Differences in postembryonic dorsal fin development resulted in phenotypic divergence in two gold fish strains, Red Cap Oranda and Ranchu
Nn Yn, Jinqin Huo, Yu-Wen Chung-Dvidson, Wenyo Cui, Weijie Hung, Wei He,Qinghu Zhng, Weiming Li, Yn Zhou,, Jinfeng Ren,
aKey Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai, 201306, China
bDepartment of Fisheries and Wildlife, Michigan State University, East Lansing, MI, 48824, USA
cDepartment of Interventional Radiology, Shanghai Sixth People’s Hospital East, Shanghai, 201306, China
Keywords:
Egg-gold fish
Postembryonic development
Fin fold
Dorsal fin
Rays and radials
eomesa
A B S T R A C T
Gold fish (Carassius auratus auratus) are important ornamental fish that have experienced extreme anthropogenic selection and exhibit diverse phenotypes. Loss of the dorsal fin is a key characteristic that distinguishes the Egggold fish from the Wen-gold fish. However, the mechanisms underlying the divergence in dorsal fin development are still unknown between the Egg-gold fish and the Wen-gold fish. In this study, we sought to unravel the postembryonic developmental processes of two gold fish strains, Red Cap Oranda, a representative of the Wengold fish, and Ranchu, a representative of the Egg-gold fish. We examined the developmental morphology of the dorsal fin in five-month-old gold fish larvae using both Alcian blue-alizarin red staining and X-ray photography. We showed that the developmental processes of the dorsal fin fold were different in Ranchu compared to Red Cap Oranda, despite similarity in their general developmental processes. We categorized the postembryonic development of Ranchu larvae into four types based on the number of the initial residual dorsal fin fold appearing at protruding-mouth stage. The number, size and position of the initial residual dorsal fin fold appeared at hatching affected and eventually determined the phenotypic variations in dorsal fin defects in Ranchu. In addition, we genotyped the eomesa gene to determine whether it would be a candidate gene responsible for the loss of the dorsal fin in Ranchu. The eomesa CDS sequence exhibited no nonsense mutation in Ranchu. In summary, the absence of the dorsal fin fold during postembryonic development eventually resulted in the loss of the dorsal fin in Ranchu. Further investigation is required to determine whether other genes and developmental pathways play important roles in the loss of the dorsal fin in Ranchu.
1.Introduction
Gold fish (Carassius auratus auratus) are important ornamental Cyprinidae fish, which have experienced extreme anthropogenic selection during their domestication history. Gold fish breeding occurred in the Song dynasty of China over 1000 years ago (Wang, 2007). At least 180 variants and 70 genetically established strains are currently produced with divergent phenotypes in body shape, coloration and scale as well as the fin, eye, and hood morphology. Such wide phenotypic diversity makes gold fish excellent models to study vertebrate development,evolution and human diseases (Omori & Kon, 2019; Ota & Abe, 2016).There are three major gold fish taxonomy systems based on four important morphological characteristics, the body shape (slender vs.egg-shaped), the dorsal fin (retention vs. loss), the eye shape (normal vs.extended), and the caudal fin (single vs. double). The three-breed system, which classifies gold fish into Grass-gold fish, Egg-gold fish and Wen-gold fish, better indicates the domestication history of gold fish than the four-breed or the five-breed systems that emphasize the eye shape(Wang et al., 2013). In the three-breed system, the Grass-gold fish differ from their ancestors only in the color of the scales, and the Egg-gold fish differ from the Wen-gold fish in the phenotypes of the dorsal fin (loss or retention) (Wang, 1985). Therefore, fin phenotypes are informative for grouping gold fish with respect to their evolutionary history (Wang et al.,2013).
As important organs for locomotion and balance, fins include median fins (dorsal, anal and caudal fins) and paired fins (pectoral and pelvic fins) in fish. Median fins evolve earlier than paired fins, and the origins of median and paired fins are 100 million years apart (Goodrich, 1906).Therefore, studies on the development of median fins can provide significant insights for evolution. Paired fins are derived from median fins and later give rise to appendages with morphological modifications such as limbs or wings during vertebrate evolution. Thus, paired fins in fish are homologous organs to limbs and wings in tetrapods. Although the morphology of vertebrate appendages has undergone tremendous changes during evolution, the developmental processes and genetic regulatory networks are conserved. Examples can be found in the studies of limbs in mice (Mus musculus), wings in chickens (Gallus gallus), and paired fins in zebra fish (Danio rerio) (Zuniga, 2015).
Unlike paired fins, the developmental mechanisms of median fins are still elusive. Few studies have shown that paired fins and median fins have different embryological origins but share similar developmental mechanisms (Freitas et al., 2006). For example, specific genes or regulatory elements essential in limb development also affect the development of the dorsal fin.Eomes, a T-box family transcription factor, plays key roles in limb formation and development (Wilson & Conlon, 2002).A nonsense mutation ofeomesain zebra fish (eomesafh105) generated a truncated protein in the T-box domain and resulted in the delay in early embryonic development and loss of the dorsal fin (Du et al., 2012). A long range limb-specific enhancer, the zone of polarizing activity (ZPA)regulatory sequence, termed ZRS, controlsSonic hedgehog(Shh) gene expression critical for normal limb development (Sagai et al., 2005). A single-nucleotide mutation within ZRS causes limb malformations in multiple vertebrate species including humans (VanderMeer & Ahituv,2011). Sequential nucleotide substitutions or losses within ZRS region contributed to functional degenerationin vivoand resulted in phenotypic variations in snake body plan, extending from a basal form with vestigial limbs to more advanced form with no limb (Kvon et al., 2016).Deletions of both ZRS and shadow ZRS in Japanese medaka (Oryzias latipes) abolishedshhexpression and eliminated pectoral fin formation,whereas deletions of ZRS resulted in complete ablation of the dorsal fin.This finding indicates that a ZRS-Shhregulatory module was shared by paired and median fins and that paired fins likely emerged by duplication and co-option of developmental programs established in the median fins, supporting the notion that paired fins originated from the median fins with shared developmental mechanisms (Letelier et al., 2018).
