早产儿视网膜病激光光凝术后并发急性呼吸窘迫综合征的危险因素分析
张静 黄学林 高彩凤 钟隽镌 任建兵 聂川
[摘要] 目的 探讨引起早产儿视网膜病激光光凝术后并发急性呼吸窘迫综合征的可能危险因素。方法 回顾性分析2015~2018年在广东省妇幼保健院因早产儿视网膜病行激光光凝术的358例患儿临床资料,将并发急性呼吸窘迫综合征的患儿作为观察组(ARDS组),无急性呼吸窘迫综合征的患儿作为对照组(NARDS组)。统计出生胎龄、出生体重、手术时矫正胎龄、手术时体重、是否合并支气管肺发育不良(BPD)、手术眼睛(单眼/双眼)、白细胞计数(WBC)、C-反应蛋白(CRP)及痰培养情况,对可能引起急性呼吸窘迫综合征的高危因素进行分析。结果 ARDS组出生胎龄及出生体重均较NARDS组低,差异均有统计学意义(均P<0.05)。两组患儿的血清C-反应蛋白比较,差异有统计学意义(P<0.01)。ARDS组痰培养阳性18例(25.71%),NARDS组痰培养阳性31例(10.76%),两组比较,差异有统计学意义(P<0.01);ARDS组诊断为BPD的有36例,NARDS组82例,两组比较,差异有统计学意义(P<0.01)。经Logistic回归分析,早产儿激光光凝术后并发急性呼吸窘迫综合征的高危因素包括:感染(痰培养阳性OR=2.69,P=0.01;C-反应蛋白OR=1.13,P<0.01),合并BPD(OR=2.37,P=0.02)。结论 ROP患儿出生胎龄越小,體重越轻,行激光光凝术后越容易发生急性呼吸窘迫综合征。其高危因素包括CRP升高、痰培养阳性及合并支气管肺发育不良基础疾病。
[关键词] 早产儿视网膜病;激光光凝术;急性呼吸窘迫综合征;支气管肺发育不良;肺损伤;氧化应激
[中图分类号] R779.7 [文献标识码] A [文章编号] 1673-9701(2022)17-0001-04
Analysis on risk factors for ARDS complicated with ROP after laser photocoagulation
ZHANG Jing1 HUANG Xuelin2 GAO Caifeng2 ZHONG Junjuan1 REN Jianbing1 NIE Chuan1
1.Department of Neonatology, Guangdong Maternal and Child Health Hospital, Guangzhou 511442, China; 2.Department of Ophthalmology, Guangdong Maternal and Child Health Hospital, Guangzhou 511442, China
[Abstract] Objective To investigate the possible risk factors causing acute respiratory distress syndrome (ARDS) complicated with retinopathy of prematurity (ROP) after laser photocoagulation. Methods The clinical data of 358 cases of children who underwent laser photocoagulation for ROP at Guangdong Maternal and Child Health Hospital from 2015 to 2018 were retrospectively analyzed. Children with complications of ARDS were selected as the observation group, and children without ARDS were selected as the control group. The gestational age, birth weight, corrected gestational age at the time of operation, weight at the time of operation, whether bronchopulmonary dysplasia (BPD) was complicated, operated eyes (single eye/double eyes), white blood cell count (WBC), C-reactive protein (CRP) and sputum culture were counted. High-risk factors that may cause ARDS were analyzed. Results The gestational age and birth weight in the ARDS group were lower than those in the NARDS group, with statistically significant differences (all P<0.01). There was statistically significant difference between the two groups in serum CRP level (P<0.01). There were 18 positive sputum cultures in the ARDS group (25.71%) and 31 positive sputum cultures in the NARDS group (10.76%), with statistically significant difference between the two groups (P<0.01). 36 children in the ARDS group and 82 children in the NARDS group were diagnosed with BPD, with statistically significant difference between the two groups (P<0.01). The Logistic regression analysis showed that, the high-risk factors for ARDS in prematurity undergoing laser photocoagulation included infection (sputum culture positive OR=2.69, P=0.01; CRP OR=1.13, P<0.01) and complicated BPD (OR=2.37, P=0.02). Conclusion The younger the gestational age and the lower the birth weight of the child with ROP, the more likely he or she is to develop ARDS after laser photocoagulation. The high-risk factors include elevated CRP, sputum culture positive, and complicated with BPD and other underlying diseases.
