The potential of Eucheuma cottonii extract as a candidate for fish anesthetic agent
Ninik Purosari, Endang Warsiki, Khaswar Syamsu, Joko Santoso
aStudy Program of Aquaculture, Politeknik Negeri Lampung, Jalan Soekarno Hatta No 10 Rajabasa Bandar Lampung, 35144, Indonesia
bDepartment of Agro-Industrial Engineering, Faculty of Agricultural Technology, IPB University, Jalan Lingkar Akademik Kampus IPB, Darmaga Bogor, 16680,Indonesia
cDepartment of Aquatic Products Technology, Faculty of Fisheries and Marine Science, IPB University, Jalan Lingkar Akademik Kampus IPB, Darmaga Bogor, 16680,Indonesia
Keywords:
Active compounds
Eucheuma cottonii
Fish anesthetic
Phenol
A B S T R A C T
Red seaweed species Eucheuma cottonii was thought to have potential as an anesthetic agent because it is shown to contain compounds with bioactivity as antimicrobial, antifungal, and also anticancer. There have been no studies regarding the active ingredients of anesthetized. The objective of the study was to examine the potential of E. cottonii extract as a candidate for fish anesthetic based on toxicity, phytochemistry, and active compounds contained in the extract. The stages of research include extraction, toxicity test extracts (BSLT), phytochemical test, quantitative test compound phytochemical results, and the identification of active compounds with LC-MS.Test results showed that E. cottonii extract contains a total of phenols of 4.60 ±0.06 mg/GAE g which is one presentation of the active ingredient in the extract. The extract had bioactivity with LC50 of 221.71 ± 5.74 μg/mL. E. cottonii extract contained favonoids compounds and saponins with a consecutive number of 24.48% and 0.22%. The results of identifying active compounds were found in several compounds that had activity asantidepressants or sedatives. E. cottonii extract can be used as a candidate for a fish anesthetic agent.
1.Introduction
Anesthesia has long been used for the handling and transportation of live fish in aquaculture activities. Anesthetize conditions during transportation will reduce the level of stress, metabolism, and friction among fish individuals so that the degree of high livelihood. As long as these anesthetic agents are used more derived from relatively expensive chemicals and leave a residue that harmful when consumed such as tricaine metana-sulfonate/MS-222 ( Bolasina et al., 2017; Chance et al.,2018), benzocaine (Bizarro et al., 2018) and 2-phenoxyethanol(Hedayati, 2018). However, synthetic agents are forbidden to be used regarding security and resistance issues as well as costs that are often a limiting factor (Brown, 2011). The use of natural agents as anesthetic agents began to develop among them tuba root (Derris elliptica), nutmegMyristica fragrans(Al-Niaeem et al., 2017), and clove oil (Bizarro et al.,2017). Clove oil proved most effective and commonly used (Akinrotimi et al., 2016; Saini et al., 2018). Nevertheless, the lack of clove oil is a narrow range of concentrations and long recovery time (Abdolazizi et al., 2011; Sutili et al., 2014). Therefore, there is a safer alternative to natural anesthesia agents, leaving no residue, as well as a wider range of doses. One of the potential natural agents that can be used as candidates for an anesthetic agent is the seaweed that has been proven to have extensive bioactivity.
