A Simple and Rapid Oxidative Stress Screening Method of Small Molecules for Functional Studies of Transcription Factor
Vanitha Adhinarayanreddy, Preethi Vijayaraghavareddy, Ashwin Vargheese, Sujitha Dadi, Akshay Uttarkar, Vidya Niranjan, Anuradha Venkatraman, Sheshshayee M. Sreeman, Ramu S. Vemanna
Letter
A Simple and Rapid Oxidative Stress Screening Method of Small Molecules for Functional Studies of Transcription Factor
Vanitha Adhinarayanreddy2, 4, Preethi Vijayaraghavareddy2, Ashwin Vargheese2, Sujitha Dadi2, Akshay Uttarkar3, Vidya Niranjan3, Anuradha Venkatraman4, Sheshshayee M. Sreeman2, Ramu S. Vemanna1
(Laboratory of Plant Functional Genomics, Regional Centre for Biotechnology, Faridabad-Gurgaon Expressway, Faridabad 121001, India; Department of Crop Physiology, University of Agricultural Sciences, Bengaluru 560065, India; Department of Biotechnology, Rashtreeya Vidyalaya, College of Engineering, Bengaluru 560059, India; Department of Biochemistry & Biotechnology, Annamalai University, Chidambaram 608002, India)
Structure-assisted reverse chemical discovery approaches are becoming attractive due to target based screening. However, Small molecules (SMs) identified by reverse chemical genetic approach require robust phenotyping assays to study the plant responses. We optimized a screening method in rice seedlings using methyl viologen (MV) induced oxidative stress under high light conditions. This method was suitable for studying the efficacy of SM targeting oxidative stress related genes. Structure-assisted drug designing approach was used to identify SM targeting DREB2A transcription factor (TF). Rice seedlings treated with pronetalol showed susceptible phenotype when exposed to oxidative stress. Pronetalol inhibited the DREB2A activity and suppressed the expression of its targetandgenes under oxidative stress. The assay is quite simple and robust and can be expanded to high throughput screening method for functional validation of TF.
SMs are low molecular weight compounds, which have high penetrating ability and their applications are advantageous in medicinal drug discovery, herbicides, fungicides, phytohormones, etc. SM probes are used to study the structure and function of genes, cells and organisms. SMs provide excellent opportunities to study multiple aspects of cell biology, cell cycle, signalling pathway and gene expression (Hicks and Raikhel, 2014). The effect of SM is often transient and reversible and can selectively alter biological system by inhibiting the function of specific proteins (Halder and Russinova, 2019). Phenomenal progress has been made in identifying SM by forward and reverse genetic approaches. Large number of SMs have been identified by forward chemical genomic approach based on phenotype specific to plant hormones (Dejonghe and Russinova, 2017; Halder and Russinova, 2019). Fipexide induces calli in rice, poplar, soybean, tomato and cucumber and acts as a useful tool in plant transformation technologies (Nakano et al, 2018). 6-(2-methoxyphenyl)-1,3-dimethyl-5-phenyl-1H-pyrrolo [3,4-d] pyrimidine-2,4(3H,6H)-dione or MDPD alters flowering time and seedling development in(Khan et al, 2017) with a potential to exploit in other crops. Compound ZA144 enhances the number of stomata inwithout inhibiting the plant growth (Ziadi et al, 2017).
To improve the stress tolerance, crop protection and yield, it is urgent to identify new SMs for agricultural use. The major challenge in identifying SMs relevant to specific phenotypes is requirement of robust phenotyping assays to study the subtle plant responses. However, screening many molecules is a laborious process and is also difficult to find a hit compound (Choi et al, 2014). Reverse chemical genomics approach utilizes protein structure to identify the molecules from chemical library databases (Blackwell and Zhao, 2003). Advances in structural biology and computational genomics provide an option for structure-assisted drug screening against a target protein, which associates with specific mechanisms. Galvestine-1 identified from high throughput screen inhibits mono galactosyl diacyl glycerol, the most abundant lipids and thylakoids and results in impairment of chloroplast development along with pollen tube elongation in(Botté et al, 2011). The small molecule ZINC12007063 acts as a potent herbicide by inhibiting transketolase enzyme (Huo et al, 2018).