Lack of the dorsal fin is a key characteristic that distinguishes the Egg-gold fish from the Wen-gold fish (Wang, 1985). The embryonic and postembryonic developmental processes in the wild-type gold fish(Wakin), and the twin-tail gold fish (RyukinandOranda, both with the dorsal fin) have been well documented (Li et al., 2015, 2019; Tsai et al.,2013). However, the differences in dorsal fin development are still unknown between the Egg-gold fish and the Wen-gold fish. In this study, we sought to elucidate the processes of dorsal fin development by comparing the postembryonic development in two gold fish strains,Red Cap Oranda, a representative of the Wen-gold fish, andRanchu, a representative of the Egg-gold fish. We hypothesized that the postembryonic developmental process ofRanchudiffered from that ofRed Cap Oranda,resulting in the loss of the dorsal fin inRanchu. We also suspected thateomesacould be a candidate gene responsible for the loss of the dorsal fin inRanchu. The results from this study are a first step in laying a foundation for future studies to elucidate the mechanisms underlying the loss of the dorsal fin in Egg-gold fish.
2.Materials and methods
2.1.Fish stocks and artificial fertilization
Sexually matureRed Cap OrandaandRanchugold fish were purchased from Nantong gold fish farm, Jiangsu Province, and then transported and maintained at Xinchang Fish Farm, Pudong New District,Shanghai. Spawning fish were transferred to the Campus of Shanghai Ocean University, and embryos were obtained through artificial fertilizations. Briefy, the eggs and sperms of gold fish were squeezed out from a mature female and male into 9-cm Petri dishes and fertilized using dry methods (Tsai et al., 2013). The fertilized eggs were spread onto the Petri dishes and hatched in a large tank (40 cm × 23 cm × 25 cm) at 26°C. The embryos hatched about 3-4 days post fertilization (dpf). The larvae were fed with brine shrimps three times per day when they began to swim after 3-4 days post hatch (dph).
2.2.Microscopic observation and photography
TwentyRed Cap Orandalarvae and 60Ranchularvae were randomly selected from the hatching tank and each larva was raised individually in one plastic cup (200 ml). To record the developmental processes, each larva was observed every 3-5 days during 3-30 dph. The larva was transferred into a hollow-ground slide containing 0.016% anesthetic MS-222 (Sigma-Aldrich Inc. USA) and imaged under a stereo microscope(Zeiss stemi 2000). The standard length (SL, in mm) of the larva was measured from the tip of the mouth to the caudal peduncle using a Zeiss ZEN 2012 software.
Five-month-old gold fish juveniles were photographed with a digital camera (Canon EOS 60D) and individual SL was measured with a vernier caliper (ResTure IP67, Harbin Measuring & Cutting Tool Group Co. Ltd.,China). The gold fish were also anesthetized and photographed with X-ray using a Mammomat Novation DR (Siemens, Germany).
2.3.Alcian blue and alizarin red staining
Skeletal staining of five-month-old gold fish was performed according to previously established methods with minor modifications (Harris et al., 2008). The anesthetized gold fish were fixed with 4% paraformaldehyde (Sigma-Aldrich Inc.) at 4°C overnight and bleached with a mixture of 1% KOH and 3% H2O2until the pigments were faded. The gold fish were then stained with 0.05% alcian blue (Sango Biotech Inc.China) at 4°C for 2 days in the dark, dehydrated with an ethanol gradient and digested with 0.1% trypsin solution (Sango Biotech Inc.) at 37°C for 2 days until the bones were clearly visible. Finally, the gold fish were stained with 0.02% alizarin red (Sango Biotech Inc.) at 4°C for 3 days in darkness, immersed in a glycerol gradient and stored in 100% glycerol (Sigma-Aldrich Inc.). The Skeletal structure was observed with a stereo microscope (Zeiss stemi 2000) and analyzed with the ZEN software.
2.4.eomesa CDS sequence cloning and analysis
Total RNA was isolated from muscle tissues ofRanchuusing TRIzol reagent according to the manufacturer’s manual (Thermo Fisher Scientific, USA). Subsequently, mRNA was reversely transcribed into cDNA using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa,Japan). The full-length CDS sequences ofeomesa1andeomesa2were amplified simultaneously using a pair of primers that were designed based on the conserved regions ofeomesa1andeomesa2(forward primer: 5′ATG CAG TTA GAA AGC ATC CTC CCT G3′, and reverse primer: 5′TTA AGG GCT GGT GTA GAA GGC GTA A3′). The PCR products were cloned into a pESI-T vector (Yesen Biotech. Inc., China),and then transformed into DH5α competent cells (TianGen Biotech. Inc.,China). The plasmids were extracted and sequenced with an ABI 3730xl DNA Analyzer.
