2008). In certain cases, vast stretches of open water bodies are found to become hypoxic, unable to sustain life. These pockets of hypoxic zones are designated as ‘dead zones’ and are not suitable for fishery resources, such as bony fishes, shrimps, crabs,shell fishes, corals, and aquatic plants (Rabalais et al., 2002). Multiple monitoring studies, made on different marine zones such as the Gulf of Mexico, California, Coastal zone of Denmark, indicated that a considerable increase in the number of hypoxic zones appear regularly along with their extension, severity, and duration (Chan et al., 2008; Conley et al., 2007; Turner et al., 2008). Combined effects of anthropogenic exploitation of fertilizers and subsequent eutrophication have been suggested to be some of the additional causes of developing hypoxic growth areas, leading to excessive production and accumulation of organic matters and simultaneous increase in the oxygen demand by the coastal ecosystems (Deininger & Frigstad, 2019). The increase in surface temperature of water bodies, caused by climatic conditions of the atmosphere, is responsible for the further enhancement of respiratory oxygen demand of the aquatic biota (Harris et al., 2006), reduction in the solubility of oxygen, and also the ventilation of coastal water mainly by affecting the patterns of stratification (Stow et al., 2005). National Oceanic and Atmospheric Administration (NOAA) has reported the creation of the largest dead zone in the United States, which is formed every spring in the northern Gulf of Mexico. In 2019, the NOAA reported the creation of a dead zone in the USA that covered more than 6900 square miles of the sea floor (https://oceanservice.noaa.gov/hazards/h ypoxia/). The largest dead zone in the world is situated in the Arabian Sea, which covers almost the entire 63,700 square miles Gulf of Oman(https://www.nationalgeographic.com/environment/article/dead-zones). Sediment cores analyses made in the Bering Sea have been made to understand a cyclical relationship between the warmer climates and sudden events of low-oxygen ‘dead zones’ in the subarctic North Pacific Ocean (Knudson et al., 2021). Hypoxia, resulting from excessive anthropogenic input of nutrients, organic matters, and fertilizers into water bodies, has been suggested to be a cosmopolitan issue (Aarnio et al., 1998; Gamenick et al., 1996; Sandberg, 1997; Wu & Lam, 1997).Hypoxic or anoxic effects have been recognized to be common problems that are found in large regions of marine water bodies of North and South America, Africa, Europe, India, Southeast Asia, Australia, Japan,and also China (Diaz, 2001; Diaz & Rosenberg, 1995), as well as in certain freshwater bodies (Jenny et al., 2016; Keister et al., 2000;Landman et al., 2005). Due to the global increase in the number of ‘dead zones’ over the years, hypoxia will increase in terms of frequency,severity, and the large areas that are getting affected with the possibility of further exacerbation shortly (Diaz & Rosenberg, 2008). An ensemble study, made as a model for the Baltic Sea, has speculated a progressive increase of hypoxic and anoxic areas until a limit set by the bottom area below the halocline (Meier et al., 2011). Eutrophication resulting from hypoxia is now considered one of the major leading threats to different aquatic bodies globally, which ultimately leads to a severe loss of aquatic biodiversity. Hypoxia-mediated constraints impact surviving organisms through various ways, such as the induction of various physiological stresses, forceful migration, reduction of suitable habitats,reduced growth rate, and reproduction, enhanced susceptibility to predation, and also disturbances in life processes (Hale et al., 2016;Vaquer-Sunyer & Duarte, 2008; Villnäs et al., 2012).
3.Overview of reproductive processes in fish
There is a great variety of reproductive processes in fish. Nearly all bony fishes reproduce by sexual reproduction. Invertebrates regulate this intricate process of sexual reproduction by the highly conserved effects of endocrine components and related pathways, which are initiated by the hypothalamus-pituitary-gonadal (HPG) axis (Levavi-Sivan et al., 2010; Zohar et al., 2010).
3.1.The hypothalamus-pituitary-gonadal (HPG) axis
The endocrine regulation of the HPG axis in bony fishes is made up of three major sites, the hypothalamus, pituitary, and gonads. The HPG axis and the controlling mechanisms of reproductive hormones synthesis and their secretion are schematically shown in Fig. 1. Environmental stimuli like photoperiod, temperature, nutritional changes, and hypoxia are presumably received from the hypothalamus, leading to decapeptide gonadotropin-releasing hormone secretion (GnRH) hormone at the intercellular space pituitary hypophysis. The GnRH is released as a neurosecretion from nerve terminals and reaches the pituitary by circulation. GnRH specifically binds to the cellular receptor of the pituitary gonadotroph, thus causing the stimulation in the synthesis of two gonadotropic hormones (GtHs), luteinizing hormone (LH) and the follicle-stimulating hormone (FSH). Two gonadotropins, transported via circulation, bind to specific G-protein-coupled membrane GtH receptors(GtH-Rs), thereby promoting the synthesis and secretion of steroid hormones via the activation of G-proteins, adenyl cyclase, and also the Ca2+- dependent signaling pathways. FSH and LH stimulate the production of female steroid hormone 17β-estradiol (E2), followed by stimulation of ovarian oocyte development, production of vitellogenin(Vtg), and the precursor protein for egg yolk in the liver (Reading et al.,2018). In the case of male fish, Leydig cells are stimulated by gonadotropin leading to the synthesis of androgens (testosterone and 11-ketotestosterone), followed by the stimulation of spermatogenesis and spermiogenesis as a consequence of activation of Sertoli cells (Schulz et al., 2010). Other than GnRH, several neurotransmitters, which are released by the brain, are also responsible for regulating the piscine reproductive functions. Some of these neurotransmitters are kisspeptins,neurokinin B (NKB), pituitary adenylate cyclase-activating peptide(PACAP), neuropeptide Y (NPY), neurokinin F (NKF), secretoneurin,ghrelin, leptin, gonadotropin-inhibitory hormone (GnIH), serotonin,dopamine, γ-aminobutyric acid (GABA), dynorphin, and spexin (Kah et al., 1993; Trudeau et al., 2000).