A few SMs promote pathogen resistance but do not inhibit plant growth (Takaoka et al, 2018). (+)-7--Jasmonoyl-l- isoleucine enhances plant resistance against diseases but causes inhibition of growth. However, many genes do not show phenotype under some conditions. Therefore, to identify the effectiveness of SMs and to study the plant response, it is important to have robust phenotyping assays.
We developed a robust method using rice seedlings to study the effect of SM identified through structure-assisted drug design approach targeting oxidative stress responsive TFs. Rice seedlings were exposed to oxidative stress by MV under low light and high light conditions. This assay is quite simple as oxidative stress can be created easily at the rice seedling stage. The assay is robust enough to study any gene associated with oxidative stress. DREB2ATF is upregulated in resistant genotype under oxidative stress (Sujitha et al, 2022). To study the role of DREB2Ain oxidative stress, SMs were designed using a predicted protein structure and virtual screening. The application of SM enhanced the sensitivity of rice seedlings exposed to oxidative stress. The molecule showed specificity to inhibit DNA binding activity of DREB2Ain target gene promoters. The method described offers a quick option to study the function of genes through chemical genomics and can be used as high throughput screening method for identifying new SMs.
Rice seedlings were grown continuously in Hoagland’s nutrient media to optimize light conditions and reactive oxygen species (ROS) inducer concentrations (Fig. S1). MV is widely used as an oxidative stress inducer since it blocks the electron transport chain, leading to increased production of ROS (Lascano et al, 2012). Different concentrations of MV (2.5, 5.0, 10.0, 15.0 and 20.0 µmol/L) were used to create oxidative stress. We custom designed a light emitting diodes (LED) light chamber with a variable light intensity controller to avoid heat generation during the assay period (Fig. S1). The light intensity and sample exposure duration were automatically controlled to study the seedling responses.
The imbalance between ROS produced and scavenged creates oxidative stress in plants. This imbalance generally occurs in both biotic and abiotic stress conditions, leading to damage of cellular components, thus causing dysfunction of many enzymes and cell death (Hasanuzzaman et al, 2020; Vemanna et al, 2020). However, to create the oxidative stress to capture the plant responses, only a few protocols such as leaf disc assays with MV treatment followed by high light exposure are generally used (Babitha et al, 2013; Vemanna et al, 2016a, b, 2017). Nevertheless, studying the seedlings responses to oxidative stress under autotropic conditions is challenging. Unlike in the leaf disc assays, actively growing plant requires simultaneous expression of many regulatory genes for optimum cellular homeostasis and growth (Rehem et al, 2012; Nisarga et al, 2017). From this context, we optimized light intensity and MV concentration to capture the stress response at the seedling stage of rice.
It is well known that MV action is highly dependent on light conditions (Brooks et al, 1988). Therefore, we provided two treatments, namely high [600 μmol/(m2∙s)] and low [150 μmol/(m2∙s)] light intensities to determine optimum conditions to capture plant responses. The MV treatment had no effect of oxidative stress on rice seedlings (Fig. S2-A), growth parameters (Fig. S2-B and -C) and chlorophyll content (Fig. S2-D) in the plants exposed to low light intensity. Furthermore, in all the MV concentrations, the membrane integrity quantified by Evan’s blue dye staining and the levels of ROS by nitro blue tetrazolium chloride (NBT) staining showed no damage (Fig. S2-E and -F). This is mainly due to the lack of light, which does not cause excitation of PSII and subsequent transfer of electrons to produce ROS (Huang et al, 2018).