[Key words] Retinopathy of prematurity; Laser photocoagulation; Acute respiratory distress syndrome; Bronchopulmonary dysplasia; Lung injury; Oxidative stress
早产儿视网膜病变(retinopathy of prematurity,ROP)是指未完全血管化视网膜的血管异常增殖,纤维组织增生,晚期出现牵拉性视网膜脱离,最终严重影响患儿视力甚至失明。早期发现、及时有效的治疗可防止视网膜脱离,降低儿童低视力率和致盲率。而激光光凝是控制早期ROP病变进展的重要手段[1]。既往研究发现ROP患儿行激光光凝术后易发生呼吸暂停、坏死性小肠结肠炎、败血症和消化道出血等相关并发症,而采取全身麻醉后实施光凝术可减少不良反应的发生[2]。芬太尼联合表面麻醉镇痛可以稳定生理状态,降低应激反应,且对胃肠功能及血流动力学影响小[3]。在既往研究的基础上通过加强激光光凝术围术期管理后,发现部分ROP患儿在光凝术后出现肺部渗出增多、呼吸衰竭等急性肺损伤的相关表现,现报道如下。
1 资料与方法:
1.1 一般资料
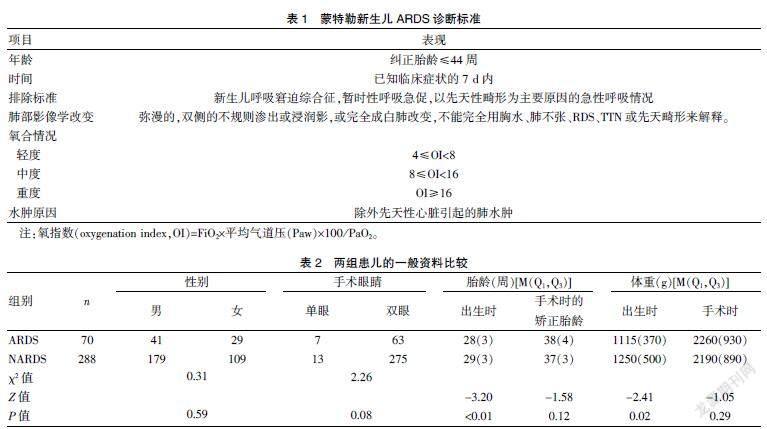
选取2015~2018年广东省妇幼保健院新生儿科收治的按照ROP国际分类标准和早产儿治疗用氧和视网膜病变防治指南标准需行激光光凝术的早产儿358例,其中男220例,女138例。诊断标准[4]:①依据2017年新生儿ARDS蒙特勒诊断标准,见表1;②支气管肺发育不良(bronchopulmonary dysplasia,BPD)的诊断基于早产儿出生后持续用氧≥28 d,术前取痰培养阳性,同时伴C-反应蛋白升高者,确认为有意义。排除标准:①矫正胎龄>44周;②术前未停氧者;③术前已明确合并有肺外严重感染、复杂型先天性心脏病、严重畸形或染色体疾病者;④术后死亡者。将入组患儿分为两组,术后72 h内出现气道分泌物增多、低氧血症、双肺弥漫性渗出增加,同时合并OI≥4者纳入急性呼吸窘迫综合征(ARDS)组;无相关临床表现者纳入非急性呼吸窘迫综合征(NARDS)组。ARDS组70例(19.56%),其中男41例,女29例;出生胎齡中位数为28周,出生体重中位数为1115 g。NARDS组288例,其中男179例,女109例;出生胎龄中位数为29周,出生体重中位数为1250 g。两组患儿的性别、手术时胎龄、手术时体重及手术眼睛(单眼/双眼)比较,差异均无统计学意义(均P>0.05);ARDS组患儿的出生胎龄及体重均低于NARDS组(均P<0.05)。见表2。本研究经广东省妇幼保健院医学伦理委员会审批通过[广东省妇幼保健院医伦第(201901165)号]。
1.2 方法
(1)围手术期管理:①术前禁食4~6 h;②术前1 h用复方托吡卡胺滴眼液散瞳;③在芬太尼镇痛、眼球表面麻醉后行气管插管及呼吸机辅助通气;④术后24 h停芬太尼,待患儿自主呼吸恢复后拔除气管插管。