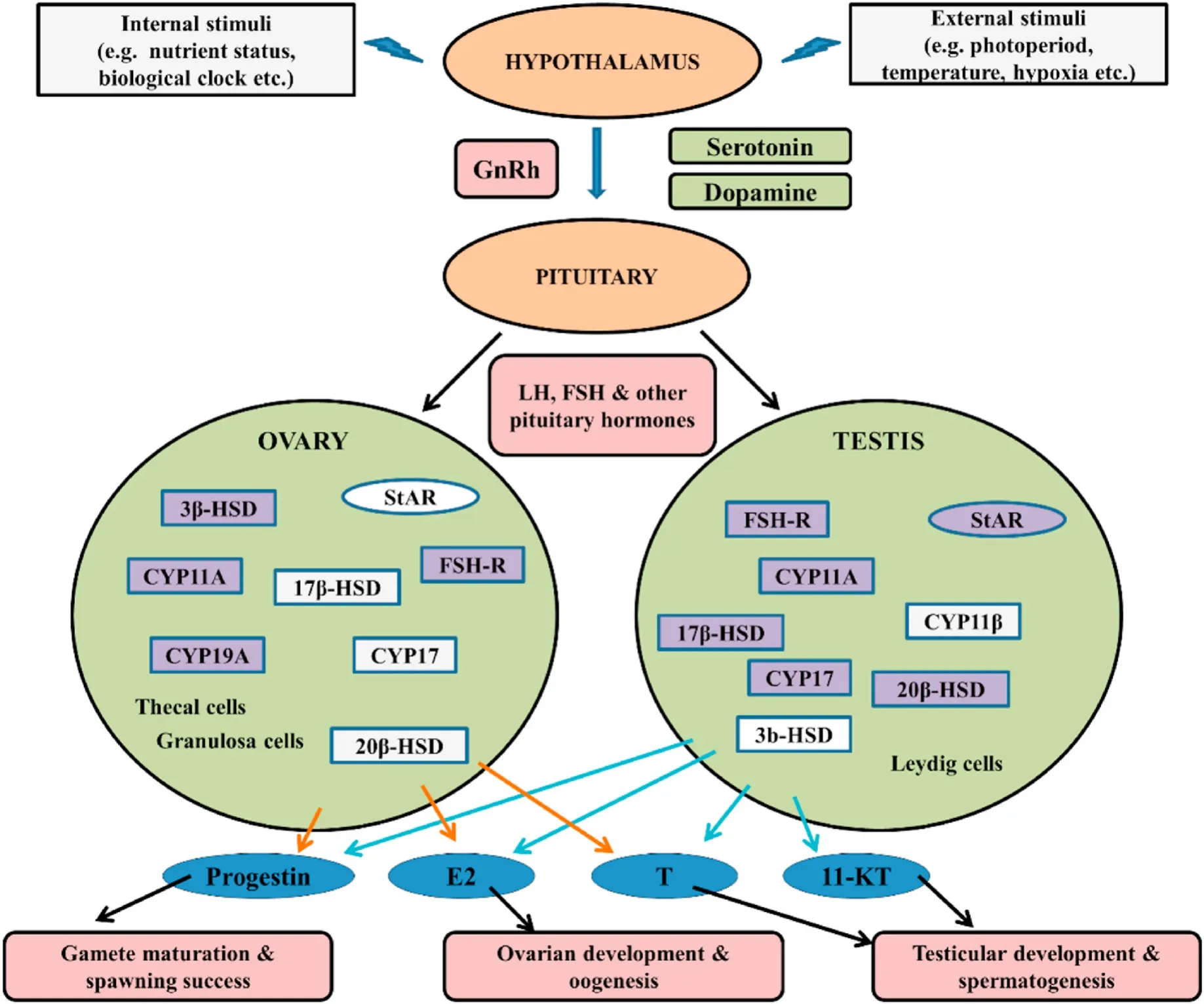
Fig. 1.Schematic representation depicting the roles of HPG axis in controlling the synthesis of reproductive hormones in bony fishes (modified from Wu, 2009).