Eucheuma cottoniiis a species of red seaweed that contains various active compounds with its bioactivity as an antioxidant (Lim et al., 2015;Wulandari et al., 2018), antibacterial and antifungal (Selvan et al.,2014), as well as the anticancer (Namvar et al., 2012). The active compounds owned byE. cottoniiare polyphenols and their derivatives such as alkaloids, favonoids, and steroids. Also, there are tannin,saponin, and terpenoids. Meanwhile, the polyphenols from the brown seaweedEclonia cavaproved to have a sedative (anesthetic) effect (Cho et al., 2012); similarly, the compound in the treatment ofLaurencia dendroidahas the effect of larvicidal and anesthesia (Neto et al., 2016).Ekeanyanwu and Njok (2014) reported that the favonoids extract ofMonodora tenuifoliaseed proved toxic for an albino rat. Saponin is a toxic compound for fish and mollusk (Abele & Lukevics, 2001). The alkaloids(Jawale, 2014), saponins, tannins, favonoids, and steroids of ground plant extracts are proven to be larvacidal and as an anesthetic ingredient(Tsuchiya, 2017). Although several active ingredients contained in theE. cottoniihave been widely reported, there have been no studies related to the active ingredient of anesthesia, either the effectiveness of the process of extraction, toxicity and the type of active compounds. Based on the reports above: (1) saponin and favonoid compound inE. cottoniiproved toxic for fish; (2) the polyphenol from other types of seaweed are toxic to fish, whileE. cottoniialso contains polyphenol compounds, it is suspected thatE. cottoniihas activity as a sedative or anesthetic material with to arrange the dose. Thus the purpose of the study was to examine the potential ofE. cottoniiextract as a candidate for a fish anesthetic agent. Indicator of anesthesia potential seen from toxicity and the presence of compounds that have anesthetic or antidepressant activity.
2.Materials and methods
2.1.Extraction and determination of total phenolic content
SeaweedE. cottoniiused was obtained from Ruguk Village Ketapang Sub-district South Lampung. It was harvested on the 45th day conforming to the best result gained from the previous study. Fresh seaweedE. cottoniiwas washed in running water then was air-dried for 3 days.Subsequently, oven drying at 50-60°C for 10 h was performed, followed by grinding using a food grinder and sieving into 100 mesh (Soliman et al., 2017). The water content of the powder was estimated according to SNI 01-2891-1992.
The aqueous extract was performed by extracting 20 gE. cottoniipowder in 200 mL water at 120 r/min for a certain time in a shaking water bath (lab Companion BS-21) (Matanjun et al., 2008). The extraction was done at 30°C and 80 min in two replicates. The extraction indicator was resulting in total phenol.
In this study, phenol was determined as a compound representing the active ingredient ofE. cottoniiextract. Total phenol content was identified using the Folin-Ciocealteu method. Determination of total phenolic content incorporated gallic acid as a standard, and therefore the result was expressed as a percentage of gallic acid equivalent (GAE).Calculation of total phenolic content was done following (Permana et al., 2016) with a slight modification.
2.2.Toxicity assay
Toxicity assay was carried out using Brine Shrimp Lethality Test(BSLT) method (Kumala & Sapitri, 2011; Meyer et al., 1982). The stages included hatchment of shrimp (Artemia salina) larvae, preparation of the assayed solution, and toxicity assay of the extract. (a) Hatchment of shrimp (A. salina) larvae. Artemia eggs were placed in a hatchment box.The box was slowly filled with seawater up to half the total volume and reaching the height of the eggs. The box was covered with aluminum foil and was placed under 5 W light (24-28°C). After 48 h, shrimp larvae were collected using a pipette and were ready for toxicity assay. (b)Preparation of assayed solution. The extract analyzed was prepared at concentrations of 1000, 500, 100, and 10 μg/mL seawater. (c) Toxicity assay of the extract. 10-15 liveA. salinawere put into a 100 μL sea water-containing test vial, and sample solution at concentrations of 10,100, 500 and 1000 μg/mL was added, followed by mixing until homogenous. Each concentration was done in three replicates. As a control, a mixture was prepared without the addition of the sample and was let to stand for 24 h, followed by calculation of died and live shrimp larvae. Mortality was calculated by comparing dead larvae with total shrimp larvae.
A graph of log concentration against mortality was plotted. LC50value was obtained from drawing a line from a 50% point of mortality axis until the intersection with the plotted line. The point of the intersection at the concentration axis was recorded, which de fined a dose required to cause death for 50% larvae, expressed as LC50. A substance is called active or toxic when its LC50≤ 1000 μg/mL (Meyer et al., 1982).
