Zinc Tolerance and Accumulation in Four Alfalfa (Medicago sativa) Varieties
Hui JING Nan HOU Shuhe WEI Huiping DAI Xiaona HOU
AbstractTo evaluate the zinc (Zn) remediation capacity of four alfalfa species, the effects of different concentrations of Zn on plant growth, Zn uptake and translocation as well as uptake of other nutrients were investigated. The results showed that the Zn tolerance index of Aohan was significantly higher than other species. Among the four species, Aohan had the highest concentration of Zn in roots, followed by Golden Empress, Sanditi and Longxi. Aohan had the highest bioconcentration factor (BCF) in leaves. Whereas, Sanditi and Longxi had the lowest BCF in stem and roots, respectively. The translocation factor of Golden Empress was significantly lower than other species. The Zn accumulation rate of Aohan was higher than other species regardless of the concentration of Zn. Longxi had the lowest allocation of Zn in leaves and Golden Empress had the lowest allocation of Zn in roots. The concentrations of other elements (Fe and Mg) in leaves were decreased with Zn additions, but the interactions between Zn and other elements in roots varied with species. These results indicated that suitable species of alfalfa could successfully be used for the phytoremediation of Zn-contaminated soils.
Key wordsZinc; Bioconcentration factor; Phytoremediation
Heavy metal contamination is an important global environmental problem that results in bioaccumulation in the food chains, posing a direct threat to animals and human health[1-4]. Among metals, zinc (Zn) is an essential element for animals and plants. However, excess Zn may cause severe toxicity, including nutrient imbalances[5-9], growth inhibition, leaf chlorosis and photosynthesis impairment[10-13]. Thus, Zn-contaminated soils can cause injury to soil microorganisms, which, in turn, reduce crop yield and are dangerous to food chains[10]. Therefore, suitable methods are urgently needed to clean Zn from Zn-contaminated soil. Phytoremediation has been effectively used to remediate inorganic and organic contaminants in soil and groundwater[14-16]. In Zn phytoremediation, plants are used either to absorb Zn from the soil and translocate it to harvestable shoots (phytoextraction) or to stabilize metal contaminants in soils by root accumulation or by precipitation within root zones (phytostabilization)[17-18]. The systematic screening and identification of plants used to remediate heavy-metal polluted areas is important.
Phytoremediation is an important technology to remove or degrade various pollutants from soils by employing plants with an exceptional capacity to accumulate metals[19]. As one of the most plants with tolerance to many toxic heavy metals, alfalfa has been suggested to be a potential phytoremediator[9]. As an important legume forage plant worldwide, alfalfa (Medicago sativa L.) has advantages in rapid growth, high biomass production, high-protein content and can be cultivated on marginal lands. Especially, alfalfa has the ability to accumulate high concentrations of nutrients and heavy metals after long-term exposure, which makes it suitable for phytoremediation projects. However, the mechanism of heavy metal accumulation and translocation in alfalfa is still unknown. So, it is very interesting to elucidate how alfalfa accumulates and translocates heavy metal. Hence, the objectives of this study were to investigate the effects of Zn on plant growth and development, Zn tolerance, Zn accumulation and translocation in different tissue organs of four alfalfa varieties, as well as the possible relationship between the effects of Zn on growth and the concentrations of Mg and Fe in plant tissues.
Materials and Methods
Plant material and growth conditions
The experiments were performed in the orchard of Shaanxi University of Technology, Hanzhong (33°34′ N, 107°28′ E), P. R. China. Seeds of four alfalfa varieties (Aohan, Golden Empress, Sanditi, Longxi) were purchased from Northwest A&F University. Seeds were surface-sterilized with 0.1% (w/v) HgCl for 10 min. The vermiculite and peat were mixed (w/w 1∶1) to prepare the culture medium and Hoagland solution was used as the nutrient solution. The nutrient solution includes 5 mM Ca(NO)·4HO, 5 mM KNO, 2 mM MgSO·4HO, 1 mM KHPO, 0.1 mM EDTA-Fe, 461 mM HBO, 9.11 mM MnCl·4HO, 0.321 mM CuSO·5HO, 0.761 mM ZnSO·7HO and 0.51 mM HMoO·HO[13]. The plants were cultured in an opaque culture plate with abundant illumination. The pH of Hoagland solution was adjusted to 6.5±0.1 with 0.1M HCl or 0.1M NaOH every day. After culture of 2 months, plants were transferred into solutions containing 0, 300, 600 and 900 μg/g Zn (added in the form of ZnSO·7HO), respectively. Six repeats were done for every treatment.