3.2.Steroidogenesis and maturation of gonad
Steroidogenesis primarily occurs in adrenal and gonadal tissues(Konkal & Ganesh, 2020; Rajakumar & Senthilkumaran, 2020). An illustration of the steroidogenic process in bony fish is represented in Fig. 2. The steroidogenesis starts with the transport of cholesterol (a common precursor molecule for all the sex steroid hormones synthesis)into the mitochondria through the membrane of mitochondria by the stereogenic acute regulatory protein (StAR), which acts as a key regulator responsible for maintaining the timing and the rate of steroidogenesis (Rathor et al., 2017). With the help of the cholesterol side-chain cleavage enzyme (P450scc, coded bycyp11a1), cholesterol is then converted to pregnenolone on the inner membrane of the mitochondria(Hanukoglu, 1992). The synthesis of sex steroids from pregnenolone like 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DP), testosterone (T),11-Ketotestosterone (11-KT), and estradiol-17β (E2), as well as corticosteroids, are known to occur through the sequential action of several steroidogenic enzymes (Senthilkumaran, 2011). Maturation-inducing steroids (MISs), 17α,20β,21-trihydroxy-4-pregnen-3-one and 17α,20β-DP have been involved in the maturation of oocytes, and also to an extent in sperm maturation of teleost fish (Senthilkumaran et al., 2004;Sreenivasulu & Senthilkumaran, 2009). Progestins, like 17α,20β-dihydroxy-4-pregnen-3-one (DHP), and 17,20β,21-trihydroxy-4-pregnen-3-one (20βS), or both together could act as regulatory molecule(s) for fish sperm maturation and spermiation (for review, see Scott et al., 2010). Sperm maturation, mediated via the DHP through an increase in cAMP level, has been well documented in Japanese eel(Anguilla japonica) (Miura et al., 1991, 1995). Involvement of DHP in sperm maturation has also been reported in masu salmon (Oncorhynchus masou) and rabbit fish (Siganus argenteus) (Miura et al., 1992; Rahman et al., 2003). The involvement of other progestin like 20βS has also been demonstrated in the sperm maturation in Atlantic croaker (Micropogonias undulates), red drum (Sciaenops ocellatus), spotted sea trout(Cynoscion nebulosusand), and southern flounder (Paralichthys lethostigma) (Thomas et al., 2009). Steroid hormone DHP-mediated changes of pH, K+, and Na+concentrations in the sperm duct of some bony fishes are reported to be the causative agents in sperm maturation. In comparison, the progestin 20βS-mediated sperm maturation is reported to be modulated by changing the intracellular concentrations of Ca2+and cAMP in the sperm cells (Asturiano, 2020).
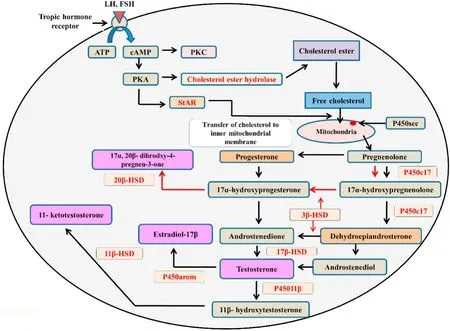
Fig. 2.A schematic diagram of the steroidogenic pathway in gonads of bony fishes. Black arrows indicate the androgen synthesis pathway, and red arrows indicate the progestogen synthesis pathway. Vital sex hormones are shaded in purple.
About 90% of Osteichthyes have distinct male and female gonads.The gonad is made up of two primary cell lineages, germ cells and surrounding the germ cells along with the somatic gonadal mesoderm.The gonadal development takes place in two phases. The first phase is the indifferent gonadal development, which occurs within the intermediate mesoderm, and arises as paired structure. The differential development of either the testis or ovary occurs in the second phase. The sexual differentiation of the fish gonad is essentially determined by either genetic or environmental factors that operate at the beginning of the developmental stages (Kobayashi & Nagahama, 2009; Siegfried,2010). Several essential genes, which are responsible for the initial differentiation steps, have already been identified, and these aredmrt1,amh, orsox9genes in male fish, andcyp19a1aorfoxl2gene in female fish (Lee et al., 2009; Nakamura et al., 2009; Wu et al., 2010). In a variety of fish species, high genetic variation has been identified, which is responsible for determining sex along with the number of genes involved in such processes and their interrelationships (Martínez et al., 2014).Out of all these genes, five master genes such asdmY,gsdf,amhy,amhr2,andsdYhave been identified, which play a vital role in sex differentiation in bony fishes. The endocrine system is known to play a crucial role in sex differentiation in bony fishes, which is mediated via the complex interaction between the BPG axis and various reproductive hormones(Devlin & Nagahama, 2002). For ovarian growth, the involvement of various hormones like gonadotropin, growth hormone, thyroid hormones, insulin, and insulin-like growth was demonstrated earlier in rainbow trout (Oncorhynchus mykiss), brown trout (Salmo trutta), and Chinook salmon (Oncorhynchus tshawytscha) (Tyler & Sumpter, 1996).However, the complex molecular and biochemical mechanisms of sex differentiation and gonadal development in fishes are not yet fully understood. Further extensive studies must be carried out to understand the exact scenario.
