Coleoptile Purple Line Regulated by A-PGene System Is a Valuable Marker Trait for Seed Purity Identification in Hybrid Rice
Du Shuanglin, Wang Zhongwei, Chen Yun, Tan Yao, Li Xiang, Zhu Wenping, He Guanghua, Lei Kairong, Guo Longbiao, Zhang Yi
Research Paper
Coleoptile Purple Line Regulated by-Gene System Is a Valuable Marker Trait for Seed Purity Identification in Hybrid Rice
Du Shuanglin1, #, Wang Zhongwei2, #, Chen Yun1, #, Tan Yao1, Li Xiang1, Zhu Wenping1, He Guanghua3, Lei Kairong2, Guo Longbiao4, Zhang Yi1
(State Key Laboratory for Conservation and Utilization of Bio-resources in Yunnan / Research Center for Perennial Rice Engineering and Technology in Yunnan / School of Agriculture, Yunnan University, Kunming 650091, China; Biotechnology Research Institute, Chongqing Academy of Agricultural Sciences, Chongqing 401329, China; Key Laboratory of Application and Safety Control of Genetically Modified Crops / Rice Research Institute, Academy of Agricultural Sciences, Southwest University, Chongqing 400715, China; State Key Laboratory of Rice Biology, China National Rice Research Institute, Hangzhou 310006, China; These authors contributed equally to this work)
In plants, a large number of anthocyanin biosynthetic genes encoding enzymes and regulatory genes encoding transcription factors are required for anthocyanin synthesis. Coleoptile purple lines are two purple lines on both sides of coleoptiles after seed germination. However, the molecular mechanism of coleoptile purple line is not clear in rice so far.In this study, two major dominant genes,(, also known as)and(), were isolated via map-based cloning, and both of them were required for anthocyanin biosynthesis of coleoptile purple line in rice. The knockout and complementation experiments confirmed thatwas required for purple color in most organs, such as coleoptile line, sheath, auricle, stigma and apiculus, whereaswas just required for coleoptile purple line.was predominantly expressed in coleoptiles, flag leaves, and green panicles, and highly expressed in young leaves, whereaswas predominantly expressed in coleoptiles, and extremely lowly expressed in the other tested organs. Loss-of-function of eitherorresulted in significant reduction of transcript levels of multiple anthocyanin biosynthesis genes in coleoptiles. Coleoptile purple line was further used as a marker trait in hybrid rice. Purity identification in hybrid rice seeds via coleoptile purple line just needed a little water, soil and a small plate and could be completed within 5 d. Molecular marker and field identification analyses indicated that coleoptile purple line was reliable for the hybrid seed purity identification. Our findings disclosed that coleoptile purple line in rice was regulated by two major dominant genes,and, and can be used as a simple, rapid, accurate and economic marker trait for seed purity identification in hybrid rice.
;; coleoptile purple line; anthocyanin; seed purity identification; marker trait
Anthocyanins, a large family of flavonoids, are very important metabolites in plants, and comprehensively distributed in various organs of plants, such as roots, leaves, flowers, fruits and seeds, and play vital roles in attracting pollinators and frugivores, resisting biotic or abiotic stresses and pathogen attacks (Lev-Yadun and Gould, 2009; Hichri et al, 2011). Anthocyanin synthesis has been well elucidated in various plants, which requires a series of anthocyanin biosynthetic genes encoding enzymes and regulatory genes encoding transcription factors. Regulators mainly contain myeloblastosis (MYB)-type transcription factors, basic helix-loop-helix (bHLH)-type transcription factors and WD40 repeat (WDR)-type transcription factors, and these transcription factors comprise a MBW (MYB- bHLH-WDR) complex to activate a series of anthocyanin biosynthetic genes (Hichri et al, 2011; Petroni and Tonelli, 2011).
In maize, anthocyanin synthesis is regulated by/gene family and/gene family, which encode the MYB and bHLH transcription factors, respectively (Hichri et al, 2011; Petroni and Tonelli, 2011). The MYB gene, which is homologous to the maizegene, is isolated from rice (Reddy et al, 1998), and is essential for purple apiculus (Fan et al, 2008; Zhao et al, 2016), purple sheath (Gao et al, 2011; Chin et al, 2016; Hu et al, 2020), purple hull (Sun et al, 2018) and purple leaf (Zheng et al, 2019). The bHLH genes, such as,,,and, also regulate anthocyanin synthesis in various rice organs. The adjacentandgenes have been identified to comprise the purple leaf locus, each of which is sufficient for purple leaf (Sakamoto et al, 2001)., the gain-of-function mutation of, resulted from a rearrangement in the promoter region, which results in black pericarp of rice (Oikawa et al, 2015).(probable) together withand/oris responsible for purple hull (with functional) or brown hull (with non-functional) (Sun et al, 2018)together withandis responsible for purple leaf in most rice species, which can be substituted forin a few black rice species (Zheng et al, 2019).together withandis responsible for purple leaf sheath in rice (Hu et al, 2020). Recently, a WD40 genehas been identified to play a vital role in anthocyanin biosynthesis in various rice organs (Yang et al, 2021).
Heterosis is widely utilized in three-line and two- line hybrid rice in China. The seed purity of hybrid rice is a key factor for rice production. Therefore, it is very important to test the seed purity and ensure the seed purity match with the national standard before rice cultivation. Field identification is one of the most standard methods to test seed purity, but it always takes a long time and is also easily affected by external environment (Zhang and Chen, 2004). Thus, a few phenotypical recessive traits are used as markers for seed purity identification, such as the pale green leaf trait (Dong et al, 1995; Song and Song, 2007), the purple leaf trait (Cao et al, 1999; Yu et al, 2003) and the green-revertible albino leaf trait (Shu et al, 2001; Zhao et al, 2004).