Sampling and measurements
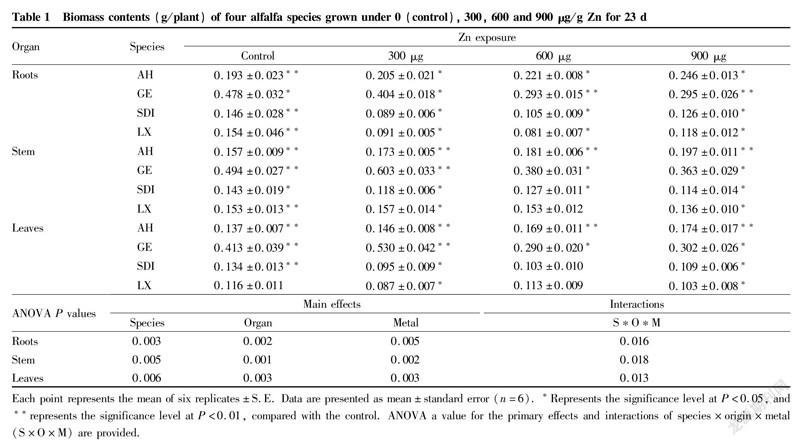
The seedlings were harvested after 23 days of treatment. The roots were immersed in 5 mM CaCl for 15 min and then the whole plants were rinsed with deionized water. The leaf and stem were separated and dried in an air oven at 80°C until a constant dry weight. The effects of different zinc concentrations on the dry weights of root, stem, and leaf were assessed by two-way ANOVA.
Measurement of bioconcentration factor, tolerance index, translocation factor and total Zn accumulation rate
The bioaccumulation factor (BCF) was calculated as: BCF= zinc concentration in plant tissues/zinc concentration in soil. The ratio of heavy metal concentration in the aerial parts to that in the root of the plant was calculated as translocation factor (TF) as: TF=zinc in the aerial (μg/g)/zinc in root(μg/g)[20]. The tolerance index(TI) was calculated as TI=Mean increase of root length in treated plants/mean increase of root length in control plants×100[21]. Total Zn accumulation rate was expressed as (mg/g)/d[20]. Consider measuring the root length after 23 d.
Statistical analysis
A completely randomized design with six replicates was used for each time point. The data were tested for normality prior to the statistical analysis. For experimental variables, three-way analysis of variance (ANOVA) was applied, with ZnSO4 (Zn), species and tissue as the three principal factors. Statistical analysis was conducted by STATISTICA 5.1 software (Statsoft Inc., United States of America). Separation of means was carried out by using Fisher's LSD test at P<0.05 and P<0.01 significance level.
Results
Biomass
During the whole experimental period, biomass increments in alfalfa plants were monitored (Table 1). Compared with controls, exposuring to 900 μg/g Zn markedly decreased the total root biomass of Sanditi and Longxi. Exposuring to 300 μg/g Zn markedly increased the stem and leaf biomass of Aohan and Longxi. Notably, Aohan had the largest biomass increment and Longxi had least biomass increment among four species.
Zn tolerance and translocation
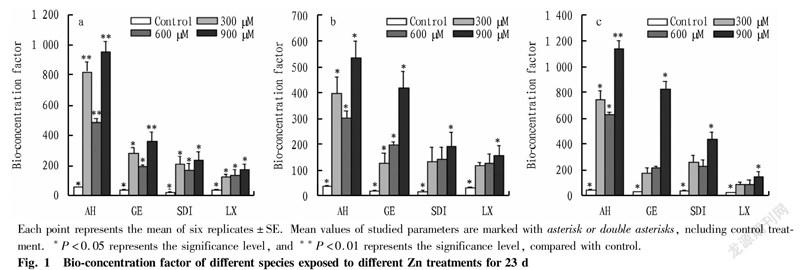
To evaluate the capability of different alfalfa species to extract and accumulate zinc, the bio-concentration factor was calculated. In Fig. 1, BCF in the aerial parts of the four species increased as the following order: Longxi<Sanditi<Golden Empress<Aohan. BCF of the aerial parts (leaves and stems) of Aohan was significantly higher than other species. At a zinc concentration of 900 μM , the BCF in the roots of four species was between 171 and 953. Aohan and Golden Empress had higher BCFs than other species. Above results indicated that Aohan had the stronger ability to accumulate Zn in leaf and stem tissues than other species.
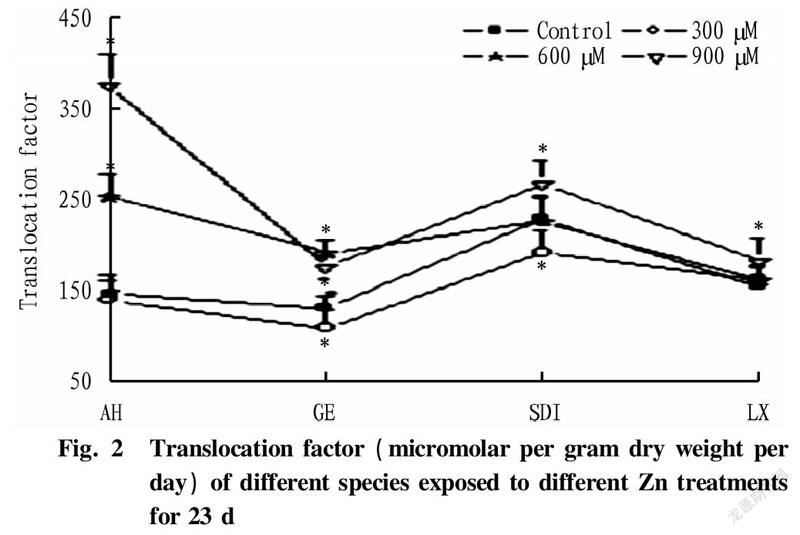
Translocation factor (TF) stands for the ratio of the absorbed metal that reached the aerial part to that present in the roots. Thus, the capability of four alfalfas to accumulate zinc in aboveground tissues (leaves and stem) was further confirmed by calculating their TF (Fig.2). Among four alfalfa species, Aohan had higher TF value (up to 374%) than other species.