4.Effects of hypoxia
Hypoxia is a severe threat to aquatic systems worldwide since it leads to alterations in fish species composition, lowering the fish population,and fish biomass (Wu et al., 2003). The reproductive success of a fish depends on several interrelated factors such as the development of mature gonads, quality of gametes, reproductive behaviour, success in fertilization, and embryonic development, including the survival rate of larvae. Hormones, secreted by the HPG axis, are primarily responsible for controlling fishes’ seasonal reproduction, including gonadal development and gametogenesis.
Different water bodies are getting degraded as a consequence of input of different industrial and agricultural chemical wastes, which are known as endocrine-disrupting chemicals (EDCs) since they cause the disrupting of the endocrine orchestra, thereby leading to the impairment of reproductive ability in fish (Arcand-Hoy & Benson, 1998; Carnevali et al., 2018). The harmful effects of acute/chronic hypoxia on the reproductive processes and subsequent changes in endocrine function,gonadal development, gamete quality, fertilization, and larval survival of fish have been recognized in various fields and laboratory studies(Table 1).
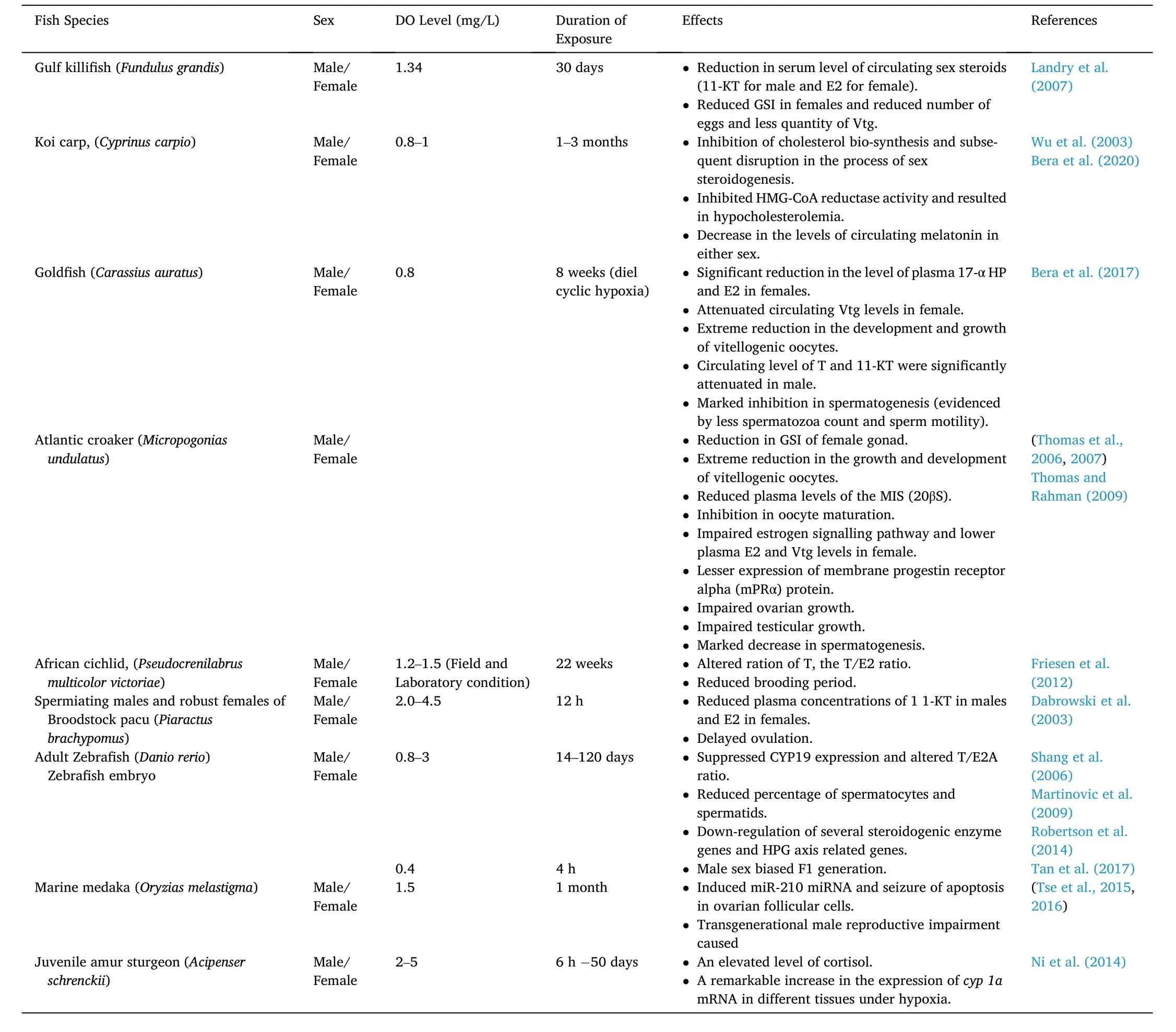
Table 1Reproductive impairments in different bony fishes exposed to hypoxia.