2.3.Phytochemical assay
The qualitative phytochemical assay was carried out on favonoids,tannins, saponins, quinones, steroids, and triterpenoids, following methods employed by (Harborne, 1987; Ningsih et al., 2016; Suratno,2016). The procedure of the assay was as follows. (a) Flavonoids. 2 mLE. cottoniiextract was diluted into 2 mL methanol, followed by the addition of mg powder and 5 drops of concentrated HCl. The presence of favonoid compounds was shown by the formation of red or orange color. (b) Alkaloids. 2 mL chloroform and 2 mL ammonia were added intoE. cottoniiextract, followed by filtration. Three to five drops of concentrated H2SO4was added into the filtrate. The solution was mixed until it formed two layers. 2.5 mL top layer was transferred into three test tubes. The three solutions were analyzed with 4 drops of Mayer,Dragendorff, and Wagner reagents. The formation of sediment indicated the alkaloid content of the sample. Reaction with Mayer reagent resulted in white sediment. Reaction with Dragendorff resulted in orange-red sediment. Reaction with Wagner reagent resulted in brown sediment.(c) Tannins. 10 drops of FeCl310% were added into a certain mLE. cottoniiextract, followed by mixing for 1 min before the addition of 2 drops HCl 1 N. The extract was said positively containing tannins when producing a blackish-green or blackish-blue color. (d) Saponins. A certain mL ofE. cottoniiextract was added into 10 mL water with 1-min shaking, followed by the addition of 2 drops HCl 1 N. The extract positively contained saponins when the formed foams remained stable for 7 min. (e) Quinones. NaOH 1 N was added into a certain amount of sample, and color change was observed. A positive reaction was shown by the formation of a yellow color. (f) Steroids and triterpenoids. 10 drops of glacial CH3COOH and 2 drops of concentrated H2SO4were added into anE. cottoniiextract. The solutions were shaken and left to stand for minutes. A positive reaction of steroids was identified when green or blue color was produced, while a positive reaction of triterpenoid was identified when red or purple color was produced.
2.4.Identification of active compounds
The identification of active compounds was carried out by quantitatively measuring metabolite compounds from the result of the phytochemical assay with spectrophotometer and LC scanner. Also,other compounds were identified with and LC-MS. Identification of the compounds in the extract used LC-MS with the following process conditions: Column ACQUITY UPLC @ BEH C18 1.7 μm; Mobile phase: (a)ammonium formate, (b) acetonitrite, (c) methanol, and (d) water; Flow:water (+0.01% FA +0.05% ammonia); Injection volume: 8 μL; Column temperature: 28.2°C; Flow rate: 0.20 mL/min; Analysis time: 23.20 min.
2.5.Data analysis
The results from an assay with LC-MS were processed in MassLynx V4.1 software. The compounds found were interpreted using Chems pider.com.
3.Results
3.1.Phenolic content as a result of extraction
The total phenolic content ofE. cottoniiextract was 4.60 ±0.06 mg/GAE g, produced at a temperature of 30°C for 80 min. Total phenolic represented the active compounds ofE. cottoniiextract.
3.2.Toxicity
The toxicity assay ofE. cottoniishowed an L50value of 221.71 ±5.74 μg/mL. This suggested that theE. cottoniiextract had bioactivity, as reported by Meyer et al. (1982) that a compound has bioactivity if its LC50<1000 μg/mL. Thus the appropriate dose settings of the extract can serve as an anesthetic agent.
3.3.Phytochemicals
Results of the phytochemical assay onE. cottoniishowed that the extracts contained secondary metabolite compounds, e.g. favonoids and saponins. The result of measuring the number of favonoids compounds using spectrophotometers and saponins uses a consecutive TLC scanner of 24.48% and 0.22%.
3.4.Active compounds in E. cottonii extract
Identification of active compounds inE. cottoniiextract carried out in LC-MS exhibited the presence of other compounds as presented in Table 1. Various compounds have been identified with various bioactive, and many more compounds that have not been exposed may still be found in this extract.