The reference CDS and the deduced amino acid sequences ofeomesa1andeomesa2genes from the reference genome of gold fish strainWakinwere downloaded from NCBI (eomesa1: XM_026289312 and XP_026145097;eomesa2: XM_026232450 and XP_026088235). The CDS sequence and the deduced amino acid sequence ofeomesagene from the zebra fish were downloaded from Ensembl (ENSDART00000122257 and ENSDARP00000111994). The nucleotide and amino acid sequences ofeomesa1andeomesa2genes from different gold fish strains were aligned with those ofeomesagene from the zebra fish using Clustal X 1.83. The sequence identity ofeomesagenes among different gold fish strains and the zebra fish were also analyzed at the nucleotide and the amino acid levels.
3.Results
3.1.Morphological comparisons of parent gold fish: Red Cap Oranda vs.Ranchu
The postembryonic developmental processes of two different goldfish strains,Red Cap Oranda, a representative of the Wen-gold fish(Fig. 1A), andRanchu, a representative of the Egg-gold fish (Fig. 1B)were investigated. These two strains of adult gold fish share many similar morphological features, including a globular body, a bifurcated caudal fin, and a well-developed warty epidermis on the head. InRed Cap Oranda, the warty epidermis grows on top of the cranium like a red cap,which is its namesake. InRanchu, the warty epidermis grows around the face and cranii (Fig. 1). In addition, theRanchustrain is distinguished from theRed Cap Orandaby the lack of the dorsal fin (Fig. 1B). Further examinations were focused on the differences in dorsal fin developmental processes between these two gold fish strains.

Fig. 1.Morphological comparison of parent gold fish between Red Cap Oranda (A) and Ranchu (B). (For interpretation of the references to color in this figure legend,the reader is referred to the Web version of this article.)
3.2.Normal postembryonic development of Red Cap Oranda larvae
Gold fish postembryonic stages comprise larval, juvenile and adult periods. In this study, we focused on the gold fish developmental processes during the larval period, which was categorized into protrudingmouth (Prot), posterior swim bladder (Psb), caudal fin ray (Cr), forked caudal fin (Fcf), anterior swim bladder (Asb), dorsal fin ray (Dr), anal fin ray (Ar), pelvic fin bud (Pb), and pelvic fin ray (Pr) stages in a previous study (Li et al., 2015). Based on the reported staging indices, we examined the postembryonic developmental processes ofRed Cap Orandalarvae.Red Cap Orandaexhibited normal larval developmental processes, similar to the twin-tail strainsRyukinandOranda, and the common strainWakin(Li et al., 2015, 2019).
Gold fish individual hatched out at 3-4 dpf and began its larval Prot stage. At this stage, the fish body truck was surrounded along the midline by a somewhat continuous median fin fold, which consisted of three lobes, the dorsal, caudal and ventral fin folds (pre- and post-anal fin folds). Most yolk remained and the swim bladder was not yet developed (Data not shown). At the early Psb stage (Fig. 2A), the residual yolk was absorbed and the posterior swim bladder appeared with silver black color and oval shape. A heart full of fowing blood under the anterior trunk and an enlarged blood island at the postcloacal region were visible. All fin folds expanded away from the body, with the pre-anal fin fold increasingly rounded. Meanwhile, the bifurcated fin folds around the tail tip were observed. At the late Psb stage (Fig. 2B), fin folds began resorbed and the dorsal fin fold and postanal fin fold were slightly reduced compared to early Psb stage. The notochord of the caudal region remained straight (Fig. 2A and B).

Fig. 2.Normal postembryonic development processes in Red Cap Oranda.Standard length (number in mm) are shown at the lower left corner of each panel. White arrow indicates the caudal fin ray; black arrows indicate the lobe of dorsal and post-anal fin folds; brown arrows indicate the pre-anal fin folds. Black arrowheads indicates the position of dorsal fin bud (A′-F′), dorsal fin rays (G′-H′) and ray segment addition (I-J′). Brown arrowhead indicates the position of anal fin bud (A′-H′), anal fin rays (I′) and ray segment addition (J′). Red arrow indicates the position of pelvic fins (J′). A′-J′are magnified view of A-J. Scale bars, 1000 μm (A-J) and 500 μm (A′-J′). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The larva reached the Cr stage at 6.4 mm SL. Typical features of this stage were an evident fection in the notochord and visible caudal fin rays at the posterior side of the notochord fection (Fig. 2C). Transparency of the larval body gradually lost with the development of the cranial and trunk muscles. Concurrently, the heart and blood vessels also became invisible gradually (Fig. 2C and D). The middle of the dorsal fin fold showed a significant resorption. Meanwhile, the anterior of the dorsal fin fold bulged out from the mid-trunk at a mesenchymal condensation spot where the dorsal fin development initiated (Fig. 2D and E).
The anterior lobe of the swim bladder was visible from the lateral view at the early Asb stage (Fig. 2F). Initially the anterior swim bladder lobe was smaller than the posterior one, and was round in shape.Gradually, the anterior lobe of the swim bladder increased in size,eventually became larger than the posterior lobe (Fig. 2F and G). The dorsal and post-anal fin folds were evidently reduced compared to the Dr stage (Fig. 2G). The dorsal fin fold at the mid trunk level was prominent,and three or four dorsal fin rays were visible (Fig. 2G’). The dorsal and post-anal fin folds mostly remained at the level of the notochord fection,and a large part of the pre-anal fin fold also remained. The caudal fin gradually switched from asymmetrical shape to almost symmetrical homocercal shape (Fig. 2G and H).