4.1.Sex hormones
Several studies suggested that hypoxia or oxygen de ficiency could act as a potential endocrine disruptor in fish (Wu, 2009; Wu et al., 2003).Hypoxia can lead to alterations in the levels of sex hormones, triiodothyronine (T3), and vitellogenin in fish, which will lead to retarded development of gonad, decrease in spawning success, stimulation of sperm motility, and hamper the fertilization, hatching rate, and larval survival as underlying mechanisms of reproductive impairments. A significant reduction in the levels of sex steroids (11-KT for males, and E2 for females) in the blood serum of estuarine fish (Fundulus grandis)was recorded during chronic hypoxic exposure (1.34 ±0.45 mg/L for 30 days) (Landry et al., 2007). But chronic hypoxia was not found to cause any changes in the levels of precursor hormone T in both the sexes, as well as in the levels of estrogen-induced protein vitellogenin in this estuarine fish. In koi carp (C. carpio) gonad, an inhibition in cholesterol biosynthesis and subsequent disruption in the process of sex steroidogenesis has been documented during exposure to chronic hypoxia (0.8±0.2 mg O2/L for 30 days) (Bera et al., 2020). Hypoxia is known to decrease serum 17α-hydroxyprogesterone (17-α HP) (a precursor of E2 and T synthesis) in both the sexes, thereby reducing the levels of 11-KT in males E2 in female koi carp. Inhibition of sex steroidogenesis was reported to down-regulate the activity of rate liming enzyme HMG-CoA reductase, involved in the mevalonate pathway for cholesterol synthesis(Eacker et al., 2008; Friesen & Rodwell, 2004). Furthermore, a recent report suggested that chronic hypoxia leads to stimulation of hypocholesterolaemia in the liver and gonad of both the sexes of koi carp due to inhibition of HMG-CoA reductase activity (Bera et al., 2020). Exposure to hypoxia (0.8 ±0.2 mg/L) for 9 h daily for 8 weeks (diel cyclic hypoxia) was reported to cause a significant decrease in the levels of plasma 17-α HP and E2 in female gold fish (Carassius auratus), thereby suggesting that these hormonal changes were associated mainly with the attenuated circulation of Vtg in female gold fish without causing any changes of plasma T levels in all the hypoxic fish (Bera et al., 2017). In male gold fish, under diel cyclic hypoxia, the circulating levels of T and 11-KT were significantly attenuated. Treatment with low DO (1.7 and 2.7 mg/L) led to an intense decrease in the growth of gonad, gametogenesis, and also the endocrine function on reproduction in both the sexes of Atlantic croaker (Micropogonias undulatus) (Thomas et al.,2007). During hypoxia, a significant decrease in the growth and development of vitellogenic oocytes along with the impairment of the estrogen signalling pathway, which is responsible for regulating the Vtg synthesis, was also noticed in female croakers. Further, the gonadal recrudescence in male croakers was significantly impaired during hypoxia, as evidenced by an apparent decline in spermatogenesis and the production of mature sperm, which was associated with decreased 11-KT signaling for regulating gametogenesis. A field survey made by Friesen et al. (2012), clearly indicated that geographical separation plays a critical role in sustaining estradiol in female African cichlid(Pseudocrenilabrus multicolor victoriae). They further suggested that hypoxia can also affect the levels of T, the T/E2 ratio, and the percentage of brooders in the female African cichlid. Out of these findings, it can be hypothesized that hypoxia affects the reproduction of fishes through a specific interruption in steroid biosynthesis. In broodstock pacu (Piaractus brachypomus), hypoxia exposure (2.0–4.5 mg/L) was associated with a significant decrease in the levels of plasma 11-KT in males and E2 in females along with their precursor T as compared to the fish kept under normoxia (Dabrowski et al., 2003). The changes in the levels of sex steroids, resulting during hypoxia, were found to be linked with the reduction in the gonadal development both in the male and female carps, and lesser success in spawning, sperm motility, fertilization,hatching rate, and also the rate of survival of larvae (Wu et al., 2003).Further, they suggested that all these adverse effects associated with the performance of reproduction in fish are an outcome of endocrine disruption caused due to hypoxia. In contradiction with the reducing tendency of sex steroids regardless of species and sex, a dramatic rise in the ratio of T/E2 was observed in female zebra fish (Danio rerio) during exposure to hypoxia (0.8 mg/L for 120 days) (Shang et al., 2006). It is to be noted that in all these above-mentioned studies, the decreases in the levels of sex hormones reported in different fish species were mainly associated with reproductive impairments. Due to having wide-range effects of hypoxia on different physiological and biochemical pathways, it has become a challenging task to find out the direct effects of hypoxia on reproduction and development in fish, and also it is very difficult to differentiate between the direct and indirect effects of hypoxia.