Coleoptile is the pointed protective sheath covering the emerging shoot in monocotyledons, such as rice. It first breaks through the hull of rice seed after germination, and when two clear purple lines gradually exhibit on both sides of coleoptile, it is designated as coleoptile purple line (Xi, 1997; Zhang et al, 2021). Coleoptile purple line is controlled by multiple nuclear genes (Xi, 1997; Zhang et al, 2004a). Additionally, the two parents both exhibit coleoptile non-purple line, while their F1population exhibits coleoptile purple line (Zhang et al, 2004a). However, the molecular mechanism for coleoptile purple line is still unclear so far. In this study, two major dominant genes,and, were isolated, which compose an(, encoding activator)(, encoding tissue-specific regulator) gene system coordinately regulating anthocyanin biosynthesis of coleoptile purple line in rice. Also, coleoptile purple line was used as a marker trait for seed purity test in three-line hybrid rice. Those results from molecular marker and field identification analyses indicated that coleoptile purple line was a simple, rapid, accurate and economic marker trait for seed purity identification in hybrid rice.
Results
Phenotype of coleoptile purple line after germination
Two clear purple lines gradually exhibited on both sides of coleoptiles in wild type R25 at 2–3 d after seed germination, which extended from the tip to the bottom of coleoptiles (designated as coleoptile purple line) (Fig. 1-A and -C). However, two clear non-purple lines exhibited on both sides of coleoptiles in YR25 (designated as coleoptile non-purple line) (Fig. 1-B and -D). Leaves, hulls and pericarps of all the rice materials used in this study exhibited non-purple color, but other organs exhibited different colors besides coleoptile purple line or coleoptile non-purple line in a few materials, which are showed in details in Table 1.

Fig. 1. Phenotypic characteristics of coleoptile purple line and coleoptile non-purple line on R25 and YR25 at the germination stage.
A andC, Phenotypes of coleoptile purple line in R25.B and D, Phenotypes of coleoptile non-purple line in YR25. Coleoptile purple line and non-purple line are indicated by black and white arrows, respectively. Scale bars, 1 mm.
Mapping and cloning of two candidate genes for coleoptile purple line
All F1plants (Zhongjiu A × R25) exhibited the same purple color as R25 in coleoptile line, sheath, auricle, stigma and apiculus. Segregation was occurred in F2population, and the segregation ratio of 3 : 1 in F2population (coleoptile purple line : coleoptile non-purple line = 585 : 183; χ2= 0.502 < χ20.05= 3.84) indicated that a single dominant locus was responsible for coleoptile purple line. The locus was designated as.
Using 384 plants exhibiting coleoptile non-purple line in F2plants (Zhongjiu A × R25), thelocus was initially mapped to chromosome 6 between markers RM587 and RM5531 (Fig. 2-A). An additional F2population with 1 020 plants exhibiting coleoptile non-purple line was used to constrain the interval containing thelocus, which was mapped between markers RM5754 and RM19665 (Fig. 2-B). In this interval, thegene encoding a R2R3-MYB transcription factor had been reported to be involved in anthocyanin biosynthesis (Saitoh et al, 2004), and to be responsible for purple sheath, stigma and apiculus (Fan et al, 2008; Gao et al, 2011; Chin et al, 2016), which were consistent with our results. Thus, the genome sequence ofwas sequenced, which revealed that a 10-bp fragment was deleted in the third exon ofin Zhongjiu A, which caused a premature stop codon (Fig. 2-C).was predicted to contain three exons with a coding sequence of 819 bp. Accordingly,was selected as the candidate gene for.

Table 1. Purple organs in different rice materials used in this study.
Cl, Coleoptile line; Sh, Sheath; In, Internode; Au; Auricle; L, Ligule; St, Stigma; Ap, Apiculus; +, Purple color; –, Non-purple color.
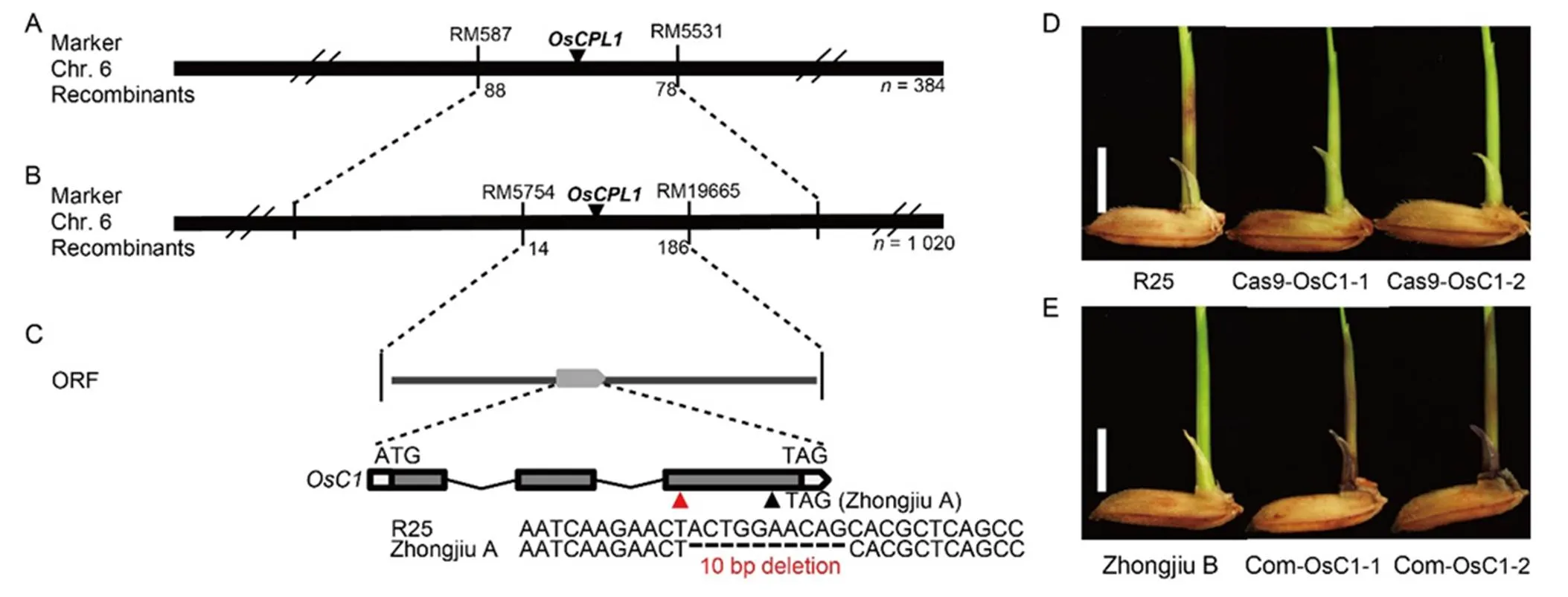
Fig. 2. Map-based cloning of.