The dorsal and anal fin folds nearly disappeared at the Ar stage (8.8 mm SL, Fig. 2I). The residual dorsal and anal fin folds were much smaller, especially the dorsal fin fold proximal to the anal fin. Both the dorsal and anal fin folds contained fin rays. Eleven dorsal fin rays and five anal fin rays could be seen in the individual. The outlines of the dorsal and twin-tail caudal fins were clear, forming a curved triangular shape (Fig. 2I). The developmental process of the anal fin was similar to that observed in the dorsal fin, but the timing was later. At the posterior end of the anal fin, part of the anal fin fold and most of the pre-anal fin fold remained (Fig. 2I). The anterior lobe of the swim bladder was visible while the posterior lobe became a stripe from the body surface as the body obtained more muscles and the intestine became semitransparent by the pelvic fin ray stage (Fig. 2J). The dorsal and anal fin folds in the caudal region remained at the caudal peduncle. Fin rays were visible in the remaining dorsal fin fold in the caudal region. The post-anal fin fold nearly disappeared but the pre-anal fin fold still remained. The pelvic fin rays were visible from the lateral view. The dorsal rays at anterior regions were elongated. The pelvic fin and its rays were easily observed on the lateral surface of the trunk (Fig. 2J).
3.3.Abnormal postembryonic development of Ranchu larvae
Ranchuexhibited similar larval developmental processes as theRed Cap Oranda, except for the dorsal fin fold development. During the early period of the fin fold development inRanchu, the ventral portion of the median fin fold developed normally, but the dorsal portion was almost absent compared toRed Cap Oranda(Fig. 3). The size of the dorsal fin fold varied at hatching, resulting in different degrees of losses in the dorsal fin.
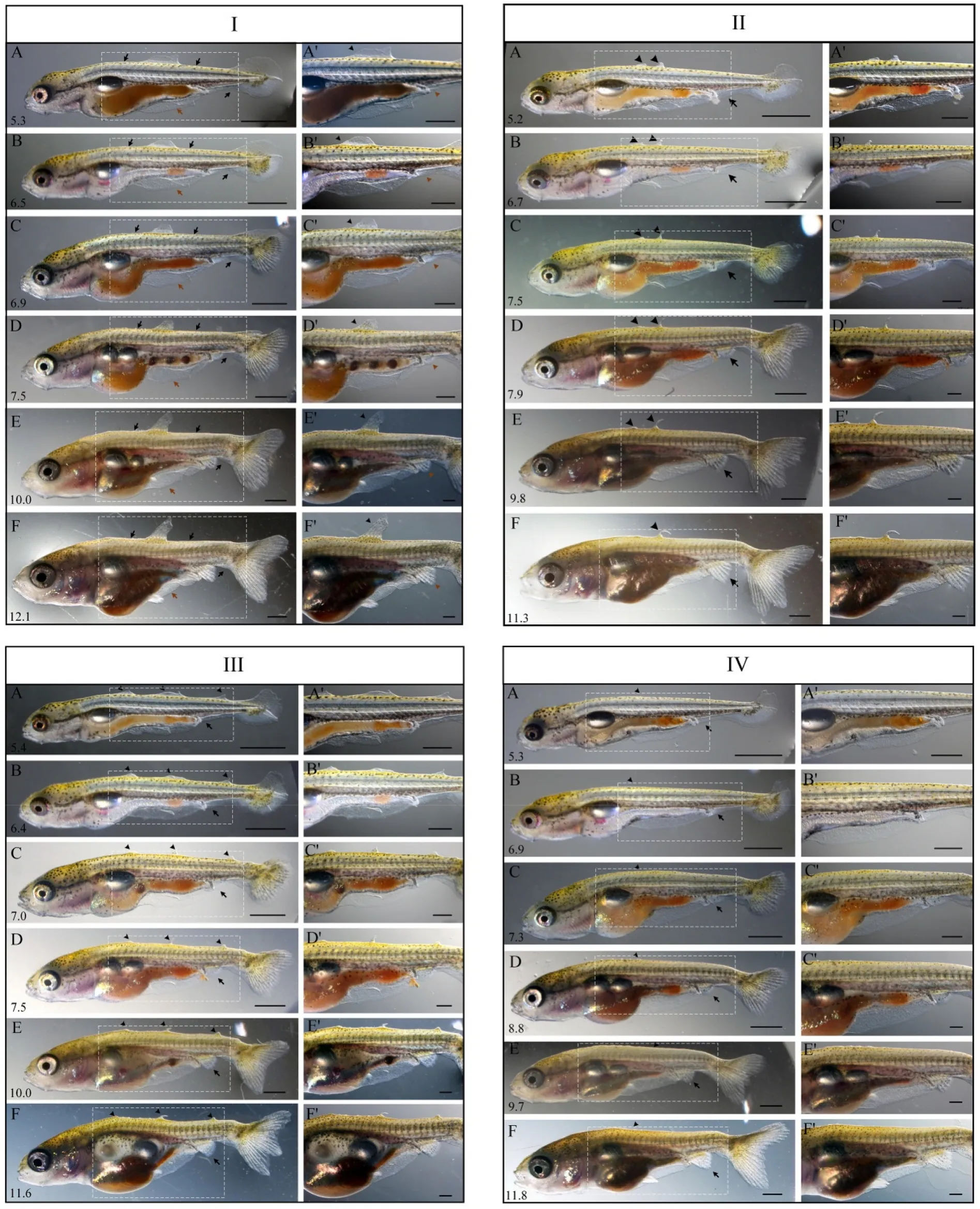
Fig. 3.Abnormal postembryonic developmental processes in Ranchu.The postembryonic developmental processes of Ranchu larvae were classified into four types (Type I, II, III and IV) based on the number of initial residuals of the dorsal fin fold appeared at Prot stage. Type I contained one residual (I), Type II contained two residuals (II), Type III contained three residuals (III) and Type IV contained no residual (IV). Standard length (number in mm) is displayed at the lower left corner of each panel. I: black arrows demarcate where the dorsal fin fold normally starts and ends; brown arrows indicate the pre-anal fin folds. Black arrowheads indicate abnormal fin folds; brown arrowheads indicate the anal fin. II-IV:black arrowheads indicate the development of residuals of the dorsal fin fold; brown arrowheads indicate the development of the anal fin. A′-F′are magnified view of A-F. Scale bars, 1000 μm (A-F) and 500 μm (A′-F′). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We categorized the postembryonic development ofRanchularvae into four types (Type I, II, III and IV) based on the number of the residual dorsal fin fold appeared at Prot stage. Type I contained one (Fig. 3I),Type II contained two (Fig. 3II), Type III contained three (Fig. 3III) and Type IV contained no residual dorsal fin fold (Fig. 3IV). In each type, the individuals could be further classified into several subtypes according to the size and position of the initial residual fin fold and the final appearance of the dorsal fin at the end of larval stage. For example, Type I was classified into three subtypes according to the size and final appearance of the initial residual fin fold (Fig. 3I and Fig. S1). The dorsal fin fold was divided into three parts: anterior, middle, and posterior,each consisted of roughly 1/3 of the dorsal fin fold. Initially, the larvae either retained a large (contained more than 3 fin-rays) residual of the anterior dorsal fin fold (Fig. 3I-A), a tiny (contained less than 3 fin-rays)residual of the anterior fin fold, or a tiny residual of the middle fin fold(Fig. S1-IA, IIA). Eventually, the large residual developed into a dorsal fin with one spine and four rays by ~12.1 mm SL (Fig. 3I-F), while the tiny residual of the anterior or middle fin fold developed into a hard spine or completely degenerated, respectively (Fig. S1-IF, IIF).