4.2.Gonad development and gametogenesis
Results obtained from multiple laboratory andfield analyses by various workers revealed that chronic hypoxia causes a significant reduction in the size of gonad and delay in gametogenesis and the development of gonad in bony fishes. After exposure to hypoxia for 8 weeks (1.0 mg O2/L), the gonadal somatic index (GSI) of adult carp(C. carpio) in male and female fish decreased significantly by 40% and 33%, respectively (Wu et al., 2003). All these events were also accompanied by a significant decrease in the number of spermatocytes and spermatids in the testicular lobules and an increase in the number of spermatogonia, thus suggesting that hypoxia in adult carp led to a retarded testicular growth and less production of sperm. Similarly, a significant decrease in the diameter of the testicular lobule was also noticed in male carp after 8 weeks of hypoxia. Similar retarded growth of gonads was also detected in female carp, with reduced gonad size and a lesser level of yolk in each egg. In hypoxic females, the stage III and IV oocytes constituted about 83.3% and 16.7%, respectively, but no stage IV oocyte was seen in any of the hypoxic females. The estrogen-mediated signalling disruption could be the possible cause of reducing the number of mature oocytes in hypoxic fish. Transcriptomic analysis of the ovary of marine fish provided further evidence that hypoxia causes impairment of the steroidogenic pathway (Lai et al., 2016). The stimulation of progesterone synthesis, inhibition of atresia of the reproductive tract,and the activation in the development of internal genitalia are considered as some of the harmful effects of hypoxia in teleost fish.
A significant reduction in the GSI caused a reduction in the production of eggs and also less quantity of Vt in the female Gulf killifish(F. grandis) during hypoxic exposure (1.34 mg/L for one month) (Landry et al., 2007). During hypoxia (0.8 mg/L for 120 days), the percentage of spermatocytes and spermatids was found to reduce significantly by 46.6% and 36.6%, respectively, in male zebra fish (D. rerio) (Shang et al.,2006). In the case of female zebra fish, oocytes remained predominantly in previtellogenic and vitellogenic stages under hypoxia, but under normoxia, oocytes reached the pre-ovulatory phase. In another study,made with female Atlantic croaker (M. undulatus), it was observed that hypoxia with 2.7 and 1.7 mg/L DO lead to shrinkage of GSI by 6.8 and 3.9%, respectively (Thomas et al., 2006, 2007). Similarly, in the case of male croaker fish, hypoxia led to severe impairment of gametogenesis,and only a tiny proportion of the spermatocytes progressed to the spermatozoan stage. The GSI value typically indicates the fish ovary’s ripeness (Tagarao et al., 2020). A higher GSI means the fish larvae are healthy and have higher survival rates (Rizzo & Bazzoli, 2020). Thus,from all these observations as mentioned above/reports, it may be contemplated that hypoxia leads to various harmful effects on fish gonadal development in both the sexes, and hypoxia-mediated disruption in gonadal development impairs fish reproduction and decrease their reproductive output and success.
4.3.Sperms and eggs quality
The motility of sperms always correlates with the positivity of reproductive success, while the less persistent movement of sperms(large de flection angle) correlates negativity of the reproductive success(Diemer et al., 2021). In adult male carp (C. carpio), the sperm motility was reported to decrease significantly following exposure to hypoxia (1 mg/L DO) for 12 weeks, thus indicating that there was an impairment of sperm quality (Wu et al., 2003). Testicular growth in Atlantic croaker(M. undulates) was found to get damaged significantly after 4 weeks of exposure to hypoxia (1.7 mg/L DO), resulting in a significant decrease in the number of motile sperms as compared to the fish kept under normoxia (Thomas & Rahman, 2009). Significant impairment of ovarian growth was also noticed in Atlantic croaker while exposed to hypoxia(1.7 mg/L DO) for 4 weeks, which resulted in a slight decrease in the size of fully grown oocytes (Thomas & Rahman, 2009). The plasma level of MIS (20βS) was also significantly reduced in Atlantic croaker after exposure to chronic hypoxia.
Further, in control fish, kept under normoxia, more than 50% of the large oocytes was reported to reach the germinal vesicle breakdown(GVBD) stage of oocyte maturation while incubating with gonadotropin for 18 h, while under hypoxia, only 20% of the total large follicleenclosed oocytes of female croaker fish experienced oocyte maturation with gonadotropin treatment for the same period (Thomas & Rahman,2009). The amount of the membrane progestin receptor alpha (mPRα)protein on the plasma membrane of large oocytes was also seen to reduce significantly during hypoxia exposure. In another study, made in Atlantic croaker, it was demonstrated that the maturation of oocytes under gonadotropin treatmentin vitrocondition also declined after exposure to hypoxia for 10 weeks (Thomas et al., 2007). Hypoxia exposure for 9 h daily caused marked inhibition (in the range of 69% compared to the normoxia group) in spermatogenesis in male gold fish(C. auratus) along with cessation of spermatozoa movement (Bera et al.,2017). Further, a dramatic reduction in the development and growth of vitellogenin oocytes was observed in the female gold fish during exposure to hypoxia. But, in the juveniles of common carp (C. carpio), no significant changes in the abundance of oocytes with tertiary yolk were observed during exposure to hypoxia (1.0 mg/L DO) (Wang et al., 2008).However, the oocyte percentage with a migrated germinal vesicle(GVM) in hypoxic female gonads was recorded to decrease significantly compared to the fish kept under normoxia. Hypoxia and re-oxygenation were reported to cause an imbalance in redox homeostasis due to overproduction of reactive oxygen species (ROS), resulting in severe oxidative stress-induced cellular damages in fish (Mustafa et al., 2011;Pei et al., 2021). Multiple biotic and abiotic factors and cellular redox homeostasis are suggested to be significant phenomena in the production of high-quality gametes in fish (Valdebenito et al., 2015).Several studies have demonstrated the direct harmful effects of oxidative stress on growth and reproduction in fish (Newman et al., 2015;Zhou et al., 2006). The hidden adverse role of oxidative stress can be contemplated on the reproductive impairment in fish during hypoxia.Exposure to hypoxia (1 mg/L DO) was found to induce oxidative stress and subsequent decrease in motility time and the number of mobile sperms in tambaqui (Colossoma macropomum) (Castro et al., 2020).ROS-mediated testicular damages and inhibition of oocyte maturation were observed in adult Koi carp (C. carpio) during chronic exposure to hypoxia (0.8 mg/L DO) (Bera et al., 2016).