A,locus is mapped to chromosome 6 (Chr. 6). B,locus is narrowed down between markers RM5754 and RM19665. C, Open reading frame (ORF) of candidate genein the mapped region. The mutation site is indicated by a red upright triangle, and the premature stop codon is indicated by a black triangle. D, Independent T1mutants generated using a CRISPR/Cas9 system. The mutants (Cas9-OsC1-1 and -2) display non-purple color in coleoptile. R25, Wild type plants. Scale bar, 0.5 cm.E, Independent T1complementary plants (Com-OsC1-1 and -2). The positive plants display purple color in coleoptile. Zhongjiu B is amutant. Scale bar, 0.5 cm.
In a previous study, another dominant locus on chromosome 11 has been identified to be responsible for coleoptile purple line (Zhang et al, 2004b). The locus was designated as. The F2population (YII-32A × R25) was used for fine-mapping of thelocus, which was further mapped between markers RM3701 and RM536 (Fig. 3-A) using 92 plants. An additional F2population with 3 500 plants exhibiting coleoptile non-purple line was used to constrain the interval containing thelocus, which was mapped to a 51.2-kb interval between markers RM26384 and C11Lz2 (Fig. 3-B). This interval contained eight putative open reading frames (ORFs)(Rice Genome Annotation Project, http://rice. plantbiology.msu.edu), including three retrotransposons, two expressed genes, one disease resistance gene, one plant thionin family gene and one anthocyanin regulatorygene (). In maize,is a member of thegene family, which is required for tissue-specific anthocyanin biosynthesis (Ludwig et al, 1989). Genome sequencing analysis revealed that nine bases were mutated in the coding region ofin YII-32B, and protein sequence alignment revealed that four mutated bases resulted in four amino acid substitution (Fig. S1).was predicted to contain eight exons with a coding sequence of 1 710 bp, which encodes a bHLH transcriptional factor. The genome coding regions ofin other rice materials exhibiting coleoptile purple line or coleoptile non-purple line were also sequenced, which showed that three amino acid substitutions could cause the loss-of- function ofin YII-32 B (Fig. 3-C; Fig. S1). Whereas, further studies are needed to validate whether one or more of the three amino acid substitutions cause the loss-of-function ofin YII-32B. In previous studies, a few bHLH transcriptional factors have been reported to regulate anthocyanin synthesis in different rice organs, such as(Oikawa et al, 2015),and(Zheng et al, 2019; Hu et al, 2020). Accordingly,was considered as the candidate gene for.
Knockout and complementation experiments of OsC1 and OsCPL2
To investigate whetheris responsible for coleoptile purple line, the non-mutatedgene of R25 was knocked out using a CRISPR/Cas9 system. A total of 12 independent homozygous T0transgenic plants were obtained (Fig. S2-A), and all of the T1homozygous mutants exhibited coleoptile non-purple line (Fig. 2-D). Besides, T1homozygous mutants also exhibited non-purple in sheath, auricle, stigma and apiculus (Fig. S3-A). Additionally, the 35S::OsC1 vector was transformed into Zhongjiu B (with the mutatedgene) calli. A total of 21 independent positive transgenic plants were obtained and all the T1positive transgenic plantsrescuedcoleoptile purple line (Fig. 2-E). Besides, T1positive transgenic plants also rescued purple in sheath, auricle, stigma and apiculus (Fig. S3-B). Taken together,was confirmed to be responsible for coleoptile purple line, but also for purple color in sheath, auricle, stigma and apiculus in rice.
To investigate whetheris responsible for coleoptile purple line, the non-mutated geneof R25 and II-32B was knocked out using the CRISPR/ Cas9 system. Each 15 and 13 T0positive transgenic plants were obtained, respectively. All the T1homozygous mutants exhibited coleoptile non-purple line (Fig. 3-D and -E; Fig. S2-B), but still exhibited purple color in sheath, auricle, stigma and apiculus (Fig. S3-C and -D). Taken together, the data indicated thatwas especially responsible for coleoptile purple line instead of purple color in other organs in rice.

Fig. 3. Map-based cloning of.
A,locus was mapped to chromosome 11 (Chr. 11). B,locus was narrowed down to a 51.2-kb interval. C, Open reading frames (ORFs) of the candidate genein the mapped region. The sense mutation sites are indicated by red upright triangles. D and E, Independent T1mutants of R25 (D) and II-32B (E) generated using the CRISPR/Cas9 system. The mutants have non-purple line in the coleoptile. R25 and II-32B are wild type plants. Scale bars, 0.5 cm.
In summary, bothandwere essential for coleoptile purple line, and loss-of-function of eitherorresults in coleoptile non-purple line in rice. Additionally,was a vital regulating gene for purple color in multiple organs, andwas a tissue-specific regulating gene for coleoptile purple line in rice.
Expression patterns of OsC1 and OsCPL2 in wild type plants
To investigate the expression patterns ofand, qRT-PCR was performed to analyze the relative transcript levels ofandin roots, coleoptiles, young leaves, sheaths, internodes, flag leaves, young panicles and green panicles in the wild type plants. The results showed thatwas predominantly expressed in coleoptiles, flag leaves, green panicles and relatively highly expressed in young leaves; however,was predominantly highly expressed in coleoptiles, and extremely lowly expressed in the other tested organs (Fig. 4-A).