(2)根据病历资料统计两组患儿的出生胎龄、出生体重、手术时矫正胎龄、手术时体重、是否合并BPD、手术眼睛(单眼/双眼)及术前血常规、C-反应蛋白及痰培养情况。
1.3 统计学方法
采用SPSS 21.0统计学软件进行数据分析,本研究中计量资料呈非正态分布,以中位数(四分位数间距)[M(Q1,Q3)]表示,两组间比较采用非参数检验;计数资料以[n(%)]表示,组间比较采用χ2检验;影响因素分析采用二分类Logistic回归分析,P<0.05为差异有统计学意义。
2 结果
2.1 两组患儿的白细胞计数、CRP、痰液培养及既往诊断BPD情况比较
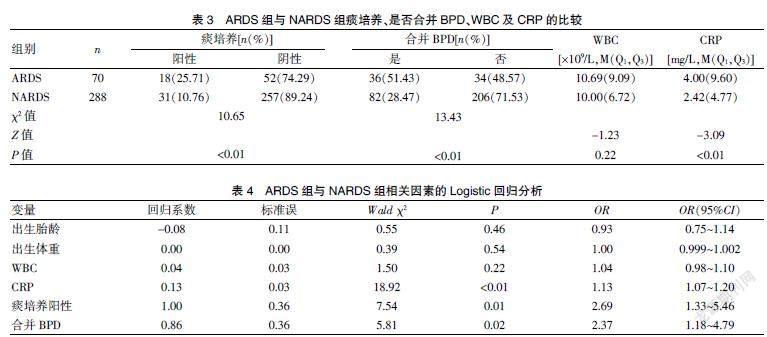
ARDS组患儿中痰培养阳性共18例(25.71%),NARDS组患儿中痰培养阳性共31例(10.76%),两组比较,差异有统计学意义(P<0.01)。ARDS组患儿中诊断为支气管肺发育不良的有36例(51.43%),NARDS组有82例(28.47%),两组比较,差异有统计学意义(P<0.01)。ARDS组患儿的术前CRP高于NARDS组,差异有统计学意义(P<0.01)。见表3。
2.2 多因素分析
以术后发生急性呼吸窘迫综合征为因变量,将出生胎龄、出生体重、白细胞计数、CRP、痰培养及是否合并BPD作为自变量,引入Logistic回归分析,结果发现CRP升高、痰培养阳性及合并BPD是早产儿激光光凝术后并发急性呼吸窘迫综合征的高危因素(均P<0.05)。见表4。
3 讨论
急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)是由各种非心源性因素引起的急性、进行性呼吸功能衰竭,其病理生理改变表现为肺顺应性降低,肺内分流增加以及通气与血流比例失调导致的顽固性低氧血症。ARDS不仅是肺部疾病,也是全身炎症反应在肺部的突出表现。目前其发病率及病死率仍较高,本研究旨在分析早产儿行ROP激光光凝术后发生ARDS的相关危险因素,为有效预防这一不良反应提供依据。
ARDS病因包括两大类,直接肺损伤因素以肺泡内病变为主,而间接肺损伤因素以血管内皮损伤和多种炎症细胞浸润为主。本研究结果显示ARDS组患儿出生胎龄小于NARDS组,且ARDS组中存在BPD基础病的患儿明显多于NARDS组。BPD是由多种因素相互作用损伤不成熟肺组织所造成的肺组织异常修复,主要表现为肺纤维化[5]。胎龄和体重越小,基础条件越差,发生BPD的机率越大,其肺部对各种刺激和损伤的耐受性更差。国外研究表明,机械通气通过触发促炎介质的释放而诱发术后ARDS的发生[6,7]。该过程涉及肺泡上皮、血管内皮、中性粒细胞、巨噬细胞和通过自分泌和旁分泌信号转导的介质释放。机械转导则是施加在肺上的物理力和导致细胞因子产生的细胞内信号通路之间的关键环节[8]。而肺泡Ⅱ型上皮细胞、血管内皮细胞的大量凋亡可引发肺水肿和肺换气功能障碍。本研究中ROP激光光凝术均在镇痛、眼球表面麻醉、气管插管及呼吸机辅助通气下由眼科医生完成。合并BPD的患儿因为肺部纤维化,顺应性下降,过高的气道压或潮气量可导致肺泡过度扩张,毛细血管内皮、肺泡上皮细胞和基底膜破裂,导致肺损伤的发生。同时,有研究指出使用低潮气量、中度呼气末压和肺复张策略可改善氧合和肺生理功能,并减少高危患儿的术后肺部并发症的发生[9]。因此,在围术期管理中呼吸机参数需个体化,可采用肺保护策略,以降低术后出现ARDS的风险。