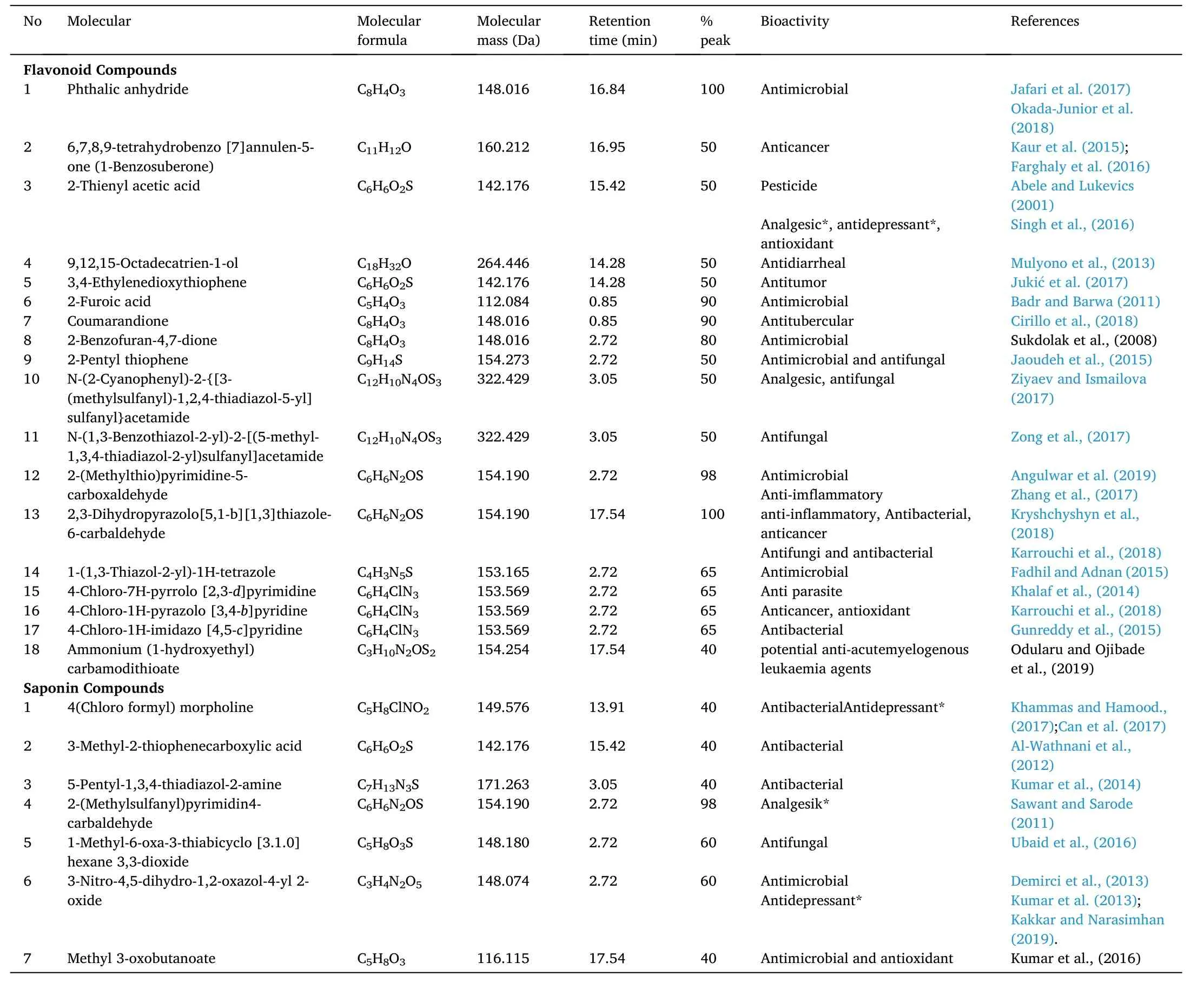
Table 1Results of E. cottonii active compound identification in LC-MS.
4.Discussion
4.1.Extraction result: total phenol
In extraction, water was selected as a solvent conforming to the preelementary study conducted. Water as a polar solvent has been shown to produce a relatively high content of active compounds fromE. cottoniiextract. Also related to its application in aqueous media, the extract with a water solvent is the best choice. When compared with the Results of existing studies, the research provides more value, where water was an effective solvent in extracting phenols as active compounds. This means that the extraction process is safer and economical. Total phenol of 4.60± 0.06 mg/GAE g accrued on 30°C and 80 min. It means the effective condition producing phenol. Extraction temperature and time affected the total phenol content (Sulaiman et al., 2017).