OsC1 and OsCPL2 regulated transcript levels of multiple anthocyanin biosynthesis genes in coleoptiles
Given thatencoding a MYB transcription factor andencoding a bHLH transcription factor,ormight regulate the transcript expression of anthocyanin biosynthesis genes in coleoptile lines. Because the coleoptile lines were too small to be separated, the whole coleoptiles were used for RNA extraction. Three upstream anthocyanin biosynthetic genes (,and) and four downstream anthocyanin biosynthetic genes (,,and) were analyzed in the coleoptiles of R25, theandmutants with qRT PCR. The transcript levels of the seven anthocyanin biosynthetic genes were all significantly reduced, and the expression levels of,andwere hardly detectable in the coleoptiles of themutants compared with those in the coleoptiles of R25 (Fig. 4-B). The transcript levels of six anthocyanin biosynthetic genes were significantly reduced exceptin the coleoptiles of themutant compared with those in the coleoptiles of R25 (Fig. 4-B). These results indicated that bothandregulated the transcript expression of multiple anthocyanin biosynthesis genes in rice coleoptile.
Coleoptile purple line was a valuable trait for seed purity identification in hybrid rice
Coleoptile purple line in rice was a trait to be identified easily at the early stage, which can be observed at 2–3 d after seed germination (Fig. 1). Thecytoplasmic male sterile (CMS) line 2081A and fiverestorer lines (Luhui 17, 06A2066, R30, R287 and R725) all exhibited coleoptile non-purple line, but their F1populations exhibited coleoptile purple line. In order to evaluate whether coleoptile purple line was a useful trait to check the purity hybrid rice seed, two independent experiments were implemented.

Fig. 4. Expression patterns ofandand transcript expression analysis of anthocyanin biosynthesis genes in coleoptiles.
A, Expression pattern analysis ofandin various organs of wild type R25. B, Transcript expression analysis of anthocyanin biosynthesis genes in the coleoptiles of R25, themutant Zhongjiu B and themutant YII-32B.() was used as the internal control. Values are Mean ± SD with three biological replicates. The asterisks indicate statistical significance between R25 and theormutant, as determined by the Student’s-test (**,< 0.01).
The cross combination between 2081A and Luhui 17 was used for molecular marker analysis. Over 500 F1seeds were seeded in wet soil in a plate and coleoptile purple line or coleoptile non-purple line was observed at 2–3 d after seed germination (Fig. 5). A total of 14 seedlings exhibiting coleoptile non-purple line were observed out of 500 F1seedlings. Then, the 500 F1plants were further analyzed with polymorphic InDel markers R02010 and R04008 between 2081A and Luhui 17. The bands of 8 out of 14 seedlings exhibiting coleoptile non-purple line were consistent with that of 2081A, and the bands of the other 6 were consistent with that of Luhui 17. All the same, 485 of 486 seedlings exhibiting coleoptile purple line showed hybrid bands, but only 1 seeding was consistent with 2081A (Fig. S4). These results indicated that all 14 seedlings exhibiting coleoptile non-purple line were off-type seedlings, and 485 of 486 seedlings exhibiting coleoptile purple line were target hybrid seedlings. One of 486 seedlings was an off-type hybrid seedling most possibly produced by neither the pollen of 2081A nor Luhui 17 instead of other pollen. These results indicated that coleoptile purple line was a very rapid and accurate trait to be used as a marker for seed purity identification in hybrid rice.

Fig. 5. Seed purity identification in hybrid rice via coleoptile purple line at 2–3 d after seed germination.
The red arrow indicates an off-type seed exhibiting coleoptile non- purple line.

Table 2. Seed purity identification of hybrid rice seeds via coleoptile purple line.
Four independent cross combinations (2081A × 06A2066, 2081A × R30, 2081A × R287 and 2081A × R725) were used for field identification analysis. Over 1 000 F1seeds from each cross combination were seededin wet soil in a plate, and then seedlings were observed via coleoptile purple line or coleoptile non-purple line at 2–3 d after seed germination. The number of the seedlings exhibiting coleoptile purple line and coleoptilenon-purple line of each cross combination was counted (Table 2) and all the seedlings exhibiting coleoptile purple line were planted in a field at the third-leaf stage for field identification analysis. The hybrid seed purity of each cross combination was calculated using the ratio of the number of seedlings exhibiting coleoptile purple line to 1 000, each of which was higher than the national standards (> 96%) (Table 2). Field identification analysis showed that all these plants exhibiting coleoptile purple line displayed hybrid phenotypes at the heading stage, indicating they were target hybrid plants. These results demonstrated that coleoptile purple line was a very accurate marker trait for seed purity identification in hybrid rice.
In summary, the whole process of purity identification in hybrid rice seeds via coleoptile purple line only took about 5 d from beginning the seeding to finishing the investigation. The seed purity identification of 1 000 F1seeds via coleoptile purple line just needed a little water, soil and a small plate. It was reliable for the hybrid seed purity identification via coleoptile purple line. Therefore, coleoptile purple line was a very simple, rapid, accurate and economic marker trait for seed purity identification in hybrid rice.
Discussion
A-P gene system regulated anthocyanin biosynthesis of coleoptile purple line in rice
According to genetic analysis, the researchers proposed that(encoding chromogen),(encoding activator),(encoding tissue-specific regulator) genes comprised the--gene system to regulate anthocyanin biosynthesis in various rice organs (Takahashi, 1957; Kondo, 1963; Kinoshita, 1984).,() and(and) are considered as,and, respectively, for anthocyanin biosynthesis in rice apiculus and leaf (Furukawa et al, 2007).,andare considered as,and, respectively, for anthocyanin biosynthesis in rice leaf (Zheng et al, 2019).,andare considered as,and, respectively, for anthocyanin biosynthesisin rice apiculi and stigma (Meng et al, 2021). However,is considered asorin different gene systems to regulate anthocyanin biosynthesis in various rice organs. Additionally, more and more studies indicated thatis not the chromogen gene for anthocyanin (Sun et al, 2018; Meng et al, 2021). Thus, it’s necessary to redefine the--gene system to regulate anthocyanin biosynthesis in various rice organs.
