Efficacy of phytogenic extracts on growth performance and health of tilapia(Oreochromis niloticus ×O. aureus)
Lumpn Poolswt, Yifeng Yu, Xioqin Li, Xu Zhen, Wenxing Yo, Pu Wng,Congyn Luo, Xingjun Leng,b,c,*
aNational Demonstration Centre for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai, 201306, China
bCentre for Research on Environmental Ecology and Fish Nutrition (CREEFN) of the Ministry of Agriculture, Shanghai Ocean University, Shanghai, 201306, China
cShanghai Collaborative Innovation Centre for Aquatic Animal Genetics and Breeding, Shanghai, 201306, China
dZhengchang Feed Science and Technology Co. Ltd., Liyang, 213300, China
Keywords:
Phytogenic extract
Tilapia
Growth
Immune response
Intestinal histology
Microbiota
A B S T R A C T
The present study was conducted to evaluate the efficacy of phytogenic extract on growth and health of tilapia.Three experimental diets were designed as the basal diet (Con) and phytogenic extract (Flourishing, F) supplemented diets at 0.5 and 1 g/kg inclusion (F-1 and F-2), which the phytogenic additive was extracted from Eucommia ulmoides, Astragalus membranaceus, Lonicera japonica and Codonopsis pilosula. The three diets were fed to juvenile tilapia (14.0 ±0.1 g) with 30 fish per cage, and three replicate cages from the three groups were placed in one indoor concrete pool (a total of three pools were used). After 8 weeks of feeding, the weight gain was significantly increased, and feed conversion ratio was decreased in F-2 group (P < 0.05) when compared to the control. Both levels of phytogenic extract significantly promoted the retentions of protein and lipid and the apparent digestibility coefficient of dry matter and protein (P < 0.05). The activity of superoxide dismutase and the levels of white blood cells, haemoglobin and glutamic pyruvic transaminase were also significantly increased by the supplementation of phytogenic extract (P < 0.05). The villus height in anterior intestine was significantly enhanced in F-1 and F-2 groups (P < 0.05). After challenging with Aeromonas hydrophila, the cumulative mortality was significantly declined in F-1 and F-2 groups (P < 0.05). In summary, the dietary supplementation of phytogenic extract at 1 g/kg feed could provide positive efficiency on growth, nutrient utilization, immunity,gut morphology and disease resistance of tilapia under the present conditions.
1.Introduction
Most of commercial tilapia farms were conducted in intensive system, which may create disadvantages to fish growth and health due to the high pressure of rearing conditions and the increasing of bacterial disease (Iwashita, Nakandakare, Terhune, Wood, & Ranzani-Paiva,2015). The dietary supplementation of phytogenic extract was eco-friendly and inexpensive techniques to promote immune functions,digestion and performance of host (Aanyu, Ondhoro, Ganda, Kato, &Basiita, 2014; Dotta et al., 2011).
Phytogenic feed additives are blended or extracted supplements from plants, herbs, spices and essential oils (Yang, Chowdhury, Hou, & Gong,2015), which can provide bene ficial effects on growth performance and health of tilapia (Acar, Kesbic, Yılmaz, Gultepe, & Turker, 2015; Zahran,Risha, Hamid, Mahgoub, & Ibrahim, 2014). Furthermore, phytogenic feed additives were also used as immunostimulants to promote the growth performance, antioxidation, immune response and disease resistance in other aquatic animals (Cristea et al., 2012).
The dietary supplementation ofCodonopsis pilosulawas reported to improve the growth, nutrient retention and digestibility in cat fish(Silurus asotus) (Han & Ma, 2006), and stimulate the inhibition ofVibrio splendidusand immune responses in sea cucumber (Apostichopus japonica) (Fan et al., 2018). TheAstragalus membranaceussupplemented diets enhanced immune parameters as the activities of catalase and lysozyme and the levels of nitrous oxide, glutathione peroxide and aspartate aminotransferase in yellow perch (Perca flavescens) (Elabd,Wang, Shaheen, Yao, & Abbass, 2016a) and Nile tilapia (Oreochromis niloticus) (Elabd, Wang, Shaheen, & Matter, 2020). In addition, the inclusion ofEucommia ulmoidesalso promoted the growth parameters as weight gain and feed utilization in grass carp (Ctenopharyngodon Idella)(Leng, Meng, Li, Li, & Hua, 2008), and increased the antioxidant activity of turbot (Scophthalmus maximusL.) (Zhang et al., 2018).
The individual supplementation ofEucommia ulmoides,Astragalus membranaceus,Lonicera japonicaandCodonopsis pilosulahave been continuously studied in aquaculture (Ardo et al., 2008; Elabd, Wang,Shaheen, Yao, & Abbass, 2016b; Fan et al., 2018; Liu, Leng, Li, Li, &Chai, 2013), but the combined effects and synergisms have been rarely considered in aquatic animals. Thus, the aim of this study was to determine the efficacy of phytogenic extract from the mixture ofE. ulmoides,A. membranaceus,L. japonicaandC. pilosulaextracts on growth, nutrient utilization, immunological parameters, microbial community, gut health and disease resistance of tilapia.
2.Materials and methods
2.1.Ethical statement
According to the care and use of scientific animals, the guidelines and recommendations on fish manipulation established by Ministry of Science and Technology of the People’s Republic of China were performed in this study. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Ocean University(Shanghai, China) before the experimental implementation.