Total phenol is made as an extraction result indicator because in this research the phenol is de fined as a compound representing the active ingredient in theE. cottoniiextract. It refers to several research Results where polyphenols from brown seaweedE.cavahave anesthetic or sedative activity (Cho et al., 2012). Some other types of seaweed are also related to polyphenols and derivatives such as alkaloids, favonoids,steroids, tannins, saponin, and terpenoids with various bioactivities(Mohapatra et al., 2016).
Total phenolic content is different in different species, and even can be different in the same species as they are affected by the growing environment of the seaweed. Various literature related to the number of phenols in various plant extracts that have anesthetic activity is presented in Table 2.
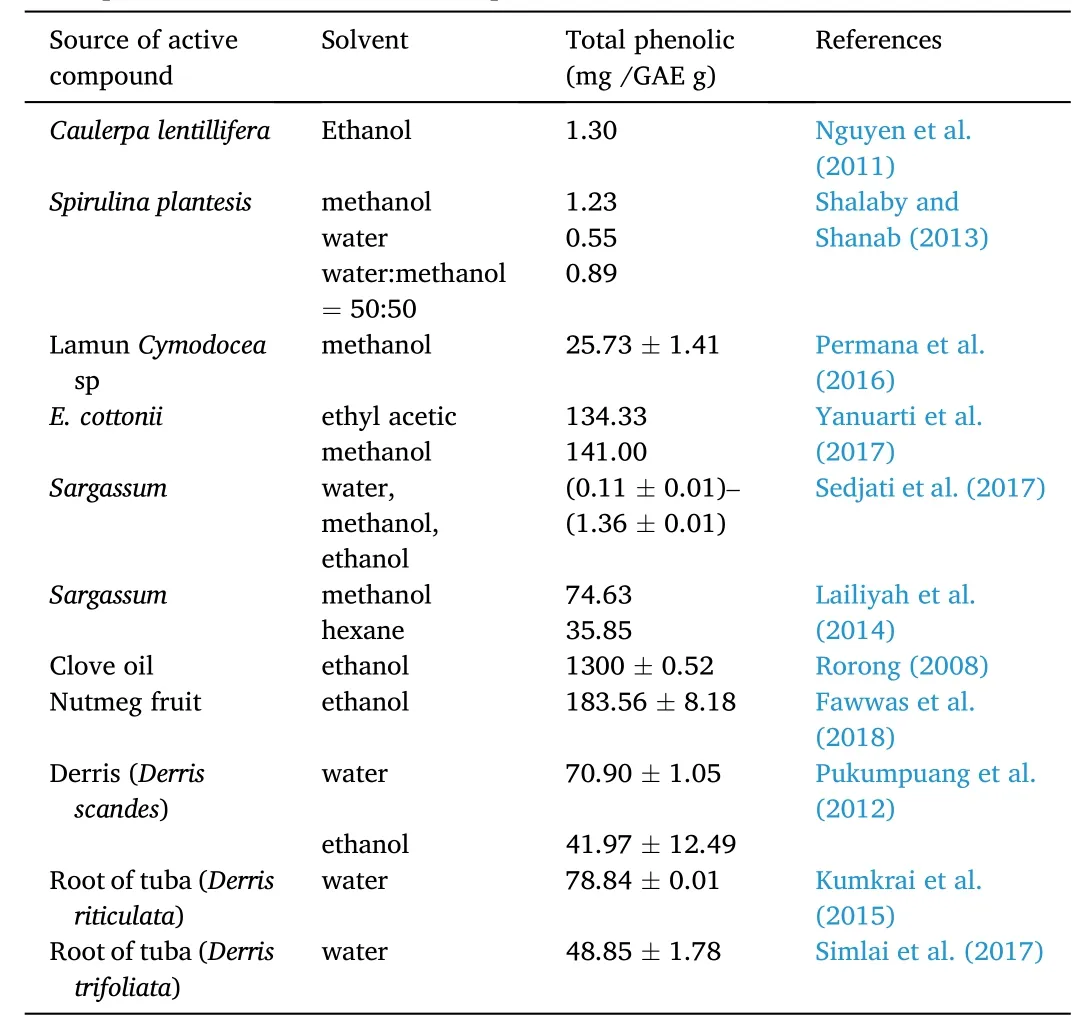
Table 2Total phenolic content of different plant extracts.