Type II was classified into four subtypes according to the size and final appearance of two initial residual fin folds (Fig. 3II, Fig. S2).Initially, the larvae either retained two tiny residuals at the anterior portion of the dorsal fin fold (Fig. 3II-A), or a tiny one at the anterior and a tiny one at the posterior portion of the dorsal fin fold (Fig. S2-IA, IIA,IIIA). These two residuals were destined for three developmental outcomes: one was absorbed and the other developed into a residual dorsal fin with one spine (Fig. 3II-F) or with one spine and rays (Fig. S2-IIF), both completely degenerated (Fig. S2-IF), or both remained(Fig. S2-IIIF).
Type III was further classified into two subtypes according to the size and final appearance of three initial residual fin folds. Initially, the larvae retained three tiny residuals of the dorsal fin fold (Fig. 3III,Fig. S3). Eventually, these tiny residuals either remained and developed into residual dorsal fins (Fig. 3III), or completely degenerated and developed into a smooth dorsum (Fig. S3). In Type IV, the majority of the developmental norm, the larvae hatched out with a smooth dorsum without the dorsal fin fold (Fig. 3IV).
The percentage of offsprings in these four types was calculated from two crossing experiments (Table 1). In the first crossing combination,the number of larvae (N =30) in each type was 12, 10, 3, and 3,respectively, recorded when the larvae were ~5.3 mm SL. The distribution significantly changed during the development. The residuals of the dorsal fin fold gradually degenerated and the number of larvae without the dorsal fin fold increased from 3 to 16 by ~10.0 mm SL. In the second crossing combination, the number of larvae (N =28) in each type was 7, 0, 1, and 20, respectively, recorded when the larvae were~5.3 mm SL. The number of larvae without the dorsal fin fold increased from 20 to 24 by ~10.0 mm SL (Table 1). Unexpectedly, we found two larvae with normal dorsal fin fold at ~5.3 mm SL, which developed into normal dorsal fin by ~11.5 mm SL (Fig. S4).

Table 1Ranchu offsprings with four types of residual dorsal fin fold from two crossing experiments.
3.4.Developmental defects in dorsal fin rays and radials of Ranchu
Alcian blue-alizarin red skeletal staining and X-ray photography revealed the cartilage and bone development of the dorsal fin inRed Cap OrandaandRanchu(Fig. 4 and Fig. S5).Red Cap Orandadisplayed a normal dorsal fin under optical observation and skeletal staining(Fig. 4A, A’). The appearance of the dorsal fin was visible with two spines, one short and one long (not segmented), and 14 fin rays(segmented and branched). Alcian blue-alizarin red staining demonstrated the complete structure of the dorsal fin with endoskeletal radials and the exoskeletal fin rays, with some cartilaginous and some ossified(bony). Each exoskeletal fin ray was paired with one endoskeletal radial,with the exception of the long spine and the first ray that were paired with one radial together.
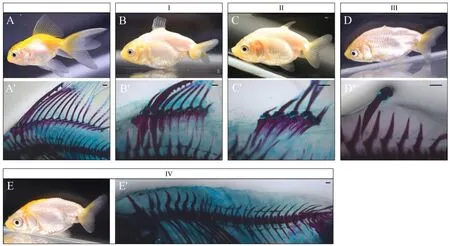
Fig. 4.Photographs of skeletal morphology of Red Cap Oranda and Ranchu with Alcian blue-alizarin red staining.Panel A and B-E (four types of dorsal fins, I-IV) are photographs of Red Cap Oranda (A) and Ranchu (B-E) by a digital camera. Panel A′and B′-E′are photographs of Red Cap Oranda (A′) and Ranchu (B′-E′) with Alcian blue-alizarin red skeletal staining. Scale bars, 500 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Ranchuexhibited an abnormal dorsal fin with residual fin rays,protrusions or nothing at five months old (Fig. 4I-IV). Alcian bluealizarin red staining con firmed various degrees of defects in dorsal fin rays and radials inRanchu. We classified the defects of the dorsal fin into four types. In Type I, the fish retained the anterior portion of the dorsal fin, namely the exoskeletal fin with the spine and several fin rays and the endoskeletal fin with several radials (Fig. 4B, B′). In type II, the fish retained the middle portion of the dorsal fin with only four residual fin rays and radials (Fig. 4C, C′). In type III, only one hard spine remained,which did not develop well and turned into a protrusions (Fig. 4D, D′). In type VI, both fin rays and radials of the dorsal fin were completely absent, forming a smooth dorsum (Fig. 4E, E’).