Anthocyanin biosynthetic genes, such as,,,and, encode fundamental enzymes for colors of anthocyanins, and mutations or ectopic expression of these genes always result in various plant colors (Zhao and Tao, 2015). Thus, these anthocyanin biosynthetic genes can be considered asfor the production of chromogen in plant organs. In maize, the MYB/genes are responsible for the developmental and light-inducible regulators of anthocyanin synthesis in seed () and other tissues (), and the bHLH/genes are responsible for tissue-specific regulators of anthocyanin synthesis (Petroni and Tonelli, 2011). In this study, the MYB genewas identified to be responsible for purple color in multiple organs, such as coleoptile line, sheath, auricle, stigma and apiculus in rice. Additionally,is also responsible for purple hull and leaf in rice (Sun et al, 2018; Zheng et al, 2019). Another MYB transcription factor,, is the candidate gene for anthocyanin biosynthesis in black rice pericarp (Maeda et al, 2014). Apparently, these rice MYB genes perform the same functions as the maize MYB/genes, which can be thus considered as thegenes acting as activators of anthocyanin synthesis in various organs. In rice, the bHLH genes, such as(Oikawa et al, 2015),(Zheng et al, 2019),/(Hu et al, 2020) and(identified in this study), are responsible for black pericarp, purple leaf, purple leaf sheath and coleoptile purple line, respectively. Apparently, these rice bHLH genes perform the same functions as the maize bHLH/genes do, which can be thus considered as thegenes acting as tissue-specific regulators of anthocyanin synthesis in various organs.
In conclusion, the anthocyanin biosynthetic genes (such as,,,and), MYB genes (such as the rice,genes and the maizegenes) and bHLH genes (such as the rice,,,genes and the maizegenes) should be considered as,and, respectively, which comprise the redefined--gene system to regulate anthocyanin biosynthesis in various rice and maize organs. According to the redefined--gene system, it is easy to elucidate the-gene system for purple hull (with functional) or brown hull (with non- functional) (Sun et al, 2018), the-gene system and the-gene system for purple leaf (Zheng et al, 2019), the/gene system for purple leaf sheath (Hu et al, 2020), the-gene system for purple apiculi and stigma (Meng et al, 2021), and the-gene system for coleoptile purple line.encoding the dihydroflavonol reductase enzyme plays a key role in anthocyanin biosynthesis in rice. In order to validate thealleles are functional, the genome coding regions ofin the rice materials used in this study were sequenced. The sequencing results indicated thatwas functional in all these rice materials (Fig. S5). Thus, we proposed that,andcomprisethe--gene system for coleoptile purple line, but further work is needed to validate this hypothesis.
So far, thegene,, has been reported to be responsible for purple color in coleoptile line, sheath, auricle, stigma, apiculus, hull and leaf in rice. We infered thatwas also probably responsible for purple internode and auricle. Thegenes, encoding the tissue-specific regulator, are responsible for anthocyanin biosynthesis in specific rice organs, such as,,and. The original promoter ofexhibits no transcriptional ability, whereas the rearrangement in the promoter region ofexhibits high transcriptional ability (Shih et al, 2008; Zheng et al, 2019), which probably enable the constitutive expression ofin various organs, such as rice pericarp, hull and leaf. We inferred that other bHLH genes might be responsible for anthocyanin biosynthesis in other rice organs, such as coleoptile, internode, auricle, ligule, stigma and apiculus. Surprisingly, two bHLH genes,and(), were recently reported to be responsible for purple apiculi and stigmas, respectively (Meng et al, 2021).
Coleoptile purple line was a valuable marker trait for seed purity identification in hybrid rice
Heterosis is widely utilized in hybrid rice breeding, which makes it possible to increase yields by about 20% (Shih-Cheng and Loung, 1980; Li et al, 2007). The low purity seeds significantly decrease the yield and reduce the grain quality, which may markedly affect the farmers’ income. Thus, it is very important to make sure that farmers get the hybrid seeds with high purity. Molecular marker assistant analysis is complex and expensive for many rice breeders. Field identification analysis is easily affected by the external environment and also takes a long time (Zhang and Chen, 2004). Therefore, some easily observed recessive marker traits, such as the pale green leaf, the green- revertible albino leaf and the purple leaf, have been introduced into male sterile lines. However, some recessive traits, such as pale green leaf trait and purple leaf trait, exhibited negative effects on male sterile lines (Song and Xiao, 2012), and it is hard to identify the off-type seeds from male parents.
Coleoptile purple line in rice shows phenotypically at the early stage. Coleoptile purple line together with the first purple incomplete leaf has been used for seed purity identification in hybrid rice by Zeng (1996). However, the female parents also exhibit coleoptile purple line (Zeng, 1996). Subsequently, Zhang et al (2004a) found that two parents both exhibit coleoptile non-purple line, while their F1population exhibit coleoptile purple lines. In this study, two major dominated genes,and, were identified to coordinately regulate anthocyanin biosynthesis of coleoptile purple line, which make it possible to use coleoptile purple line as a marker trait for more efficient seed purity identification in hybrid rice.
According to our experience, mostCMS lines used in China are the(represents, andrepresents, the same below) genotypes (exhibiting coleoptile purple line), and mostrestorer lines are thegenotypes (exhibiting coleoptile non-purple line). The sequencing results of the genome coding regions ofandin a series ofCMS lines and restorer lines exhibiting coleoptile purple line or coleoptile non-purple line confirmed that this judgement was correct (Figs. S1 and S6), and the sequencing results of the promoter regions ofshowed that they appeared to be functional (Fig. S7). Therefore, it is possible to breed CMS lines with thegenotypes or thegenotypes. Thus, these CMS lines with thegenotypes and restorer lines with thegenotypes exhibit coleoptile non-purple line, but their F1hybrid seeds with thegenotypes will exhibit coleoptile purple line. Once this idea is implemented, the F1hybrid seed purity identification could be rapidly, accurately and economically conducted.