2.2.Experimental fish
Tilapia were derived from Yueqiangfeng Aquaculture Farm,Guangzhou, China. After an acclimation of 2 weeks to experimental conditions using basal diet (Con), fish (14.0 ±0.1 g) were randomly divided into 9 cages (1.5 m × 1.2 m × 1.2 m) with 30 fish per cage. The triplicate cages from one group were separately placed in three indoor concrete tanks (5.0 m × 3.0 m × 1.2 m). The feeding trial was conducted at Binhai Aquaculture Station of Shanghai Ocean University (Shanghai,China). Fish were fed experimental diets three times per day (8.00,12.00 and 16.00 h) until satiation for 8 weeks. Faeces were removed every day after 2 h of the last feeding. The cultured water was renewed every 3 days with sedimented and filtered pond water. The water temperature (28-32°C), ammonia (≤0.2 mg/L), pH (7.5-9.5) and dissolved oxygen (≥6 mg/L) were measured every 7 days prior to the first feeding in the morning.
2.3.Experimental diets
Phytogenic extract (Flourishing: FL211) was provided by Zhengchang Feed Science and Technology Co. Ltd. (Jiangsu, China), which was produced from the mixture ofE. ulmoides,A. membranaceus,L. japonicaandC. pilosulaextracts at a ratio of 3:2:3:1, respectively. After drying and grinding, the mixture was extracted by hot water (1:12) at 60°C for 3 h, then the paste was mixed with corncob meal at the ratio of 2:1, and dried at 60°C until 100 g/kg moisture content. Three experimental diets were formulated as the basal diet (Con) and the phytogenic extract supplemented diets at 0.5 (F-1) and 1 (F-2) g/kg feed, respectively. Yttrium oxide was used as a chemical maker for nutrient digestibility analyses. All protein feedstuffs were supplied by Yuehai Feed Group Co., Ltd., Zhejiang, China. After grinding and screening with 0.425 mm sieve, feed ingredients were homogeneously mixed, then extruded to sinking pellets, and dried in oven at 40°C for 12 h. All experimental diets were preserved at 4°C until use. The dietary ingredient and chemical composition of experimental diets are shown in Table 1.

Table 1Dietary ingredient and chemical composition of experimental diets (as fed basis,g/kg).
2.4.Growth and body physical indices
After 8 weeks feeding, the final weight and number of fish in each cage were recorded for measuring growth performances after 24 h of starvation.
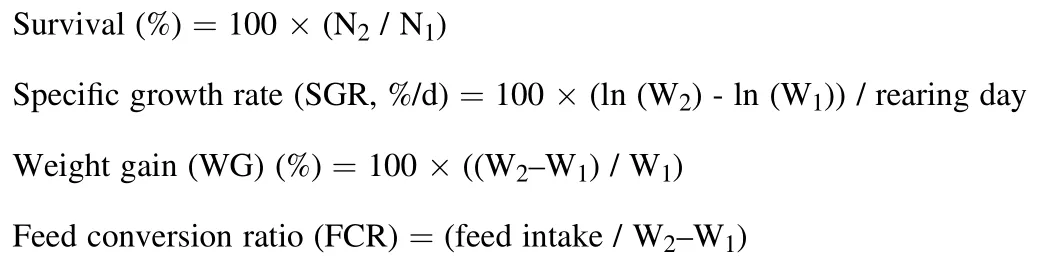
where, N1and N2are initial and final number of fish, and W1and W2are initial and final weight of fish.
Tricaine methanesulfonate (Jinjiang Shengyuan Aquatic Products Co., Ltd., Fujian, China) was used to anaesthetize experimental fish at 100 mg/L. Whole fish body (3 fish/cage) was refrigerated at -20°C for chemical composition analyses. Another three fish per cage were individually recorded body weight and length, and dissected to determine liver, digestive tract and intraperitoneal fat weights. The body morphometric indices were calculated according to the below equations:

2.5.Chemical composition analysis
The experimental diets, whole fish and faeces were measured moisture, ash and protein contents according to the standard methods of Association of Of ficial Analytical Chemists (AOAC) (1995). The lipid content was determined according to the indirect method of Folch, Lees,and Sloane Stanley (1957).
2.6.Nutrient utilization
Nutrient retention was considered according to the below equations:
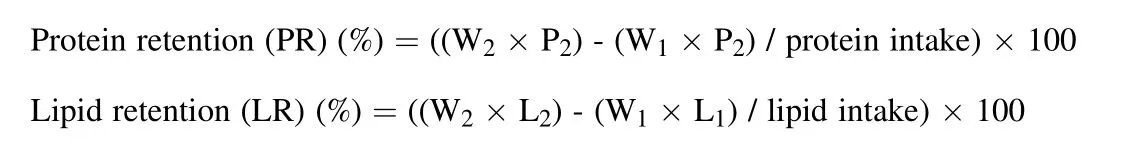
where, P1and P2are protein content in initial and final body of fish, L1and L2are lipid content in initial and final body of fish, and W1and W2are initial and final weight of fish.
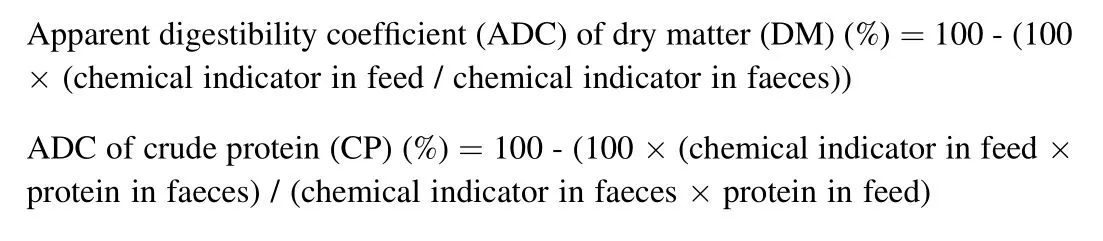
In the last two-weeks of the feeding trial, faeces in each cage was collected by hand net after 2 h of the second feeding. Yttrium oxide content in experimental diets and faeces were measured using the method described by Poolsawat, Li, He, Dong, and Leng (2020) through the inductively coupled plasma atomic emission spectroscopy (ICP-AES;Vista MPX, Varian, Palo Alto, California, USA) analysis. Nutrient digestibility was measured using the below equations:
2.7.Biochemical parameters
Whole blood (3 fish/cage) was sampled from caudal vein with anticoagulant for haematological parameters evaluation. The red blood cells (RBCs) and white blood cells (WBCs) were evaluated using haematocytometer. The haemoglobin (Hb) was analysed according to cyanmethemoglobin method. The haematocrit (Ht) was investigated using haematocrit method. The heterophils, lymphocytes, monocytes and phagocytosis activity (PP) were determined according to the indirect method of Martins et al. (2004).