3.5.Normal CDS of eomes gene in Ranchu
The nucleotide and amino acid sequences ofeomesagenes from two gold fish strainsWakinandRanchuand those from WT and mutant zebra fish were aligned and analyzed. Gold fish genome contained two copies ofeomesa, namelyeomesa1andeomesa2due to a recent whole genome duplication that occurred approximately 14 million years ago before gold fish and common carp (Cyprinus carpio) branched out from their common ancestor (Chen et al., 2019). We cloned the CDS regions ofeomesa1andeomesa2inRanchuand found no nonsense mutation in the CDS regions. Theeomesafh105allele, a nonsense allele generated by TILLING in zebra fish, contained a single point mutation that replaced a tyrosine (TAC) residue at position 100 to a premature stop codon (TAA)(Fig. S6), resulting in a truncated Eomesa protein (Du et al., 2012). We compared the CDS and deduced amino acid sequences ofRanchuwith those ofWakinand WT zebra fish. The nucleotide and amino acid sequences ofeomesa1andeomesa2inRanchuwere identical with those inWakin. Gold fisheomesa1CDS was 1989 bp in length and encoded a protein with 662 amino acids, whereaseomesa2CDS was 1995 bp long and encoded a protein with 664 amino acids, two amino acids (AA insertion) longer thaneomesa1(Fig. 5). Zebra fisheomesaCDS was 1986 bp in length and encoded a protein with 661 amino acids, three amino acids (SGGTAL deletion plus GPV insertion) shorter thaneomesa2in gold fish (Fig. 5). Both insertion and deletion occurred outside of the T-box domain, located from 225th to 405th amino acid residues in Eomesa2 (Fig. 5).

Fig. 5.Alignment of Eomesa amino acid sequence among two gold fish strains and zebra fish.The insertion (blue triangle) and deletion (black triangles) of amino acid sequences occurred in Eomesa1 and Eomes relative to Eomesa2. The position of T-box domain predicted with ScanProsite is indicated by red lines. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We also compared the identity of these sequences at the nucleotide and amino acid levels. The nucleotide and amino acid sequences ofeomesa2inRanchuwere identical to those ofeomesa2inWakin(Table 2).The sequence identity ofeomesa1 betweenRanchuandWakinwas 98.89% (1967/1989) and 99.24% (657/662) at the nucleotide and amino acid level, respectively. Five nonsynonymous mutations occurred outside of the T-box domain (from 225th to 405th amino acid residues)and located at positions 26th (E to G), 61st (T to S), 73rd (S to T), 198th(T to S) and 588th (T to A) inRanchuEomesa1 compared to the reference sequence ofWakin. We further analyzed the sequence conservation at these sites among gold fish, zebra fish, medaka, fugu fish and three strains(German mirror, Hebao red and Huanghe) of common carps. The first substitution site (E to G) observed inRanchucompared toWakinwas not found in other fish but a different substitution (E to S) at the same position was found in the medaka and fugu. Four other substitution sites found inRanchuEomesa1 also existed in other fish species (Fig. S7). The nucleotide sequence ofeomesa1 was 87-88% identical between zebrafish and gold fish while the peptide sequence was 93-94% identical(Table 2).
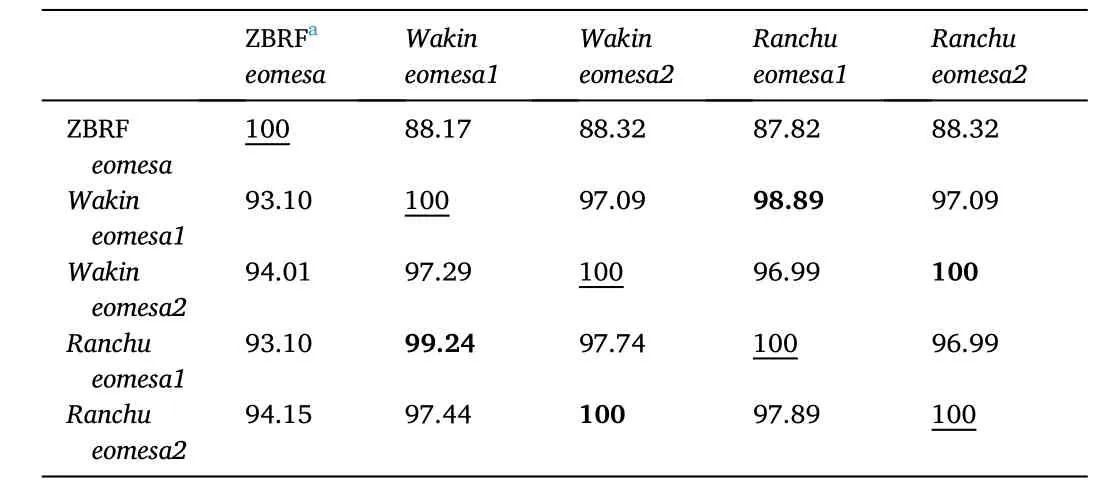
Table 2The sequence identity of eomesa gene between gold fish and zebra fishb.