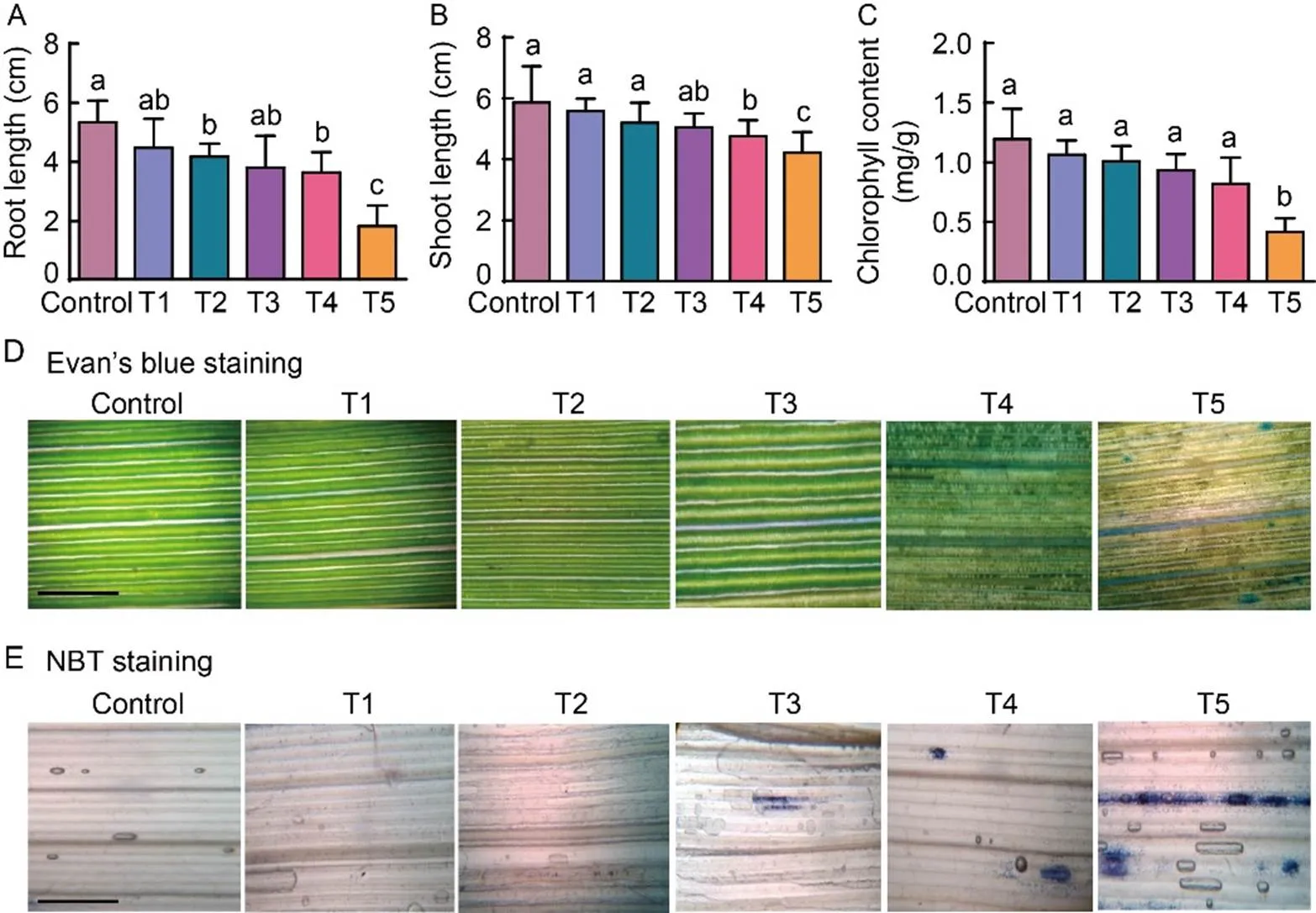
Fig. 1.Methyl viologen (MV) inducedoxidative stress of rice seedlings under high light.