The serum of tilapia was collected after centrifuging at 1512 g for 15 min at 4°C. Supernatants were used to determine immunological parameters as alkaline phosphate (ALP), glutamic oxaloacetic transaminase (GOT), lysozyme, catalase (CAT), glutamic pyruvic transaminase (GPT) and superoxide dismutase (SOD) following the manufacturer’s procedure of kits from Nanjing Jiancheng Bioengineer Institute (Nanjing, China).
2.8.Intestinal histology
The anterior intestines (3 fish/cage) were collected to investigate intestinal structure using the protocol of haematoxylin and eosin staining described by Yang et al. (2018). The villus height (VH), villus width(VW) and muscular thickness (MT) were resulted under compound microscope (Nikon YS100, Japan) via Image J14.0 software.
2.9.Microbiota diversity in digesta using 16 S rRNA sequencing
After 24 h of the last feeding, three fish per cage in the control and F-2 groups were aseptically collected digesta throughout intestine, and stored at -80°C until analyses. The microbial diversity was determined according to the manufacturer’ s procedures by Sangon Biotech Co., Ltd.(Shanghai, China). The digesta samples were extracted microbial DNA using the E.Z.N.A™ Mag-Bind Soil DNA Kit (Omega Bio-Tek, M5635-02,Georgia, USA), and the Qubit 3.0 DNA Kit (Invitrogen, Q10212, California, USA) was used to quantify genomic DNA.
The PCR amplification was generated using 2 ×Taq Master Mix Kit(Vazyme Biotech, P111-03, Nanjing, China). The microbial 16S rRNA gene was amplified using forward and reverse primers as “CCCTACACGACGCTTCCGATCTG (barcode) CCTACGGNGGCWGCAG” and“GACTGGAGTTCCTTGCACCCGAATTCCA GACTACHVGTATCCC”,respectively. The thermal cycling of PCR reaction was conducted by T100™ Thermal Cycler (BIO-RAD, California, USA). The quality of PCR product was examined using 2% agarose gel electrophoresis. The DNA libraries were purified using Magic Pure Size Selection DNA Beads(Transgen Biotech, EC401-03, Beijing, China) for Illumina MiSeq sequencing.
The 16S rRNA data were analysed following the QIIME 1.8.0 software (Caporaso et al., 2010). The effective sequences were continued to classify the operational taxonomic units (OUTs) using the clustering USEARCH 5.2.236 program at 97% similarity. All OUTs were specified taxonomic category by the Ribosomal Database Program (RDP) classifier with con fidence threshold of 80%.
2.10.Disease resistance
The strain ofA. hydrophilawas supplied by National Pathogen Centre for Aquatic Animals (Shanghai, China). The bacterial dilution (LD50 =3 ×107CFU/mL) was prepared to intraperitoneally inoculate into tilapia (10 fish/cage) at 100 μL after sample collection. All treatments were continuously fed the experimental diets, and observed the cumulative mortality for 10 days.
Cumulative mortality (%) =100 ×(the number of dead fsh / the number of injected fsh)
2.11.Statistical analysis
The results were indicated mean ±standard deviation (SD) using Statistical Package for the Social Sciences (SPSS) 22.0 statistical software (Chicago, IL, USA). One-way analysis of variance (ANOVA) and Turkey’s HSD Post Hoc tests were used to examine the significant differences among the groups (P <0.05).
3.Results
3.1.Growth parameters, body physical indices and whole body composition
As shown in Table 2, the WGR and SGR were significantly increased,and FCR was reduced by phytogenic extract supplementation at 1 g/kg(F-2 groups) (P <0.05). The dietary supplementation of phytogenic extract significantly promoted VSI and K (P <0.05). There were no significant differences in survival, HSI and IPF among the three groups(P >0.05). The protein, lipid, ash and moisture contents of whole body showed no significant difference among the three groups (P >0.05)(Table 3).