In conclusion, two major dominated genes,and, were isolated in this study, which encode a MYB transcription factor and a bHLH transcription factor, respectively. These two genes coordinately regulated anthocyanin biosynthesis of coleoptile purple line in rice. We redefined the--gene system to regulate anthocyanin biosynthesis in rice, which can be comprehensively elucidate anthocyanin biosynthesis in various rice organs. Coleoptile purple line was also developed as a valuable marker trait on hybrid rice. Molecular marker and field identification indicated that coleoptile purple line was reliable for the hybrid seed purity identification. Our findings disclosed that coleoptile purple line in rice was regulated by two major dominant genes,and, which can be used as a simple, rapid, accurate and economic marker trait for seed purity identification of rice hybrids.
methods
Rice materials and growth conditions
Thesubsp.materials II-32A, II-32B, Zhongjiu A and Zhongjiu B were kindly provided by China National Rice Research Institute, Hangzhou, China. Thematerials NJ7B, R725 and Luhui 17 were kindly provided by Neijiang Academy of Agriculture Sciences, Mianyang Academy of Agriculture Sciences and Sichuan Academy of Agriculture Sciences, respectively. Thematerials 2081A, 2081B, 06A2066, R25, YR25, R30 and R287 were self-breeding lines. II-32A, 2081A, YII-32A and Zhongjiu A are CMS lines. II-32B, YII-32B, NJ7B and Zhongjiu B are maintainer lines. Luhui 17, 06A2066, R25, YR25, R30, R287 and R725 are restorer lines.
Thematerials R25 and YR25 were selected from breeding lines based on coleoptile purple line and coleoptile non-purple line. YII-32B was derived from the crosses and backcrosses between NJ7B and II-32B (the recurrent parent). YII-32A was derived from the crosses and backcrosses between II-32A and YII-32B (the recurrent parent). All rice plants used in this study were grown in fields in Chongqing or in growth chambers.
Map-based cloning of two candidate genes for coleoptile purple line
Zhongjiu A was crossed with R25, and the plants from the F2population with 1 404 plants exhibiting coleoptile non-purple line were used for genetic analysis and gene mapping of. YII-32A was crossed with R25, and the plants from the F2population with 3 592 plants exhibiting coleoptile non-purple line were used for gene mapping of. Sequence polymorphisms betweensubsp.Nipponbare and subsp.93-11 were used to develop insertion/deletion and simple sequence repeat molecular markers (Shen et al, 2004). Gene annotation and primer design for DNA and cDNA sequencing were performed on the basis of information obtained from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih. gov/), Gramene (www.gramene.org) and Rice Genome Annotation Project (http://rice.plantbiology.msu.edu) databases. Sequence alignment was performed with Vector NTI Advance 10 (Invitrogen, USA; http://www.invitrogen.com/). All primers used in this study are listed in Table S1.
Vector construction for CRISPR/Cas9 system and genetic complementation
A modified CRISPR/Cas9 system was used for knockout ofandin the wild type, and the procedures were followed as previously described(Wang et al, 2018). Independent gRNA target sites (AGCACGCTCAGCCGCAAG ATfor, GCAGCTGGGTTGCGGATCCCandccgtatcacggcgtcgttatfor) were designedto construct the OsC1-Cas9 and OsCPL2-Cas9 vectors, respectively. The OsC1-Cas9 vector was transformed into the wild type R25 calli, and OsCPL2-Cas9 vector was transformed into the wild type R25 and II-32B calli usingan-mediated method (Hiei and Komari, 2008).
For complementation of themutant, thegene in the pCAMBIA1301 vector was substituted with a full-length coding sequences ofby usingII andEII, which generated the 35S::OsC1 vector. The 35S::OsC1 vector was transformed into the Zhongjiu B (themutant) calli usingthe-mediated method (Hiei and Komari, 2008).
Protein sequence analysis
Protein structure prediction was performed using NCBI, PROSITE (http://prosite.expasy.org/prosite.html) and Smart (http://smart.embl-heidelberg.de/). Protein sequence alignment was performed with Vector NTI Advance 10.
RNA preparation and qRT-time PCR analysis
Total rice RNAs were extracted from roots, coleoptiles and young leaves of the wild type plants at the second-leaf stage and sheaths, internodes, flag leaves, young panicles (white) and green panicles of the wild type plants at the heading stage using a RNAprep Pure Plant Kit (Tiangen Biotech, Beijing, China). First-strand cDNA was synthesized from 1 µg total RNA using a PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time, TaKaRa, CA, USA). qRT PCR was conducted using a TB Green Premix ExII (Tli RNaseH Plus, TaKaRa, CA, USA) on a Bio-Rad CFX96 system according to the manufacturer’s instructions.() was used as the internal control, and relative transcript levels were analyzed using the 2–ΔΔCTmethod (Livak and Schmittgen, 2001). Three biological replicates were performed. Microsoft Office Excel 2016 was used to analyze data, and the Student’s two-tailed-test was used to determine the statistical significance of data.
Seed purity identification in hybrid rice
Molecular marker analysis was as followed. New polymorphic InDel markers between 2081A and Luhui 17 were developed. The F1seeds were harvested from the cross between 2081A and Luhui 17. A total of 500 F1seeds were seeded in wet soil in a plate, and then the germinated seedlings were separated by coleoptile purple line or coleoptile non-purple line at 2–3 d after seed germination. Individual DNA was extracted from 500 seedlings and used to analyze with new polymorphic InDel markers between 2081A and Luhui 17. Each band of every individual DNA was analyzed compared with each other.