4.Discussion
4.1.Dorsal fin development in Ranchu is different from other gold fishes
The dorsal fin is an important organ for fish to maintain balance when swimming. Without the dorsal fin, most fishes cannot stay upright(Drucker & Lauder, 2001; Tytell, 2006). On the other hand, the dorsal fin can regulate body temperature by removing excess heat in some fish such as the sail fish (Dement, 2010), and the heat transfer in fins has been simulated in engineering (Turkyilmazoglu, 2014, 2018). Lack of the dorsal fin in gold fish was first documented in Qing dynasty (1726 A.D.),after the documentation of the twin tail fins in Ming dynasty (1596 A.D.).Therefore, the Egg-gold fish have a more recent origin than the twin-tail gold fish. The attainment of the twin tail fins probably compensate for the loss of dorsal fins (Wang et al., 2013).
Li et al. (Li et al., 2015) first de fined the postembryonic developmental stages of gold fishWakinthat is referred to the staging tables of zebra fish. Later, they observed and described the postembryonic developmental processes ofRyukinandOrandaaccording to the established staging indices (Li et al., 2019). In this study, we first observed and described the postembryonic development processes of Egg-gold fishRanchuand compared it withRed Cap Oranda. Our observations indicated that the postembryonic developmental processes ofRed Cap Orandawere highly similar to those observed inRyukinandOranda(Li et al., 2019). The postembryonic developmental processes ofRanchuwas generally similar to those observed inRyukinandOranda(Li et al.,2019) andRed Cap Oranda, except for the dorsal fin fold development.During the early period of the fin fold development inRanchu, the ventral portion of the fin fold developed normally, but the dorsal fin fold was lost to different degrees at hatching, resulting in phenotypic variations in the loss of the dorsal fin. We categorized the postembryonic development ofRanchularvae into four types (Type I, II, III and IV)based on the number of the initial residual dorsal fin fold. The number,size and position of the initial residual dorsal fin fold appeared at Prot stage affected and eventually determined the phenotypes of the dorsal fin inRanchu, including the formation of a spine, a spine with rays, only rays or complete absence of the dorsal fin (smooth dorsum). The statistical percentage of each type during the developmental processes also demonstrated that the number of larval progenies significantly changed during the development. The residuals of the dorsal fin fold gradually degenerated and the number of larvae without the dorsal fin fold increased (Table 1). Both Alcian blue-alizarin red staining and X-ray photography con firmed the characteristic of aberrant dorsal fin rays and inner radials inRanchucompared toRed Cap Oranda.
Unexpectedly, we found that two larvae contained a normal dorsal fin fold at ~5.3 mm SL, and exhibited a normal dorsal fin by ~11.5 mm SL (Fig. S4). They obviously differed from their parents in morphology.The abnormal development was also observed in a previous study. The parents ofOrandacontained two anal and two caudal fins (2A2C);however, the progenies exhibited one anal and two caudal fins (1A2C),or one anal and one caudal fin (1A1C) (Li et al., 2019).
4.2.Genotyping and phenotypic comparison with zebra fish eomesafh105
The phenotype of no dorsal fin has been reported in artificially mutated zebra fish with a nonsenseeomesafh105allele generated by TILLING and in medaka with deletions in ZRS enhancers generated by CRISPR/Cas9 (Du et al., 2012; Letelier et al., 2018). Lack of the dorsal fin also naturally occurred in all Gymnotiformes (knife fishes) and some Siluridae (cat fishes) (Mabee et al., 2012). However, the developmental processes of this distinguishing feature have not been investigated in these fishes yet.
The phenotype of no dorsal fin in gold fish was naturally mutated and under artificial selection over 300 years since Qing dynasty (Wang,2007). We suspected that the mutation of a gene led to the trait without the dorsal fin in Egg-gold fish. In a previous study on twin-tail gold fish,Abe et al. compared embryonic and adult twin-tail gold fish with zebra fish fin mutants identified so far. They found that twin-tail gold fish anddinozebra fish mutant in which thechordinwas mutated shared three characteristic phenotypes: accumulation of blood cells, bifurcated fin folds and skeletal defects in the vertebral column (Abe et al., 2014).They genotyped twochordingeneschdAandchdBin gold fish, identified a mutated allele (designated aschdAE127X) containing a stop codon at the 127th amino acid residue, and demonstrated that thechdAE127Xallele contributed to the twin-tail phenotype in gold fish (Abe et al., 2014).
To examine whethereomesawas involved in the phenotype of no dorsal fin inRanchu, we genotyped the CDS regions ofeomesagenes,eomesa1andeomesa2. Since the sequences of the genotyping primers were located within the start and stop sites of the CDS, it is possible that the potential mutations at the first and last 25 nucleotides could not be detected. However, we cloned all exons and the fanking regions ofeomesa1andeomesa2fromRanchu, and did not find any nonsense mutations in these regions in a previous study (Data not shown). In this study, we did find five amino acid nonsynonymous mutations inRanchuEomesa1 compared to the reference sequence ofWakin. The analysis of sequence conservation among five fishes showed that four out of these five amino acid substitutions inRanchualso existed in other fish species(Fig. S7). Therefore, the nonsynonymous mutations of Eomesa1 inRanchuare unlikely to affect its protein function.