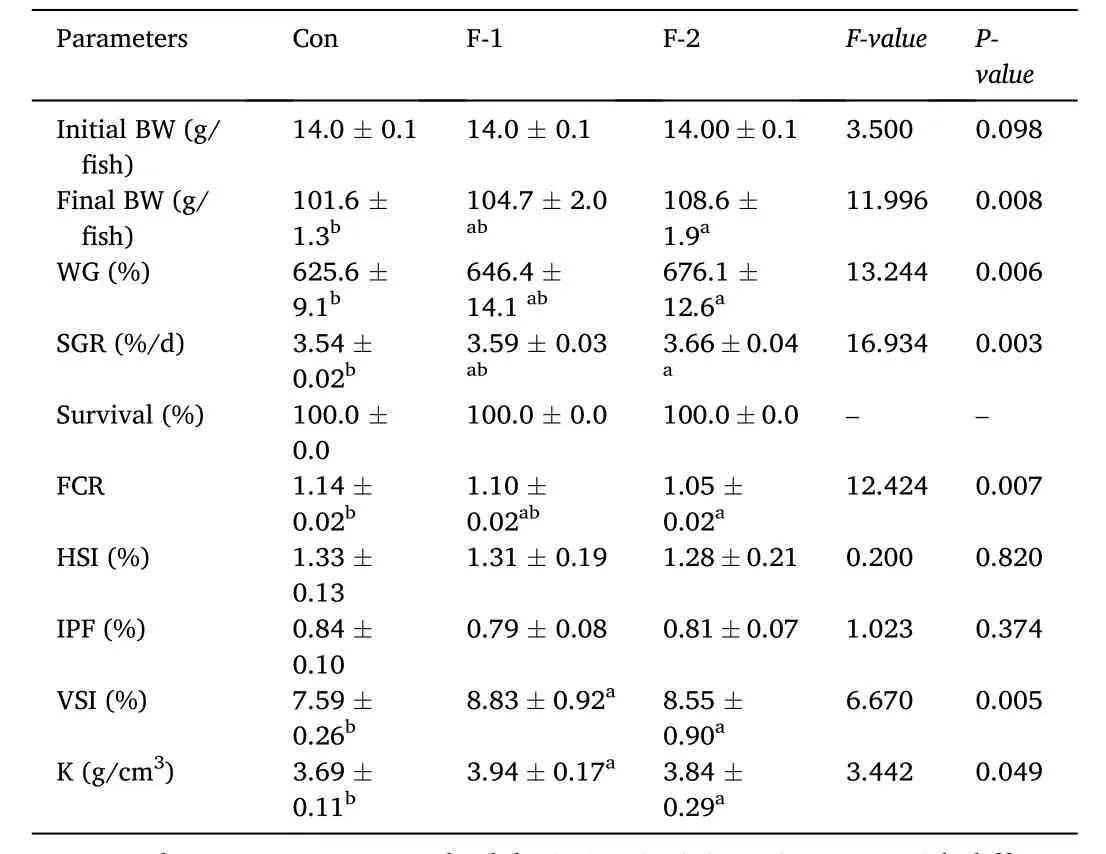
Table 2Growth and body physical indices of tilapia fed diets containing phytogenic extracts.
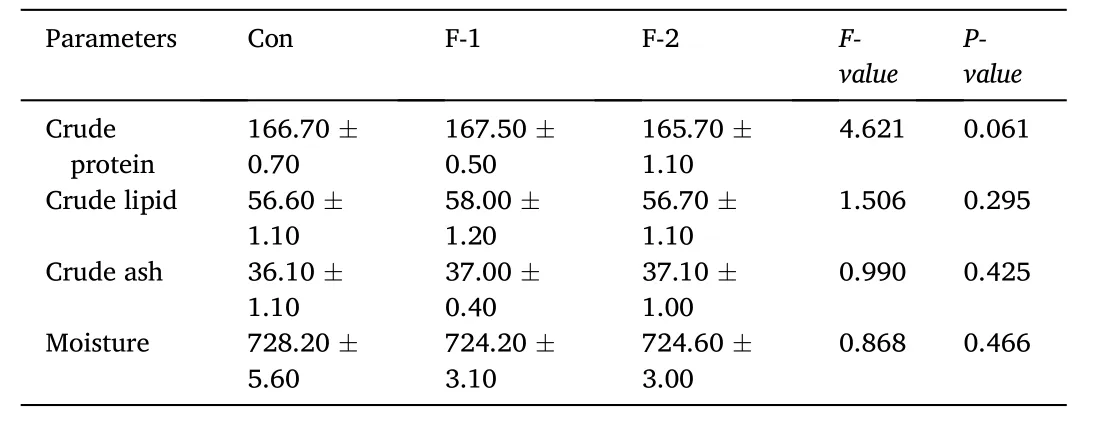
Table 3Whole body composition of tilapia diets containing phytogenic extracts (g/kg,wet weight).
3.2.Nutrient utilization
As shown in Table 4, all phytogenic extract supplemented groups showed significantly higher PR (except F-1 group), LR (except F-2 group), ADC of DM and CP than the control group (P <0.05). The ADC of CL was not significantly promoted by the supplementation of phytogenic extract (P >0.05).

Table 4Nutrient retention and digestibility of tilapia fed diets containing phytogenic extracts (%).
3.3.Biochemical parameters
As shown in Table 5, the RBCs, WBCs, Ht, heterophils, lymphocytes,monocytes, PP, ALP and lysozyme were not significantly enhanced by dietary phytogenic extract (P >0.05). The supplementation of phytogenic extract significantly improved Hb, GOT (except F-1 group),GPT, CAT (except F-2 groups) and SOD (P <0.05).

Table 5Haematological and immunological parameters of tilapia fed diets containing phytogenic extracts.
3.4.Intestinal histology
As shown in Table 6, the villus height in anterior intestine was significantly increased in F-2 group (P <0.05). There were no signi ficant differences in villus width and muscular thickness among the three groups (P >0.05). The anterior intestinal histology of tilapia was shown in Fig. 1.

Table 6Anterior intestinal morphometry of tilapia fed diets containing phytogenic extracts.