Field identification analysis was as follows. Seeds were harvested from the female plants of four cross combinations (2081A × 06A2066, 2081A × R30, 2081A × R287 and 2081A × R725). A total of 1 000 F1seeds from each cross combination were divided evenly into 20 groups, and then the seeds from each group were seeded in wet soil in a plate. All seedlings from each cross combination were observed and separated by coleoptile purple line or coleoptile non-purple line at 2-3 d after germination. The number of seedlings exhibiting coleoptile purple line from each cross combination was counted. The hybrid seed purity from each cross combination was calculated using the ratio of the number of seedlings exhibiting coleoptile purple line to 1 000. Then, the off-type seedlings exhibiting coleoptile non-purple line were discarded, and the hybrid seedlings with coleoptile purple line were planted in a field for further hybrid phenotype identification at the heading stage, which was used to evaluate the accuracy of hybrid seed purity via coleoptile purple line.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31701390 and 31370349), Special Project on Performance Incentive Guidance of Chongqing Scientific Research Institution, China (Grant No. cstc2018jxjl80021), Chongqing Agriculture Development Fund (Grant No. NKY-2021AC003) and Recruitment Announcement for High-level Talents of Yunnan University (Grant No. KL180018). We thank Dr. Shao Qiming for improving the English of the manuscript.
Supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1.genotypes in differentcytoplasmic male sterile maintainer lines and restorer lines.
Fig. S2. Targeted mutations at genomic sites inandloci in CRISPR/Cas9 plants.
Fig. S3. Phenotypic characteristics ofandknockout plants generated using a CRISPR/Cas9 system and positive transgeniccomplementation plants of.
Fig. S4. Molecular marker analysis of F1seedlings from the cross between 2081A and Luhui 17.
Fig. S5.genotypes in different rice materials.
Fig. S6.genotypes in differentcytoplasmic male sterile maintainer lines and restorer lines.
Fig. S7.promoters in differentcytoplasmic male sterile maintainer lines and restorer lines.
Table S1.Primer sequences used in this study.
Cao L Y, Qian Q, Zhu X D, Zeng D L, Min S K, Xiong Z M. 1999. Breeding of a photo-thermo sensitive genie male sterilerice Zhongzi S with a purple-leaf marker and the hoterosis of its hybrid rice produced with it., 25(1): 44–49. (in Chinese with English abstract)
Chin H S, Wu Y P, Hour A L, Hong C Y, Lin Y R. 2016. Genetic and evolutionary analysis of purple leaf sheath in rice., 9(1): 8.
Dong F G, Zhu X D, Xiong Z M, Cheng S H, Sun Z X, Min S K. 1995. Breeding of a photo-thermoperiod sensitive genic male sterilerice with a pale-green-leaf marker., 9(2): 65–70. (in Chinese with English abstract)
Fan F J, Fan Y Y, Du J H, Zhuang J Y. 2008. Fine mapping of() gene in rice., 15(1): 1–6.
Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Takamure I, Kadowaki K I. 2007. Theandgenes are involved in proanthocyanidin synthesis in rice pericarp., 49(1): 91–102.
Gao D Y, He B, Zhou Y H, Sun L H. 2011. Genetic and molecular analysis of a purple sheath somaclonal mutant inrice., 30(5): 901–911.
Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. 2011. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway., 62(8): 2465–2483.
Hiei Y, Komari T. 2008.-mediated transformation of rice using immature embryos or calli induced from mature seed., 3(5): 824–834.
Hu W, Zhou T H, Han Z M, Tan C, Xing Y Z. 2020. Dominant complementary interaction betweenand two tightly linked genes,and, controls the purple leaf sheath in rice., 133(9): 2555–2566.
Kinoshita T. 1984. Gene analysis and linkage map.: Tsunoda S, Takahashi N. Biology of Rice. Amsterdam, Netherlands: Elsevier: 187–274.
Kondo A. 1963. Fundamental studies on rice breeding through hybridization between Japanese and foreign varieties: VII. Identification of the gene system controlling anthocyanin coloration in Japanese and foreign varieties., 13: 92–98.
Lev-Yadun S, Gould K S. 2009. Role of anthocyanins in plant defence.: Winefield C, Davies K, Gould K. Anthocyanins: Biosynthesis, Functions, and Applications. New York, USA: Springer: 21–48.
Li S Q, Yang D C, Zhu Y G. 2007. Characterization and use of male sterility in hybrid rice breeding., 49(6): 791–804.
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod., 25(4): 402–408.
Ludwig S R, Habera L F, Dellaporta S L, Wessler S R. 1989., a member of the maizegene family responsible for tissue- specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region., 86(18): 7092–7096.
Maeda H, Yamaguchi T, Omoteno M, Takarada T, Fujita K, Murata K, Iyama Y, Kojima Y, Morikawa M, Ozaki H, Mukaino N, Kidani Y, Ebitani T. 2014. Genetic dissection of black grain rice by the development of a near isogenic line., 64(2): 134–141.
Meng L Z, Qi C Y, Wang C H, Wang S, Zhou C L, Ren Y L, Cheng Z J, Zhang X, Guo X P, Zhao Z C, Wang J, Lin Q B, Zhu S S, Wang H Y, Wang Z H, Lei C L, Wan J M. 2021. Determinant factors and regulatory systems for anthocyanin biosynthesis in rice apiculi and stigmas., 14(1): 37.
Oikawa T, Maeda H, Oguchi T, Yamaguchi T, Tanabe N, Ebana K, Yano M, Ebitani T, Izawa T. 2015. The birth of a black rice gene and its local spread by introgression., 27(9): 2401–2414.
Petroni K, Tonelli C. 2011. Recent advances on the regulation of anthocyanin synthesis in reproductive organs., 181(3): 219–229.
Reddy V, Scheffler B E, Wienand U, Wessler S R, Reddy A R. 1998. Cloning and characterization of the rice homologue of the maizeanthocyanin regulatory gene., 36(3): 497–498.
Saitoh K, Onishi K, Mikami I, Thidar K, Sano Y. 2004. Allelic diversification at the() locus of wild and cultivated rice: Nucleotide changes associated with phenotypes., 168(2): 997–1007.