A‒C, Root (A) and shoot (B) lengths, and total chlorophyll content (C) of rice seedlings at different MV concen- trations. Ten seedlings with three replications were used for each con- centration. One way ANOVA was used to assess the statistical significance. Lowercase letters above the bars indicate significant differences at the 0.01 level. D and E, Membrane integrity measured by Evan’s blue staining (D) and levels of singlet O2·̄ by nitro blue tetrazolium chloride (NBT) staining (E). Scale bars, 25 μm.Seven-day-old rice seedlings were exposed to MV oxidative stress at different concentrations under high light conditions [600 µmol/(m2∙s)] for 16 h. T1 to T5 refer to treatments with MV concentrations of 2.5, 5.0, 10.0, 15.0 and 20.0 μmol/L, respectively.
Under high light conditions, the root and shoot growth of rice seedlings was reduced in all the MV concentrations compared to the untreated seedlings. Among all the concentrations, the root and shoot lengths in 20.0 µmol/L MV reduced by 59.1% and 24.5% over control (Fig. 1-A and -B). In addition, the chlorophyll content of seedlings exposed to high light conditions significantly reduced in 20.0 µmol/L MV compared to the low concentrations (Fig 1-C). Under high light intensity, increased in membrane damage as measured by Evan’s blue dye was observed and it was significant at 15.0 and 20.0 µmol/LMV (Fig. 1-D). Similarly, the levels of superoxide accumulation in higher concentrations of MV were clearly evidenced by more NBT stain (Fig 1-E). These data suggested that 20.0 µmol/L MV and high light conditions are more optimum to capture stress response.
To inhibit the activity of TF, structure-assisted drug designing approach was followed. The 3D protein structure of DREB2ATF was predicted using the homology modelling with the Pfam database and iterative execution of the Threading ASSEmbly Refinement (I-TASSER) tool, subsequently refined by using the Schrodinger tool (Fig. S3-A). The predicted model had all the accepted dynamics as it passed the Ramachandran plot (Fig. S3-B). The predicted structure was used for high throughput virtual screening of molecules using the ZINC library. Based on the docking score and binding energy, we selected 1-(naphthalen-2-yl)-2-[(propan-2-yl) amino] ethanol or pronetalol having a docking score of -7.668 binds toat DNA binding region for further studies (Fig. S3-C). If the molecule binds to the DNA binding domain of DREB2A, the protein may not bind to the specific-elements on target gene promoters, thus inhibiting the TF activity (Rodríguez-Martínez et al, 2010).
TFs act as key molecular switches in plant development, cell cycle, cell signalling and stress responses. TFs which are induced under stress conditions regulate the gene expression through binding to the specific-elements of promoters in target genes, thereby modulating gene expression (Javed et al, 2020; Sujeeth et al, 2020). Oxidative stress is ubiquitous and coordinated expression of several TFs is essential to achieve desired levels of oxidative stress tolerance (Babitha et al, 2013; Yoon et al, 2020). However, comprehensive approaches are crucialto study the function of genes involved in oxidative stress. OsDREB2A is known to improve salt, drought and low temperature stress tolerance in transgenic soybean (Zhang et al, 2013). Induced overexpression ofusing ABRE promoter improves drought tolerance in rice (Cui et al, 2011). Using SMs, the TF activity may be transiently regulated and which is reversible depending the stability of the compound.