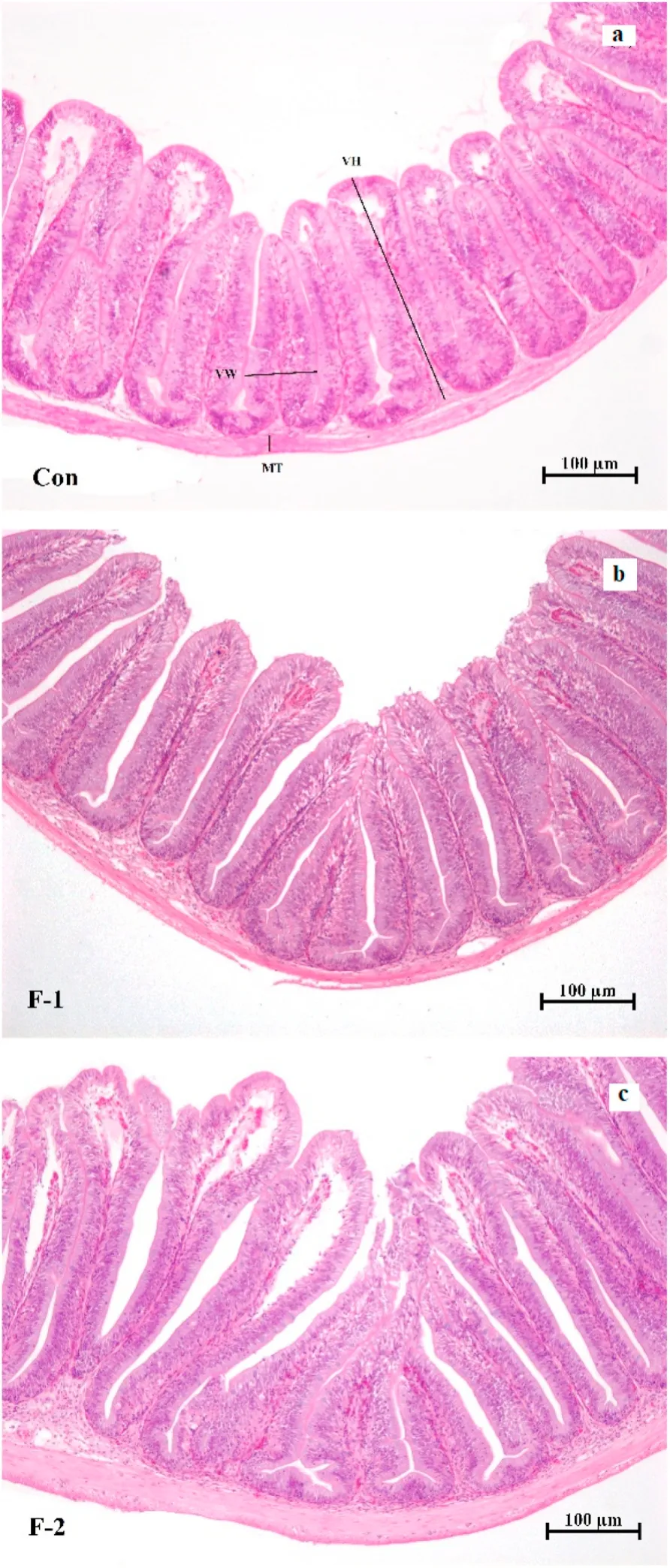
Fig. 1.The intestinal morphology of tilapia fed diets containing phytogenic extract. (a; the basal diet (Con), b; phytogenic extract supplemented diet at 0.5 g/kg (F-1), c; phytogenic extract supplemented diet at 1 g/kg (F-2); VH, villus height; VW, villus width; MT, muscular thickness).
3.5.Microbiota diversity in digesta using 16 S rRNA sequencing
A total of 256,285 sequences with an average length of 406 bp were obtained from 280,192 raw sequenced reads. The effective tags, operational taxonomic units (OUTs) number, richness (ACE and Chao1 indices) and diversity (Shannon, Simpson and Good’s coverage indicators) of microbial community showed no significant differences between two groups (P >0.05) (Table 7). The principal coordinate analysis (PCoA) observed OUT distances through the fraction of branch lengths using weighted UniFrac (Fig. 2a). The boxplot presented sequence distance of digesta microbiota via unweighted UniFrac(Fig. 2b). The non-metric multidimensional scaling (NMDS) explained the similarity distances between two groups (Fig. 2c). The taxonomic classification showed the relative abundance of microfora at phylum(Fig. 2d) and genus levels (Fig. 2e), and there were no significant differences in top 5 phyla (Planctomycetes, Chlorofexi, Proteobacteria,Firmicutes and Actinobacteria) and genera (Caldilinea,Blastopirellula,Aquisphaera,Clostridium sensu strictoandGemmobacter) of both groups(P >0.05) (Table 8). The KEGG pathway indicated the significant differences in metagenomics pro files of digesta microbiota between control and phytogenic extract supplemented groups (Fig. 2f).
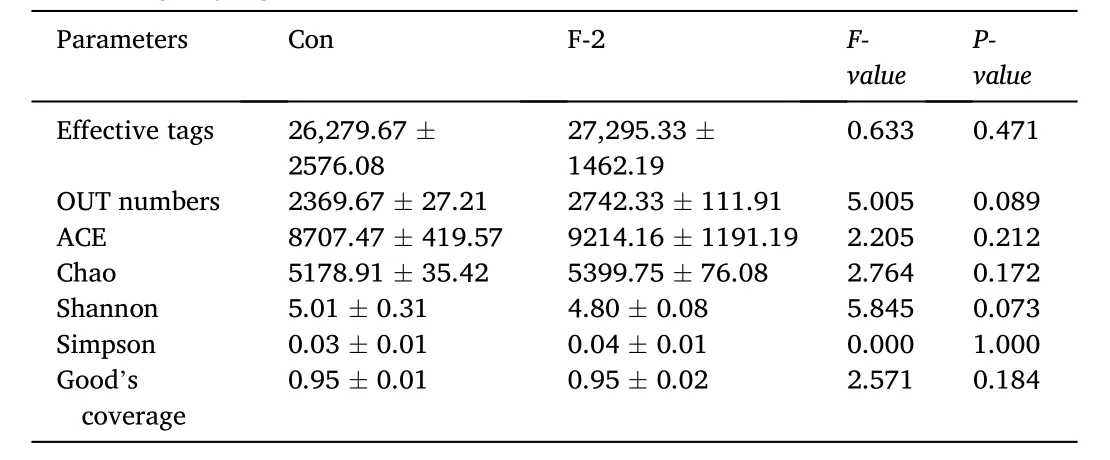
Table 7The microbial community richness and diversity in digesta of tilapia fed diets containing phytogenic extracts.