Sakamoto W, Ohmori T, Kageyama K, Miyazaki C, Saito A, Murata M, Noda K, Maekawa M. 2001. The() locus of rice: ThePlallele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis., 42(9): 982–991.
Shen Y J, Jiang H, Jin J P, Zhang Z B, Xi B, He Y Y, Wang G, Wang C, Qian L, Li X, Yu Q B, Liu H J, Chen D H, Gao J H, Huang H, Shi T L, Yang Z N. 2004. Development of genome- wide DNA polymorphism database for map-based cloning of rice genes., 135(3): 1198–1205.
Shih C H, Chu H, Tang L K, Sakamoto W, Maekawa M, Chu I K, Wang M F, Lo C. 2008. Functional characterization of key structural genes in rice flavonoid biosynthesis., 228(6): 1043–1054.
Shih-Cheng L, Loung P Y. 1980. Hybrid rice breeding in China.: Innovative Approaches to Rice Breeding: Selected Papers from the 1979 International Rice Research Conference. Los Banos, the Philippines: International Rice Research Institute: 35–51.
Shu Q Y, Chen S F, Wu D X, Shen S Q, Cui H R, Xia Y W. 2001. Studies on breeding of a new type of cytoplasmic male sterile rice line Quanlong A., 34(4): 349–354. (in Chinese with English abstract)
Song K B, Song Z G. 2007. Discovery and preliminary research of the yellowish leaf mutant Annongbiao 810S in rice., 22(6): 71–73. (in Chinese with English abstract)
Song K B, Xiao J P. 2012. Progress in breeding of male sterile lines with recessive yellowish leaf color marker in rice., 27 (2): 15–17. (in Chinese with English abstract)
Sun X M, Zhang Z Y, Chen C, Wu W, Ren N N, Jiang C H, Yu J P, Zhao Y, Zheng X M, Yang Q W, Zhang H L, Li J J, Li Z C. 2018. The--gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice., 69(7): 1485–1498.
Takahashi M E. 1957. Analysis on apiculus color genes essential to anthocyanin coloration rice., 50(3): 266–362.
Wang Z W, Lv J, Xie S Z, Zhang Y, Qiu Z N, Chen P, Cui Y T, Niu Y F, Hu S K, Jiang H Z, Ge S Z, Trinh H, Lei K R, Bai W Q, Zhang Y, Guo L B, Ren D Y. 2018.encodes a pentatricopeptide repeat protein essential for early chloroplast development and seedling growth in rice., 84(2): 249–260.
Xi J M. 1997. The discovery and primary research of coleoptile purple lines in rice., (6): 41. (in Chinese)
Yang X H, Wang J R, Xia X Z, Zhang Z Q, He J, Nong B X, Luo T P, Feng R, Wu Y Y, Pan Y H, Xiong F Q, Zeng Y, Chen C, Guo H, Xu Z J, Li D T, Deng G F. 2021., a WD40 repeat gene, regulates anthocyanin biosynthesis in rice., 107(1): 198–214.
Yu X Q, Wu P L, Zhao F S, Jin W K, Chen H S. 2003. Improvement of recessive purple leaf rice and its utilization., 31(3): 3–7. (in Chinese with English abstract)
Zeng Y H. 1996. Studies on the verification of the truth and purith of hybrid seeds of Hsier-type rice., 18(1): 119–124. (in Chinese with English abstract)
Zhang F, Chen W. 2004. Review of methods for the purity identification of hybrid rice., 23(9): 55–58. (in Chinese)
Zhang G C, Liu Z M, Liu Y H, Kuya N, Hua Y C, Shi H R, Zhao W L, Han Y Q, Yamamoto T, Chen W F, Sun J. 2021. iTRAQ-based proteomics investigation of critical response proteins in embryo and coleoptile during rice anaerobic germination., 28(4): 391–401.
Zhang Y, Li Y F, Liu X F, Lin M X, Shen F C, He G H, Yang Z L, Yang G W. 2004a. Analysis on the inheritance of coleoptile purple line in rice., 37(11): 1693–1698. (in Chinese with English abstract)
Zhang Y, Shen F C, Yang G W, He G H, Zhang Z S, Yang Z L. 2004b. Analysis on the inheritance of inhibition and anti-inhibition of coleoptile purple line in rice and the SSR location of(t) gene., 31(8): 830–835. (in Chinese with English abstract)
Zhao D Q, Tao J. 2015. Recent advances on the development and regulation of flower color in ornamental plants., 6: 261.
Zhao H J, Wu D X, Shu Q Y, Shen S Q, Ma C X. 2004. Breeding and characteristics of photo-thermo sensitive genic male sterile rice Yutu S labeled with green-revertible albino leaf marker., 18(6): 515–521. (in Chinese with English abstract)
Zhao S S, Wang C H, Ma J, Wang S, Tian P, Wang J L, Cheng Z J, Zhang X, Guo X P, Lei C L. 2016. Map-based cloning and functional analysis of the chromogen genein rice (L.)., 59(5): 496–505.
Zheng J, Wu H, Zhu H B, Huang C Y, Liu C, Chang Y S, Kong Z C, Zhou Z H, Wang G W, Lin Y J, Chen H. 2019. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves., 223(2): 705–721.
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/
8 September 2021;
18 February 2022
s:Zhang Yi (zhangyi6116@ynu.edu.cn); Guo Longbiao (guolongbiao@caas.cn); Lei Kairong (leikairong@126.com)
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Genetic Dissection of Grain Size Traits Through Genome-Wide Association Study Based on Genic Markers in Rice
- Identification of New Allele of FLOURY ENDOSPERM2 in White-Core Endosperm Mutant of Rice
- Computer-Assisted Real-Time Rice Variety Learning Using Deep Learning Network
- Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation
- Improve Anthocyanin and Zinc Concentration in Purple Rice by Nitrogen and Zinc Fertilizer Application
- Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding