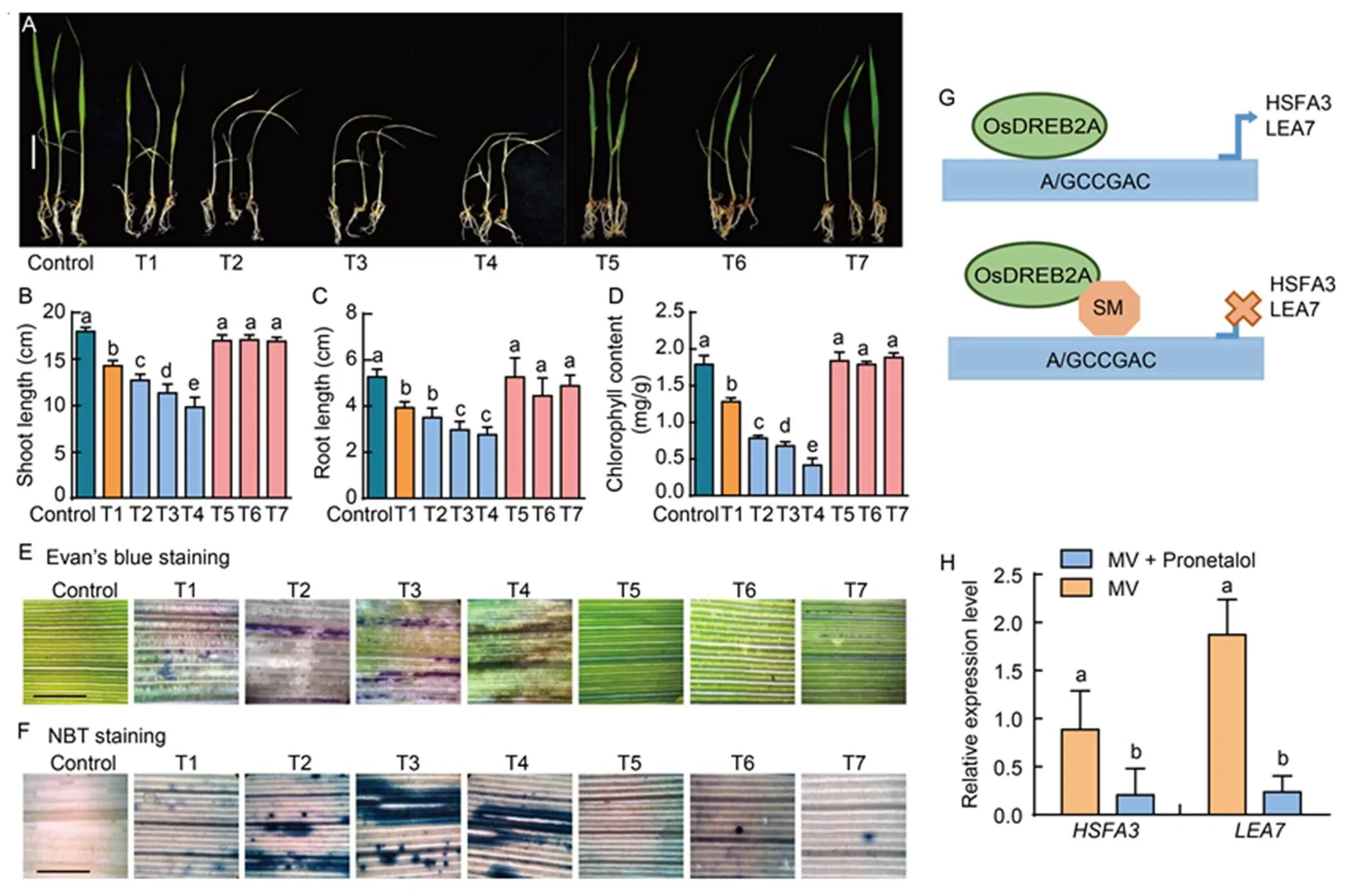
Fig. 2.Responses of rice seedlings treated with pronetalol (PRO) and methyl viologen(MV), and expression of OsDREB2A transcription factor target genes.
A, Seedling growth at different PRO and MV concentrations. Scale bar, 1 cm. B‒D,Shoot (B) and root (C) growth at different PRO and MV concentrations, and reduction in total chlorophyll content (D) over control. Ten seedlings with three replications were used for each concentration. E and F, Microscopic observation was taken for membrane integrity by Evan’s blue staining (E), and for levels of O2·̄ by nitro blue tetrazolium chloride (NBT) staining (F). Oxidative stress was imposed by MV at 20.0 µmol/L along with PRO (25.0, 50.0 and 75.0 µmol/L) for 10-day-old rice seedlings under high light conditions. Scale bars, 25 μm. G, Model depicting OsDREB2A binding to-elements of the target gene and small molecule (SM) interferences with-elements of target genes. H, Expression of OsDREB2A target genesandby qRT-PCR. Minimum three replicates were used for each sample, and rice housekeeping genewas used for normalization. One-way ANOVA was used to assess the statistical significance. Different lowercase letters above the bars indicate significant differences at the 0.01 level.T1, 20.0 μmol/L MV; T2, 25.0 μmol/L PRO + 20.0 μmol/L MV; T3, 50.0 μmol/L PRO + 20.0 μmol/L MV; T4, 75.0 μmol/L PRO + 20.0 μmol/L MV; T5, 25.0 μmol/L PRO; T6, 50.0 μmol/L PRO; T7, 75.0 μmol/L PRO.
To assess the effect of pronetalol, rice seedlings treated with 20.0µmol/L MV along with 25.0, 50.0 and 75.0 µmol/L pronetalol were exposed to high light intensity. Inhibited OsDREB2A TF activity by pronetalol led to hypersensitive phenotype under oxidative stress. The seedlings treated with MV and pronetalol showed highly susceptible phenotype to oxidative stress (Fig. 2-A). Treatments of pronetalol at 25.0, 50.0 and 75.0 µmol/L along with 20.0µmol/LMV showed 29.0%, 36.7% and 45.1% of reduction in shoot length over the cotrol, respectively. Similarly, the reduction in root lengths were 33.5%, 43.7% and 47.5% in MV and pronetalol treated seedlings compared to the control (Fig. 2-B and -C). The chlorophyll content at 25.0, 50.0 and 75.0 µmol/Lpronetalol along with MV was reduced by 38.8%, 47.0% and 67.6% compared to the control (Fig. 2-D). There was no difference observed in shoot and root lengths or chlorophyll content between the control and pronetalol alone treated rice seedlings. Pronetalol and MV treated seedlings showed a higher level of superoxide (O2·̄)accumulation and membrane damage compared to the MV alone treatment (Fig. 2-E and -F).
The seedling bio-assay clearly demonstrated that pronetalol enhanced the susceptibility of rice seedlings to oxidative stress. To assess the susceptibility of rice seedlings, the expression of target genes of OsDREB2A was analyzed. The DREB2A-element A/GCCGAC was found in() and()promoters (Fig. 2-G) (Sakuma et al, 2006; Morimoto et al, 2013). The expression of these genes were induced in the MV treated seedlings, while reduced in case of the pronetalol treated seedlings (Fig. 2-H). The reduced transcript levels ofandwere mainly due to the inhibition of DREB2A TF activity by pronetalol. HSFA3 is known to improve thermo- tolerance, salt sensitivity and oxidative stress tolerance (Song et al, 2016; Wu et al, 2018).andplay an important role in drought tolerance inand rice (Xiao et al, 2007; Wei et al, 2021).
Overall, our study demonstrated that the method described to study the effect of SMs at the rice seedling stage was very helpful to screen a large number of plants. The assay was highly reproducible for functional validation of genes associated with oxidative stress. The assay provided an option to screen SMs targeting known TFs or proteins.
AcknowledgementS
This study was supported by Regional Centre for Biotechnology core grant, Ramanujan fellowship (Grant No. SB/S2/RJN-046/ 2016) to Ramu S. Vemanna. Vanitha Adhinarayanreddy acknowledges the Department of Science and Technology Women Scientist fellowship (Grant No. WOSA/355/2017). All the authors indebted to Late Professor M. Udayakumar for his valuable inputs and support. We thank Mr. Chandrashekar from Spura Agro-tech, Bengaluru for helping in developing light chamber.
Supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Response of seedlings exposed to oxidative stress.
Fig. S2. Response of rice seedlings to methyl viologen induced oxidative stress under low light conditions.
Fig. S3. Protein structure of OsDREB2Aand binding pocket showing small molecule interaction.
Babitha K C, Ramu S V, Pruthvi V, Mahesh P, Nataraja K N, Udayakumar M. 2013. Co-expression ofandconfers resistance to abiotic stress in., 22(2): 327–341.
Blackwell H E, Zhao Y D. 2003. Chemical genetic approaches to plant biology., 133(2): 448–455.
Botté C Y, Deligny M, Roccia A, Bonneau A L, Saïdani N, Hardré H, Aci S, Yamaryo-Botté Y, Jouhet J, Dubots E, Loizeau K, Bastien O, Bréhélin L, Joyard J, Cintrat J C, Falconet D, Block M A, Rousseau B, Lopez R, Maréchal E. 2011. Chemical inhibitorsof monogalactosyldiacylglycerol synthases in., 7(11): 834–842.
Brooks A, Portis A R, Sharkey T D. 1988. Effects of irradiance and methyl viologen treatment on ATP, ADP, and activation of ribulose bisphosphate carboxylase in spinach leaves., 88(3): 850–853.
Choi H, Kim J Y, Chang Y T, Nam H G. 2014. Forward chemical genetic screening., 1062: 393–404.
Cui M, Zhang W J, Zhang Q, Xu Z Q, Zhu Z G, Duan F P, Wu R. 2011. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice., 49(12): 1384–1391.
Dejonghe W, Russinova E. 2017. Plant chemical genetics: From phenotype-based screens to synthetic biology., 174(1): 5–20.
Halder V, Russinova E. 2019. Understanding the language of drugged plants., 15(11): 1025–1028.
Hasanuzzaman M, Borhannuddin Bhuyan M H M, Zulfiqar F, Raza A, Mohsin S M, Mahmud J A, Fujita M, Fotopoulos V. 2020. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator., 9(8): 681.
Hicks G R, Raikhel N V. 2014. Plant chemical biology: Are we meeting the promise?, 5: 455.
Huang W, Yang Y J, Zhang S B, Liu T. 2018. Cyclic electron flow around photosystem I promotes ATP synthesis possibly helping the rapid repair of photodamaged photosystem II at low light., 9: 239.
Huo J Q, Zhao B, Zhang Z, Xing J H, Zhang J L, Dong J G, Fan Z J. 2018. Structure-based discovery and synthesis of potential transketolase inhibitors., 23(9): 2116.
Javed T, Shabbir R, Ali A, Afzal I, Zaheer U, Gao S J. 2020. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement., 9(4): 491.
Khan B R, Faure L, Chapman K D, Blancaflor E B. 2017. A chemical genetic screen uncovers a small molecule enhancer of the-acylethanolamine degrading enzyme, fatty acid amide hydrolase, in Arabidopsis., 7: 41121.
Lascano M, Muñoz N, Robert G, Rodriguez M, Melchiorre M, Trippi V, Quero G. 2012.Paraquat: An oxidative stress inducer.: Hasaneen M N. Herbicides: Properties, Synthesis and Control of Weeds. IntechOpen: 135–148.
Morimoto K, Mizoi J, Qin F, Kim J S, Sato H, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. 2013. Stabilization ofDREB2A is required but not sufficient for the induction of target genes under conditions of stress., 8(12): e80457.
Nakano T, Tanaka S, Ohtani M, Yamagami A, Takeno S, Hara N, Mori A, Nakano A, Hirose S, Himuro Y, Kobayashi M, Kushiro T, Demura T, Asami T, Osada H, Shinozaki K. 2018. FPX is a novel chemical inducer that promotes callus formation and shoot regeneration in plants., 59(8): 1555–1567.
Nisarga K N, Vemanna R S, Kodekallu Chandrashekar B, Rao H, Vennapusa A R, Narasimaha A, Makarla U, Basavaiah M R. 2017.() improves seed longevity in tobacco and rice by detoxifying reactive cytotoxic compounds generated during ageing., 10(1): 11.
Rehem B C, Bertolde F Z, de Almeida A A F. 2012. Regulation of gene expression in response to abiotic stress in plants.: BubulyaP. Cell Metabolism: Cell Homeostasis and Stress Response. Rijeka, Croatia: InTech: 13–28.
Rodríguez-Martínez J A, Peterson-Kaufman K J, Ansari A Z. 2010. Small-molecule regulators that mimic transcription factors., 1799: 768–774.
Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2006. Functional analysis of antranscription factor, DREB2A, involved in drought- responsive gene expression., 18(5): 1292–1309.