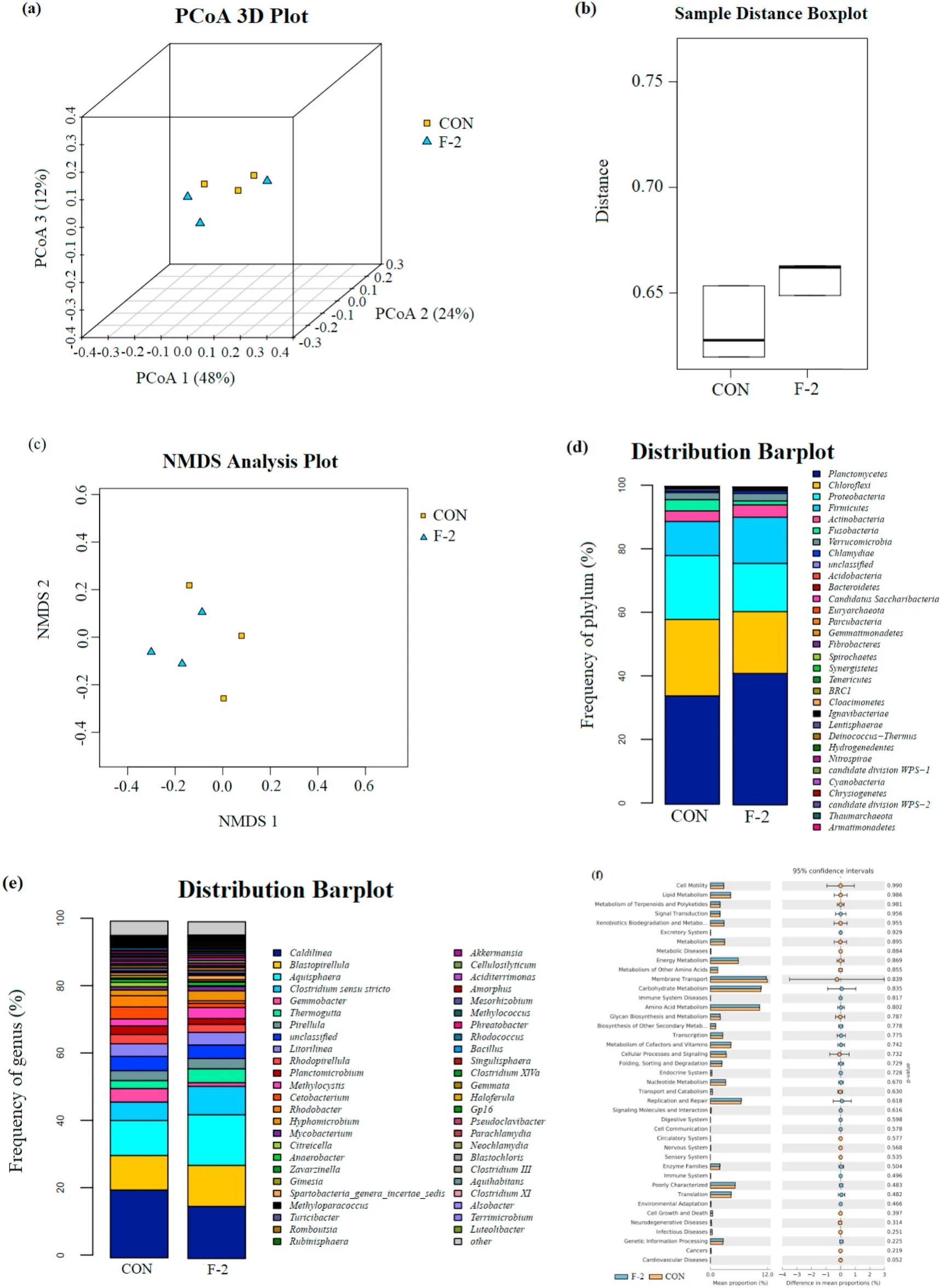
Fig. 2.The impacts of control and phytogenic extract supplemented diets on microbial diversity in digesta of tilapia. (a) Principal coordinate analysis (PCoA) based on the fraction of branch lengths (Weighed UniFrac), (b) Boxplot based on sequence distance (Unweighted UniFrac), (c) Non-metric multidimensional scaling(NMDS) based on OTU distance, (d) Relative abundance at phylum level, (e) Relative abundance at genus level, (f) KEGG pathway analysis identifying metagenomics pro files in digesta microbiota of tilapia. CON and F-2, fish fed the basal diets supplemented with 0 and 1 g/kg phytogenic extract, respectively.
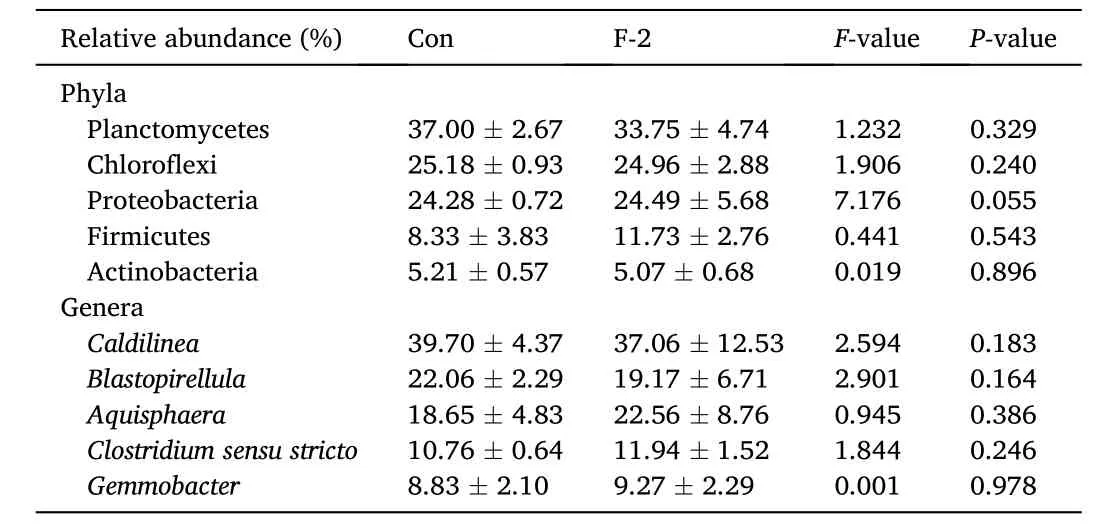
Table 8The relative abundance of top 5 phyla and genera in digesta of tilapia fed diets containing phytogenic extracts.