According to Results from previous studies presented in Table 2, theE. cottoniiextract showed a lower total phenolic content than that from the same species reported by (Yanuarti et al., 2017). Besides, the extract studied exhibited a higher total phenolic content when compared to extract of different seaweeds, either green seaweed Caulerpa (Shalaby &Shanab, 2013) or seagrass plants (Permana et al., 2016). However, the results of this study were considered better in terms of economic, safety,and environmental aspects, as the present study used water as a solvent while previous studies used organic solvents which were relatively expensive and left residues. Based on Table 2, it was noticed that terrestrial plant materials showed higher phenolic contents as compared to aquatic products, even though some materials were extracted using organic solvents (Zhu, 2009). The best effectivity was suggested by derris root with water as an extraction solvent. Therefore, following the background of this study in exploring the potential of aquatic products,the use of water solvent could be recommended for the extraction ofE. cottoniias a prospective anesthetic.
4.2.Toxicity of E.cottonii extract
The toxic properties ofE. cottoniiextract allow its use as an anesthetic ingredient by regulating its dose (Stehly & Gingerich, 2001). The toxicity ofE. cottoniiextract of 221.71 ± 5.74 μg/mL was higher than control, clove oil, showing LC50of 268.99 ± 1.60 μg/mL. Based on these data, the extract ofE. cottoniiis expected to give a higher toxic effect than the clove. Likewise,E. cottoniiextract is also expected to give a higher anesthetic effect than the widely used clove oil. The toxicity shown was higher than that shown by another natural ingredient, bee pollen, reporting a range of 292.53 ± 60.70 μg/mL (Chowdhury et al.,2017) and was nearly similar to the toxicity of derris root (Derris trifoliate), 211.31 μg/mL (Jiang et al., 2012). As derris root has been proven to serve as a fish anesthetic, it can be suggested thatE. cottoniiextract is also a prospective anesthetic agent for fish.
4.3.Compounds of phytochemical analysis
The phytochemical test results showed that the extract ofE. cottoniicontains saponins and favonoid compounds. The use of water as a solvent extract in this study suggests that both of these compounds can be taken effectively by water. However, different findings were reported by previous studies. Secondary metabolite favonoid has been effectively extracted with ethyl acetic solvent (Ebrahimzadeh et al., 2018) and methanol (Chai et al., 2015; Ganesan et al., 2008). Flavonoids are the largest polyphenol group which have a broad spectrum and biological activity associated with free-radical scavenging and antioxidant (Panche et al., 2016). Flavonoids have amphiphilic structures as well as local anesthetics (Tsuchiya, 2017).
Saponins are glycoside compounds showing a soap-like property due to its capability to form foams when being shaken in the water, for which it is called as a natural surfactant. In addition to the water, other solvents that can be used for saponin extraction are hexane, ethyl acetic, and methanol (Septiana & Asnani, 2012). Saponins can kill protozoans and mollusks and act as antifungal and antiviral agents (Addisu & Assefa,2016). Saponin components are responsible for local anesthetic effects for fish and mollusca. It can cause the plasmolysis of the red blood cells and death to the fish, while favonoids can render a calming effect(Tsuchiya, 2017).
The existence of the compound favonoids and saponins in the extract ofE. cottoniiin this study showed that the extract can be used as a candidate as a fish anesthetic agent. It supported several previous research results where favonoids serve as antioxidants, anti-cancer,anti-infammatory, and also is a modulation neuroreceptor that will affect the brain and nerve control that regulates conditions awareness(related to anesthesia). Another example, the favonoids in propolis are shown to be instrumental in increasing some of the biochemical markers associated with oxidative stress in the fish brain. Saponins are toxic to fish and amphibians. Saponins can also kill protozoa and mollusks and act as antiviral and anti-fungal (Addisu & Assefa, 2016).