Song C, Chung W S, Lim C O. 2016. Overexpression of heat shock factor geneincreases galactinol levels and oxidative stress tolerance in., 39(6): 477–483.
Sujeeth N, Mehterov N, Gupta S, Qureshi M K, Fischer A, Proost S, Omidbakhshfard M A, Obata T, Benina M, Staykov N, Balazadeh S, Walther D, Fernie A R, Mueller-Roeber B, Hille J, Gechev T S. 2020. A novel seed plants gene regulates oxidative stress tolerance in., 77(4): 705–718.
Sujitha D, Jalendra Kumar H G, Thapliayal G, Vanitha P A, Uttarkar A Niranjan V, Rayalcheruvu U, Vemanna R S. 2022. Master regulators of oxidative stress associated genes improves stress adaptation in rice.,(in press).
Takaoka Y, Iwahashi M, Chini A, Saito H, Ishimaru Y, Egoshi S, Kato N, Tanaka M, Bashir K, Seki M, Solano R, Ueda M. 2018. A rationally designed JAZ subtype-selective agonist of jasmonate perception., 9(1): 3654.
Vemanna R S, Swetha T N, Sheela S H, Babitha K C, Rohini S, Reddy M K, Tuteja N, Reddy C P, Prasad T G, Makarla U. 2016a. Simultaneous expression of regulatory genes associated with specific drought-adaptive traits improves drought adaptation in peanut., 14(3): 1008–1020.
Vemanna R S, Paramanantham A, Ramegowda V, Mohan-Raju B, Makarla U, Senthil-Kumar M. 2016b. Transcriptome analysis of sunflower genotypes with contrasting oxidative stress tolerance reveals individual- and combined-biotic and abiotic stress tolerance mechanisms., 11(6): e0157522.
Vemanna R S, Babitha K C, Solanki J K, Reddy V A, Sarangi S K, Makarla U. 2017. Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds., 113: 177–186.
Vemanna R S, Vijayaraghavareedy P, Nisarga K N, Srivastava K R, Sheshshayee M S, Mysore K S, Makarla U. 2020. Carbonyl cytotoxicity affects plant cellular processes and detoxifying enzymes scavenge these compounds to improve stress tolerance., 68(23): 6237–6247.
Wei T L, Guo D L, Liu J H. 2021. Overexpression of, a late embryogenesis abundant family gene from, confers enhanced drought tolerance by enhancing antioxidant capacity., 8(2): 236–246.
Wu Z, Liang J H, Wang C P, Zhao X, Zhong X H, Cao X, Li G Q, He J N, Yi M F. 2018. Overexpression of lilyinconfers increased thermotolerance and salt sensitivityvia alterations in proline catabolism., 69(8): 2005–2021.
Xiao B Z, Huang Y M, Tang N, Xiong L Z. 2007. Over-expression of agene in rice improves drought resistance under the field conditions., 115(1): 35–46.
Yoon Y, Seo D H, Shin H, Kim H J, Kim C M, Jang G. 2020. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants., 10(6): 788.
Zhang X X, Tang Y J, Ma Q B, Yang C Y, Mu Y H, Suo H C, Luo L H, Nian H. 2013. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean., 8(12): e83011.
Ziadi A, Uchida N, Kato H, Hisamatsu R, Sato A, Hagihara S, Itami K, Torii K U. 2017. Discovery of synthetic small molecules that enhance the number of stomata: C-H functionalization chemistry for plant biology., 53: 9632–9635.
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/
Ramu S. Vemanna (ramu.vemanna@rcb.res.in)
18 December 2021;
15 February 2022
- Rice Science的其它文章
- Breeding Effects and Genetic Compositions of a Backbone Parent (Fengbazhan) of Modern indica Rice in China
- Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding
- Coleoptile Purple Line Regulated by A-PGene System Is a Valuable Marker Trait for Seed Purity Identification in Hybrid Rice
- Improve Anthocyanin and Zinc Concentration in Purple Rice by Nitrogen and Zinc Fertilizer Application
- Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation
- Computer-Assisted Real-Time Rice Variety Learning Using Deep Learning Network