3.6.Disease resistance
As shown in Fig. 3, the supplementation of phytogenic extract showed significantly lower cumulative mortality than the control group after the infection ofA. hydrophila, and the F-2 group provided the lowest mortality in the last day of post challenge (P <0.05).
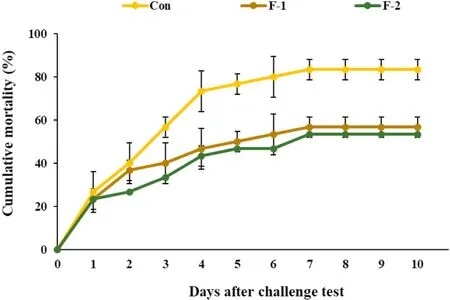
Fig. 3.The cumulative mortality of tilapia injected with Aeromonas hydrophila(Con, F-1 and F-2, fish fed the basal diets supplemented with 0, 0.5 and 1 g/kg phytogenic extract, respectively).
4.Discussion
Many phytogenic supplements have been used as immunostimulants,antimicrobial reagents, growth promoters and vaccines in aquaculture(Wang, Yang, & Liu, 2010). In this study, phytogenic extract was produced by the mixture ofE. ulmoides,A. membranaceus,L. japonicaandC. pilosulaextracts, which were traditional herbal medicines for stimulating immunity, antioxidation, disease resistance, metabolism and intestinal functions (Direkbusarakom, 2011; Pu, Li, Du, Cui, & Xu, 2017).The polysaccharides were the main bioactive compounds inC. pilosulaandA. membranaceus, which can provide positive effects on feed palatability, nutrient digestion and intestinal functions of host (Wang et al., 2015; Pu et al., 2017; Wu, 2018). Similarly, the enhanced growth performance and nutrient utilization were mentioned in bluegill sun fish(Lepomis macrochirus) fed diets supplemented withC. pilosula(Elabd et al., 2016b), and in cat fish, Nile tilapia and crucian carp fed diets supplemented withA. membranaceus(Han & Ma, 2006; Zahran et al.,2014; Wu, 2020). The geniposide and chlorogenic acids inE. ulmoideswere also mentioned to be growth promoters in grass carp (Meng et al.,2007), crucian carp (Shi, Leng, Li, Li, & Hu, 2008) and white shrimp(Litopenaeus vannamei) (Liu et al., 2013). In addition, the mixture of herbal medicines (C. pilosula,A. membranaceus,E. ulmoidesand other ingredients) also advanced growth performance of Japanese sea bass(Lateolabrax japonicus) (Wang et al., 2017). In this study, the weight gain rate, the apparent digestibility coefficient of dry matter and protein and the retentions of protein and lipid were promoted by the supplementation of phytogenic extract at 1 g/kg feed (Tables 2 and 4), and the advantages on growth and nutrient utilization may relate to the synergism of bioactive compounds as polysaccharides, geniposide and chlorogenic acids. Consistently, the previous studies also mentioned that the various types of polysaccharides in herbal medicines can play valuable effects on palatability, feed consumption, metabolism, protein synthesis and digestive enzyme activity (Pu et al., 2017; Wang, Ji, & Hu, 2010; Yang et al., 2015). The studies of Nobakht and Mehmannavaz (2010) and Luo(2012) reported that the bioactive ingredients as pectin and polysaccharides in herbal medicines can promote intestinal digestive enzyme secretion and motility, which connected with digestive function, feed intake and growth of host. Besides, the dietary supplementation ofC. pilosulaandE. ulmoideswas also mentioned to improve nutrient digestion and absorption of host (He et al., 2014; Ng, Liu, &Wang, 2004).
The present study indicated that the dietary supplementation of phytogenic extract provided bene ficial effects on haematological and immunological functions (Table 5), and it also decreased the cumulative mortality of tilapia after challenged withA. hydrophila(Fig. 3). Similarly, the infection ofA. hydrophilawas decreased by dietaryL. japonicaandA. membranaceusin Nile tilapia (Ardo et al., 2008), byAstragalus radixandGanoderma lucidumin grass carp (Yin et al., 2009) and byAstragaluspolysaccharides in largemouth bass (Micropterus salmoides)(Lin et al., 2017). The resistance againstVibrio splendiduswas promoted when sea cucumber (Apostichopus japonicus) consumed diets containingCodonopsis pilosula(Fan et al., 2018). In addition, the dietaryAstragaluspolysaccharides also promoted phagocytic activity, superoxide dismutase and lysozyme in Nile tilapia (Zahran et al., 2014), and the immune parameters as acid phosphate, superoxidase dismutase, phenoloxidase and phagocytic activity were improved byC. pilosulasupplementation in diets of sea cucumber (Fan et al., 2018). The dietary supplementation ofE. ulmoideswas reported to enhance total antioxidant capacity, superoxidase dismutase and catalase activities in turbot (Zhang et al., 2018).Furthermore, Yang, Liu, and Sun (2004) described that the dietary supplementation ofA. membranaceuscould directly affect humoral and cellular immune responses, which related to the health enhancement of fish. The study of Zhang et al. (2011) recorded that the dietary supplementation ofC. pilosulacan be anti-infammatory agent to balance stress responses of fish after disease outbreak. The general bioactive chemicals in phytobiotics as favonoids, polyphenols, polysaccharides, phenolic acids, alkaloids, tannins, chlorogenic acid and organic acids can stimulate the secretion of immunological substances for inhibiting harmful bacteria, and promote health bene fits to host (Meng et al., 2019;Pu et al., 2017; Zhu, 2020).