4.4.Compounds active in E. cottonii extract
Subsequently, an assay to investigate other compounds contained in theE. cottoniiextract was performed in LC-MS. Table 1 showed the various active compound found in theE. cottoniiextract. The numerous active compounds and extensive bioactivity in theE. cottoniiextract have supported several existing research results. The earliest alleged extracts that contain antifungal compounds and pesticides also antidepressant compounds that have an anesthesia activity. The active compound “andrographolide” in sambiloto (Andrographis paniculata)leaves are often used as a fish anesthetic in addition to giving a larvicidal activity (Thangavel et al., 2015). Derris root showing larvicidal function in addition to serving as an insecticide (Jiang et al., 2012; Yi et al., 2012)is also frequently used as a fish anesthetic (Olufayo, 2009).
The main components of the compounds shown are calculated based on the percentage of peaks in the chromatogram. The compounds discussed are the favonoid and saponin class compounds that are thought to be responsible for the anesthetic process, namely their bioactivity as an antidepressant or analgesic. The main favonoid class compounds were 2-Thienyl acetic acid with a high peak of 50% at 15.42 retention time, while the main saponin class compounds were 2- (Methylsulfanyl)pyrimidin4-carbaldehyde, 3-Nitro-4,5-dihydro-1,2-oxazole-4-yl 2-oxide, and (Chloro formyl) morpholine with peak lengths of 98%, 60%,and 40% at retention times 2.72, 2.72 and 13.91, respectively (Table 1).This study showed that theE. cottoniiextract contains several antidepressant or anesthetic compounds so it makes one of the reasons thatE. cottoniican be used as a candidate for an anesthetic agent.
A variety of compounds identified has raised a new question whether the compounds would show an anesthetic activity following saponins and favonoids or would show other activities. The presence of compounds with antidepressant activity strengthens the chance of this extract as a candidate for anesthetic. Besides that, a great number of active molecules found inE. cottoniiextract with wide bioactivity makesE. cottoniia good source for pharmacological and health substances. This becomes a new opportunity for future studies.
However, in general, the results of this research can be said to be better than the economical, safety, and environmental-friendly side because the solvent used is water, where the results of other studies using organic solvents that have a higher price and leave a residue.Based on the background of this research, to explore the results of the water, thenE. cottoniiwith solvent water can be recommended as a total source of phenol which is potentially used as anesthesia.
5.Conclusions
The effective conditions for producing phenol fromE. cottoniiextract were extraction at 30°C and 80 min with the highest amount of phenol of 4.60 ± 0.06 mg/GAE g. The extract’s toxicity was 221.71 ± 5.74 μg/mL, indicating that the extract had bioactivity. The phytochemical results showed that the extract contained 24.48% and 0.22% of favonoids and saponins, respectively. The identification results of the compounds in the extract indicated the presence of several compounds in the saponin and favonoid groups that were antidepressants. Based on these conditions,E. cottoniiextract can be used as a safe natural ingredient as an anesthetic agent.
CRediT author statement
Ninik Purbosari: Conceptualization, Methodology, Investigation,Validation, Data curation, Software, Writing - original draft; Endang Warsiki:Conceptualization, Investigation, Validation, Data curation,Writing - review & editing; Khaswar Syamsu: Conceptualization,Investigation, Validation, Data curation; Joko Santoso: Conceptualization, Investigation, Validation, Data curation.
Declaration of competing interest
The authors declare no confict of interest.
Acknowledgment
We appreciate our collegue, Tabligh Permana for his assistance during data analysis.
 Aquaculture and Fisheries2022年4期
Aquaculture and Fisheries2022年4期
- Aquaculture and Fisheries的其它文章
- A review on fishing gear in China: Selectivity and application
- atg7 and beclin1 are essential for energy metabolism and survival during the larval-to-juvenile transition stage of zebra fish
- Association between single nucleotide polymorphisms of nLvALF1 and PEN2-1 genes and resistance to Vibrio parahaemolyticus in the Pacific white shrimp Litopenaeus vannamei
- Comparative analysis of the morphology, karyotypes and biochemical composition of muscle in Siniperca chuatsi, Siniperca scherzeri and the F1 hybrid (S. chuatsi ♀ × S. scherzeri ♂)
- Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia
- Differences in postembryonic dorsal fin development resulted in phenotypic divergence in two gold fish strains, Red Cap Oranda and Ranchu