Theeomesbgene is a paralog ofeomesa. It is possible thateomesbmay exhibit the same conserved function aseomesain dorsal fin development. We knocked outeomesbwith CRISPR/Cas9 technology in zebra fish. The homozygous mutants ofeomesbdid not show the phenotype of no dorsal fin. Thus, we inferred thateomesbmay not be involved in dorsal fin development (Hu et al., 2020). In addition, a previous study reported thateomesbmight be involved in the immune responses in zebra fish (Takizawa et al., 2007). Therefore, it is unlikely thateomesbcontributes to the phenotype of no dorsal fin inRanchu.
Even thoughRanchuand zebra fish mutants showed the same phenotype of no dorsal fin, there are some differences in their dorsal fin developmental processes. The development of the median fins has three phases. Phase 1, the larval body has a continuous asymmetrical fin fold along the dorsal and ventral midline. Phase 2, the median fin buds appear in the presumptive fin-forming field of the fin fold. As the fins develop, the fin fold degenerates. Phase 3, endoskeletal radials appear under the fin bud, and then exoskeletal fin rays appear at the position proximal to the corresponding endoskeleton (Mabee et al., 2002). The malformation of the dorsal fin inRanchuoccurred at Phase 1 with the loss of the dorsal fin fold to different extents. However, the malformation of the dorsal fin in zebra fish mutant occurred at Phase 2, where the dorsal fin fold developed normally but the fin bud failed to form (Data not shown). Ineomesafh105mutant, the truncatedeomesafh105protein is unlikely to function, as it lacks the critical DNA-binding T-domain (Du et al., 2012). The lack of the dorsal fin is intriguing given the known roles of other T-box proteins Tbx5 and Tbx4 in forelimb and hindlimb development, respectively (Sheeba & Logan, 2017).
4.3.Signaling pathways involved in the developmental process of the median fin fold
The phenotype ofRanchuwithout the dorsal fin is, in fact, de ficient in development of the median fin fold (MFF), a rudiment of the median fins. Despite the evolutionary significance of the median fin, the mechanisms underlying the development of MFF and the median fin in early embryo remain largely unknown. There are some reports on artificial mutants with an abnormal MFF in zebra fish and medaka (Ishikawa, 2000; van Eeden et al., 1996) that provide insights into molecules involved in the fin development (Abe et al., 2007). Some zebra fish mutants contained no ventral MFF, but never a complete loss of the dorsal MFF (Abe et al., 2007). Some loss-of-function and gain-of-function assays in zebra fish revealed that fibroblast growth factor (Fgf) signaling plays important roles in both steps of early MFF development, the specification of a presumptive fin fold and the construction of the actual fin fold structure (Abe et al., 2007).
Double gene knockdown ofand1andand2in zebra fish embryos resulted in the absence of actinotrichia and impaired fin folds. This result suggests that actinodins are not only necessary for actinotrichia synthesis but are also essential for proper growth of the fin fold, and may play some roles in MFF development (Zhang et al., 2010).
Although the differentiation processes of the larval fin fold and adult fins are largely independent (van Eeden et al., 1996), we speculate that the development of fin folds may be regulated by similar key molecules and their involved signaling pathways in fins/limbs development. The key molecules includes the Fgfs, Wnts, Shh, retinoic acid (RA) and bone morphogenetic proteins (Bmps) (Sheeba et al., 2016). Spatiotemporally coordinated cellular and molecular interactions by these molecules will sculpt the fin fold development including its initiation, three dimensional outgrowth/patterning and termination just as the fins/limbs develop. In addition, some transcriptional factors, such ashoxgenes,de finitely play important roles in fin fold development as they specify the limb field (Tanaka, 2016). Knockdown ofhoxa13ain zebra fish resulted in the absence of the caudal fin fold (Crow et al., 2009). Thus,genes infuencing the development of the fins/limbs may relate to the loss of the dorsal fin inRanchu.
To summarize, the feature with the lack of the dorsal fin inRanchuhas been investigated in this study. However, the mutated gene that affected the normal development of the dorsal fin fold and eventually resulted in the phenotype of no dorsal fin has not been identified inRanchu. Further investigation to illuminate the affected gene would have great significance in understanding the regulatory mechanisms of dorsal fin (fold) development at molecular level.
Ethics statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Ocean University,China.
CRediT authorship contribution statement
Nan Yan: Data curation, Writing - original draft, Formal analysis.Jinqian Huo: Data curation, Formal analysis. Yu-Wen Chung-Davidson: Writing - review & editing. Wenyao Cui: Methodology, Resources.Weijie Huang: Methodology. Wei He: Resources. Qinghua Zhang:Writing - review & editing. Weiming Li: Writing - review & editing. Yan Zhou: Data curation, Writing - original draft, Formal analysis. Jianfeng Ren: Conceptualization, Project administration, Data curation, Writing -original draft, Formal analysis.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work was financially supported by the SHOU&MSU Joint Research Center grant.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aaf.2020.07.009.
 Aquaculture and Fisheries2022年4期
Aquaculture and Fisheries2022年4期
- Aquaculture and Fisheries的其它文章
- A review on fishing gear in China: Selectivity and application
- atg7 and beclin1 are essential for energy metabolism and survival during the larval-to-juvenile transition stage of zebra fish
- Association between single nucleotide polymorphisms of nLvALF1 and PEN2-1 genes and resistance to Vibrio parahaemolyticus in the Pacific white shrimp Litopenaeus vannamei
- Comparative analysis of the morphology, karyotypes and biochemical composition of muscle in Siniperca chuatsi, Siniperca scherzeri and the F1 hybrid (S. chuatsi ♀ × S. scherzeri ♂)
- Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia
- Chemical characteristics changes associated with extracted protein and salt addition and effect of different additives in the curing process on sensory acceptance of liquid fermented fish from tilapia frame