本研究还发现ARDS组患儿CRP定量及痰培养阳性率均高于NARDS组,差异有统计学意义(均P<0.001)。提示ARDS组肺部感染率明显高于NARDS组。基础研究已发现在炎症和/或感染因素刺激下,被激活的多形核白细胞(PMN)聚集、黏附于肺泡毛细血管内皮细胞,导致机体释放大量炎性介质,损伤肺泡上皮细胞和肺毛细血管内皮细胞,引发肺水肿和肺换气功能障碍[10~12]。同时,病源菌产生的细菌内毒素可刺激机体产生过量TNF-α,可直接导致细胞坏死,从而引起脏器损害[13]。ARDS组痰培养中检出肺炎克雷伯菌3例,大肠埃希菌2例,金黄色葡萄球菌2例,产气肠杆菌2例,鲍曼不动杆菌2例,铜绿假单孢菌2例,阴沟肠杆菌1例,流感嗜血杆菌1例,黏质沙雷菌1例,白色念珠菌2例。以上大部分为条件致病菌,在患儿基础状态差或免疫功能低下时,可转变为致病菌。因此,需早期识别并有效控制肺部感染,以降低ROP光凝术后出现ARDS的风险。因此,围术期是否预防性应用抗菌药物以防术后出现ARDS需进一步研究。
有研究显示在激光光凝术过程中患儿受到应激源刺激后产生应激反应[14]。氧化应激是指机体受内源性或外源性因素刺激后产生大量的氧自由基,从而导致DNA损伤、细胞凋亡、蛋白质及酶功能失调和细胞膜脂质过氧化等[15]。而炎症、缺血和细胞因子等多种因素也可导致高水平的自由基,增加机体发生氧化应激损伤的风险[16]。由此可推测,机械通气及激光光凝术引起的不适与疼痛可诱发机体的氧化还原反应失调,大量生成的活性氧可损伤肺血管内皮细胞和/或肺泡上皮细胞,引起肺毛细血管通透性增加及肺泡表面活性物质减少,造成肺水肿和肺不张,进而发展为ARDS。为减少疼痛和机械牵拉所致的应激反应,需加强围术期镇静、镇痛管理,保证生命体征平稳,降低术后出现ARDS的风险。
急性呼吸窘迫综合征源于多重危险因素的交叉影响,目前并无有效治疗方法。将患儿的出生胎龄、出生体重、白细胞计数、CRP、痰培养及是否合并BPD作为自变量,引入Logistic回归分析发现,CRP升高、痰培养阳性和合并BPD基础疾病是ROP患儿術后发生急性呼吸窘迫综合征的危险因素。为降低其发病率,在ROP患儿行激光光凝术前,需详细评估患儿的基础情况,积极做好围术期管理,控制肺部感染,个体化调整呼吸参数,减少对患儿的刺激,在控制原发病的基础上,采用保护性通气策略、加强液体管理及抗炎治疗等措施,尽可能减少ARDS的发生。
[参考文献]
[1] Fierson WM,American Academy of Pediatrics Section on Ophthalmology,American Academy of Ophthalmology,et al. Screening examination of premature infants for retinopathy of prematurity[J].Pediatrics,2013,131(1):189-195.