In this study, the villus height was significantly promoted by the supplementation of 0.5 and 1 g/kg phytogenic extract, but there were no significant differences in villus width and muscular thickness (Table 6 and Fig. 1). DietaryAstragaluspolysaccharides were also reported to produce bene ficial effect on microvillus in tilapia (Huang et al., 2010)and large yellow croaker (Larimichthys crocea) (Liu et al., 2020). The polysaccharide inA. membranaceusincreased villus height, crypt depth,muscular thickness and mucous cell, which related to growth performance and feed utilization (Huang et al., 2010). The chlorogenic acid inE. ulmoidesconsolidated intestinal barrier to prevent oxidative damage in gut, which connected to health enhancement of host (Zhao, Deng, &Liu, 2019). The increased villus height and width can directly indicate the efficiency of nutrient digestion and absorption (Aanyu et al., 2014).Some chemical components in phytogenic feed additives may cooperate to encourage intestinal morphology via the enhancement of intestinal barrier and eco-friendly environment (Chang, Teng, Lee, & Yu, 2019).However, the study of Zahran et al. (2014) indicated that the villus number and length of Nile tilapia were not affected by the dietary supplementation ofAstragaluspolysaccharides, and the combined supplementation ofA. membranaceusandL. japonicaeven decreased epithelial fold size in the middle intestine of juvenile pikeperch (Sander lucioperca) (Zakes, Kowalska, Demska-Zakes, Jeney, & Jeney, 2008).
The plate count agar was a traditional technique to investigate the number of living bacteria, which normally classified specific species by the observation of microbial morphology, and the protocol was limited by types of bacterial culture media (Manaka, Tokue, & Murakami,2017). Therefore, the present study was conducted to use 16s rRNA sequencing analysis for microbiota diversity, which can precisely recognise the complex community of microfora via the gene sequences in taxonomic tools (Schulza-Schweifing, Banerjee, & Wade, 2014).Although the results showed that the microbiota diversity parameters of tilapia digesta have no significant differences between the control and F-2 groups (Table 8). However, the previous studies have been reported that the dietary supplementation ofAstragaluspolysaccharides promoted microbiota in phyla Proteobacteria and Bacteroidetes of sea cucumber (Song, Feng, Zhang, & Zhu, 2019), and the intestinal microfora of fish was also increased by Chinese medical herbs, especiallyA. membranaceus(Wu et al., 2018). Some bioactive substances in phytogenic extracts may regulate chemical secretion in digestive tract,which can support the growth and metabolism of microbiota, and improve intestinal epithelial barrier and environment, and the bene ficial microbiota also degraded some nutrients in phytobiotic to proper molecules for metabolic absorption of host (An et al., 2019).
It is worth noting that a completely randomized design was not conducted in the present study, due to the limited space that a pool could not hold all the cages from the three treatments. Thus, three replicates from the three treatments were placed in one concrete pool to achieve equal environment across treatments. Such a design may affect the actual variances within treatment and among treatments. Therefore, a further study is needed to con firm the present result with a completely randomized design in the future.
5.Conclusion
Under the present conditions, the dietary supplementation of phytogenic extract at 1 g/kg feed could promote the growth performance,nutrient utilization, immune response, intestinal histology and disease resistance of tilapia. A further study is needed to con firm the present result with completely randomized design.
Data availability statement
All data used to support the findings of this study are included within the article.
CRediT authorship contribution statement
Lumpan Poolsawat: Project administration, Data curation, Formal analysis, Methodology, Software, Writing - original draft, Validation.Yifeng Yu: Project administration, Data curation, Formal analysis,Methodology, Software, Writing - original draft, Validation. Xiaoqin Li:Funding acquisition, Conceptualization, Investigation, Writing - review& editing, Supervision. Xu Zhen: Methodology, Formal analysis, Validation. Wenxiang Yao: Methodology, Formal analysis, Validation. Pu Wang: Methodology, Formal analysis, Validation. Congyan Luo:Funding acquisition, Conceptualization, Investigation, Writing - review& editing, Supervision. Xiangjun Leng: Funding acquisition, Conceptualization, Investigation, Writing - review & editing, Supervision.
Declaration of competing interest
None.
Acknowledgements
This study granted the financial supports from Zhengchang Feed Science and Technology Co. Ltd., Jiangsu, China and the Shanghai University Knowledge Service Platform, Shanghai Ocean University Aquatic Animal Breeding Centre (ZF1206), Shanghai, China.
 Aquaculture and Fisheries2022年4期
Aquaculture and Fisheries2022年4期
- Aquaculture and Fisheries的其它文章
- A review on fishing gear in China: Selectivity and application
- atg7 and beclin1 are essential for energy metabolism and survival during the larval-to-juvenile transition stage of zebra fish
- Association between single nucleotide polymorphisms of nLvALF1 and PEN2-1 genes and resistance to Vibrio parahaemolyticus in the Pacific white shrimp Litopenaeus vannamei
- Comparative analysis of the morphology, karyotypes and biochemical composition of muscle in Siniperca chuatsi, Siniperca scherzeri and the F1 hybrid (S. chuatsi ♀ × S. scherzeri ♂)
- Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia
- Differences in postembryonic dorsal fin development resulted in phenotypic divergence in two gold fish strains, Red Cap Oranda and Ranchu