4.4.Sex differentiation, sex development, and sex determination
Recent reports suggested that environmental factors like temperature, pH, density, and hypoxia can significantly in fluence sex and sex differentiation in teleost fish (Rajendiran et al., 2021). Theoretically, the variation in sex determination by the in fluence of environmental factors occurs much before the critical period of sex differentiation. To date, the exact underlying molecular mechanisms on the participation of the environment in sex determination remain unexplored. Sex determination is often biased to developing female characteristics in teleost fish,while fish living in captivity in a limited space and under high density usually result in a male sex-biased population (Davey & Jellyman,2005). The high density of fish population living under captivity are well known to face the problem of hypoxia (Abdel-Tawwab et al., 2014).Undifferentiated gonads of zebra fish (D. rerio) (24 hpf) showed a male sex-biased population (72.5% male) under hypoxia (0.4 mg/L DO),while fishes (36 hpf), kept under anoxia, are also reported to lead to the development of 71% male-biased population as compared with 41% males in the control group (Robertson et al., 2014). Results obtained from various studies demonstrated that exposure to chronic hypoxia during sex differentiation and sex development could be a guiding factor in altering the sex ratio in the fish population, thus leading to a male-biased F1generation. Hypoxia activates the stress axis, or the HPG axis as a molecular mechanism, which ultimately stimulates the expression of cortisone, followed by the conversion of cortisone to cortisol via the 11β-hydroxysteroid dehydrogenase (11β-HSD) enzyme,thereby participating in androgenic pathways (Strüssmann et al., 2021).The elevated circulating cortisol level leads to an increase of 11-KT level,which finally induces the development of the male-biased fish population. In response to hypoxia (2–5 mg/L DO), the cortisol level was reported to increase significantly in the juvenile of Amur sturgeon(Acipenser schrenckii) (Ni et al., 2014). Thus, it can be hypothesized that the negative feedback of cortisol leads to the masculinization of fish during sex differentiation by activating the pathway associated with the development of male gonads by inhibiting the expression of aromatase(Gardner et al., 2005). The de finite perception of hypoxia-mediated alterations in sex determination and differentiation remains mysterious to date and warrants further study.
4.5.Expression of steroidogenic enzyme genes
Several studies have been conducted to understand various hypoxia responses in gene expression better using cDNA microarray technology.Some of the responsive genes to hypoxia underpin several fundamental processes related to the reproduction and development of fish. All organisms are endowed with the ability to respond against hypoxia with certain changes in the expression of some specific genes. Homeostasis under hypoxia stress in animals, including fish, is known to maintain exclusively by one of the transcription factors, the α, β-heterodimeric hypoxia-inducible factor (HIF) (Semenza, 2000). Under hypoxia, the hypoxia-inducible factor-1α (HIF-1α) gets entry into the nucleus, followed by dimerization with HIF-1β to form a heterodimeric complex,which ultimately binds to the hypoxia-responsive elements (HREs) in the promoters to regulate the expression of multiple genes that are working against the harmful effects of hypoxia at the cellular level, and subsequently, at a systemic level for better adaptation to hypoxia(Wilkins et al., 2016). The regulatory role of HIF-1 on steroidogenesis and expression of the StAR gene has already been well established under hypoxia (Kowalewski et al., 2015). Hypoxia is known to cause signi ficant metabolic alterations in the gonad of mature fish. A microarray analysis was performed to decipher the hypoxia-associated response in gonads of adult zebra fish (D. rerio), which revealed that multiple genes are getting expressed differentially (Martinovic et al., 2009). Exposure to hypoxia for 4 days led to differential expression of 1520 genes in the testis and 480 genes in the ovary of zebra fish. After 14 days of exposure to severe hypoxia, 524 and 1627 genes in the testis and ovary, respectively, were found to express differentially as compared with those fish kept under normoxia. This study further demonstrated the downregulation of about 76% of HPG axis-related genes with upregulation of only a few HPG-axis related zebra fish genes under hypoxia. Prolonged exposure to hypoxia for 14 days caused the repression of cellular lipid metabolism-related genes and the genes involved in steroid hormones synthesis in testis. Hypoxia caused the suppression of thestargene(responsible for the transport of cholesterol, a rate-limiting enzyme for steroidogenesis) in the ovary and other genes involved in steroid hormones production (hsd11b,cyp19a1b). The regulatory effects of overexpression and knockdown of HIF-1 on specific steroidogenic enzymes genes, such asstar,cyp11a,cyp11b2,cyp17a1,3β-hsd,17β-hsd2,hmgcr,cyp19a, andcyp19bwere investigated in adult zebra fish (D. rerio) under hypoxia (1.0 mg/L DO) (Tan et al., 