[2] Kaur B,Carden SM,Wong JN,et al. Anesthesia management of laser photocoagulation for retinopathy of prematurity. A retrospective review of perioperative adverse events[J].Pediatric Anesthesia,2020,30(11):1261-1268.
[3] Sindhur M,Balasubramanian H,Srinivasan L,et al. Intranasal fentanyl for pain management during screening for retinopathy of prematurity in preterm infants: A randomized controlled trial[J].J Perinatol,2020,40(6):881-887.
[4] De-Luca D,van-Kaam AH,Tingay DG,et al. The montreux definition of neonatal ARDS:Biological and clinical background behind the description of a new entity[J].Lancet Respir Med,2017,5(8):657-666.
[5] Thebaud B,Goss KN,Laughon M,et al. Bronchopulmonary dysplasia[J].Nat Rev Dis Primers,2019,5(1):78.
[6] Hoegl S,Burns N,Angulo M,et al. Capturing the multifactorial nature of ARDS--"Two-hit" approach to model murine acute lung injury[J].Physiol Rep,2018,6(6):e13 648.
[7] Koutsogiannaki S,Huang SX,Lukovits K,et al. The characterization of postoperative mechanical respiratory requirement in neonates and infants undergoing cardiac surgery on cardiopulmonary bypass in a single tertiary institution[J].J Cardiothorac Vasc Anesth,2021,36(1):215-221.
[8] Ding X,Tong Y,Jin S,et al. Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: Role of WISP1-alphavbeta5 integrin pathway in TLR4-mediated inflammation and injury[J].Crit Care,2018,22(1):302.
[9] Liu S, Zhang L. Research progress in perioperative ventilator-induced lung injury[J].Zhong Nan Da Xue Xue Bao Yi Xue Ban,2019,44(4):346-353.
[10] Rebetz J,Semple JW,Kapur R,et al. The pathogenic involvement of neutrophils in acute respiratory distress syndrome and transfusion-related acute lung injury[J].Transfus Med Hemother,2018,45(5):290-298.
[11] Sharma NS,Lal CV,Li JD,et al. The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome[J].Am J Physiol Lung Cell Mol Physiol,2018,315(5):L653-L661.
[12] Herrero R,Sanchez G,Lorente JA,et.al. New insights into the mechanisms of pulmonary edema in acute lung injury[J].Ann Transl Med,2018,6(2):32.
[13] Pooladanda V,Thatikonda S,Bale S,et al. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-alpha mediated NF-kappaB and HDAC-3 nuclear translocation[J].Cell Death Dis,2019,10(2):81.
[14] Jiang JB,Strauss R,Luo XQ,et al. Anaesthesia modalities during laser photocoagulation for retinopathy of prematurity: A retrospective,longitudinal study[J].BMJ Open,2017,7(1):e013 344.
[15] Falsaperla R,Lombardo F,Filosco F,et al. Oxidative stress in preterm infants: Overview of current evidence and future prospects[J].Pharmaceuticals (Basel),2020,13(7):10
[16] Marseglia L,D'Angelo G,Manti S,et al. Oxidative stress-mediated damage in newborns with necrotizing enterocolitis: A possible role of melatonin[J].Am J Perinatol,2015, 32(10):905-909.
(收稿日期:2021-05-12)