2017). Further, they demonstrated the regulatory function of HIF-1in embryonic development and steroidogenesis since it regulates the expression of several steroidogenic enzyme genes like astar,cyp11a,3β-hsd,andcyp19ain zebra fish.Seizure of apoptosis in ovarian follicular cells of marine medaka (Oryzias melastigma) has been reported as a consequence of the induction of miR-210 miRNA under hypoxia, which ultimately causes reproductive impairment of fish under hypoxia (Tse et al., 2015). Tse et al. (2016)demonstrated the possible roles of hypoxia-responsive miRNAs on testis functions in marine medaka (O. melastigma). They further suggested that alterations of multiple factors genes that were observed to be mediated via the hypoxia-responsive miRNAs under hypoxia ultimately lead to transgenerational male reproductive impairment. Integrated omics analysis revealed that epigenetic changes in the methylation pattern of the sperm genome are triggered due to exposure to hypoxia, which ultimately alters the expression of genes and proteins responsible for spermatogenesis and gene silencing, thereby leading to the reduction of sperm motility and sperm quantity in F0, F1, and also in F2 generations of fish (Wang et al., 2016).
5.Conclusion
It is a well-established fact that fishes are regularly exposed to the environment of changing oxygen concentrations in their natural aquatic habitats. They respond to hypoxia with various behavioural, physiological, and cellular responses to maintain homeostasis and organismal functions. Long-term severe hypoxia disturbs cellular homeostasis and affects normal physiological processes, including the reproductive system. Hypoxia can impair the reproductive success of fish by targeting the HPG axis and affects the subsequent downstream processes. Chronic hypoxia affects the overall reproductive coordination by affecting steroidogenesis, reproductive behaviours, production of quality sperms and eggs, fertilization, embryogenesis, success in hatching, and finally leading to malformation in fish. The harshness of hypoxic effects on fish reproduction depends on the degree of the hypoxic condition and the duration of hypoxia exposure. Pieces of evidence obtained from several studies, clearly indicated that hypoxia could act as one of the endocrine disruptors, thus affecting the HPG axis and the suppression of steroidogenesis, which ultimately affects the proper gonadal development in bony fish (Fig. 3). Changes in the levels of androgens and estrogens by hypoxic exposure have been demonstrated in several fish species both under laboratory and field conditions, thus suppressing both ovarian and testicular growth. Many laboratory and field studies revealed that hypoxic exposure leads to a significant decrease in the size of the fish gonad, accompanied by a delay in gametogenesis and gonadal development. Production of fewer spermatozoa counts, cessation in spermatozoa movement, and decrease in the development and growth of vitellogenic oocytes have been observed in fish under hypoxia exposure.Furthermore, suppression of CYP19 and alteration in the ratio of T to E2 during early development in zebra fish, observed during exposure to hypoxia, ultimately favour male-biased F1 generation. Similar effects of hypoxia, which were observed in fish reproduction and development,could also occur in other (higher) vertebrates since the endocrine regulation of the HPG axis, including the enzymes for steroidogenesis,are highly conserved processes everyday phenomena in all vertebrates.Additionally, various results obtained fromin vitroandin vivostudies in higher vertebrates also support this phenomenon. Reports on reproductive impairments and also different adverse effects on the overall physiology of fish, observed under hypoxia, reveal that hypoxia could be a severe threat in sustaining the natural fish population. This problem of hypoxic stress on the fish population is likely to be exacerbated shortly since the hypoxic environment in water bodies is continuously spreading over vast areas worldwide. Our knowledge of the cellular responses to long-term hypoxia exposure and the degree of reproductive impairments in bony fishes is still not adequate to figure out the actual underlying mechanisms. An in-depth multi-omics investigation is required to increase the present understanding of hypoxia-induced reproductive impairments in bony fish.

Fig. 3.Possible harmful effects of environmental hypoxia on the reproductive system in bony fishes.
CRediT authorship contribution statement
N·S.: conceptualization, writing original draft, supervision, visualization, review & editing. D.K.: conceptualization, writing, data compilation. R.S.: data compilation, writing.
Acknowledgments
This study was supported by a project sanctioned to NS by the Science and Engineering Research Board, New Delhi (CRG/2021/005588).
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Understanding the impact of stress on teleostean reproduction
- Control of gonadal maturation and sex in grouper
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
- Germ cell markers in fishes - A review
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Reproductive farming technology in Japanese eel and chub mackerel