Concentrations of nutrients in plant tissues
The nutrient concentrations in tissues were significantly influenced by species and Zn treatments except for the concentrations of
Each point represents the mean of six replicates±SE. Mean values of studied parameters are marked with asterisk or double asterisks, ncluding control treatment. *P<0.05 represents the significance level, and **P<0.01 represents the significance level, compared with control.
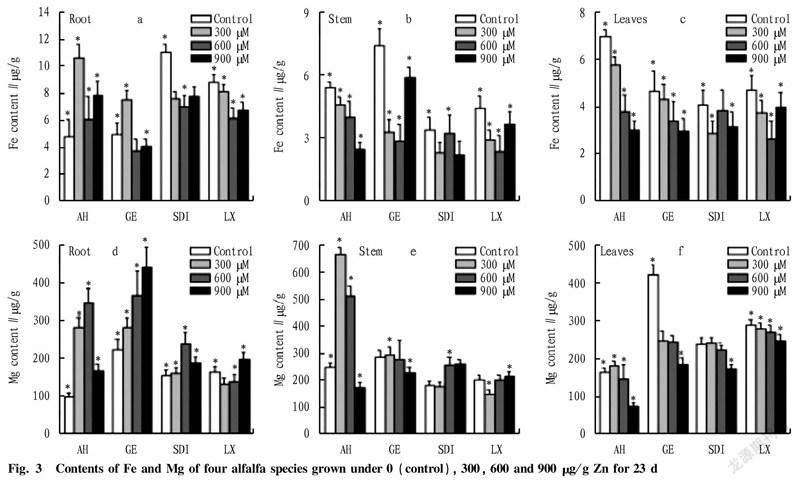
Fe and Mg in roots, stems and leaves. Significant interactions between species and Zn treatments were found for the concentrations of Fe and Mg in leaves, stems and roots (Fig. 3). In general, the concentrations of Fe and Mg in stems decreased with the increase in Zn concentrations. However, the concentrations of Fe in roots increased except the concentrations of Fe in Golden Empress and Longxi, Sanditi. While the concentrations of Mg in roots and leaves (except Golden Empress and Longxi) increased with the increase in Zn concentrations. The concentrations of Zn in roots increased at low Zn levels, but decreased at higher Zn levels. The concentrations of Fe and Mg in roots of Aohan increased with the increase in Zn concentrations (Fig. 3).
Discussion
To study Zn hyperaccumulators is dual. On one hand, there is a fundamental interest in acquiring the mechanisms underlying environmental adaptation and the evolution of complex traits. On the other hand, there is a fundamental interest in acquiring cellular mechanisms involved in the detoxification of one of the most toxic trace metals for living organisms[22-23]. In this study, we have integrated plant biomass and Zn accumulation capacity to assess the Zn tolerance of four alfalfa species. Our findings indicated that dry mass accumulation could be induced by long-term Zn exposure in Aohan among four identified zinc hyperaccumulators. Despite the toxicity of heavy metals, plants usually show the ability to accumulate heavy metals without visible changes in their appearance or biomass[12,14]. Based on the threshold (Zn>10 000 μg/g) for Zn hyperaccumulation defined in shoots[25], the concentrations of metals in the roots and shoots of the studied plants were higher than the corresponding soil. Furthermore, the BCF in root and aboveground of all the studied plants was>1. Several authors have reported that BCF>1 indicated a special ability to extract and transport metals from substrate to plant parts[26-27]. Such plant species can be considered as hyperaccumulator and may be applied for the phytoextraction of metals[28]. The tested species in this study showed different tolerances to Zn toxicity and capacity of Zn uptake, accumulation and translocation. Root growth is more sensitive to heavy metals than shoot[29]. This evidence was correlated with our results, which showed that Aohan had significantly higher root length after Zn treatments, compared with the control (i.e., tolerance index). Differences in Zn accumulation capacity and localization are major factors in determining plant tolerance to Zn exposure[30]. Among the four species, Aohan had a relatively higher accumulation rate, higher translocation factor and the highest tolerance index. In the present study, Zn accumulated predominantly in aboveground parts of the tested plants, but the different species had significant differences in the distribution percentage of Zn in different tissues. Aohan distributed the highest Zn in the leaves. For Zn accumulation and translocation, the comparisons among tested species have revealed that different species could be used for different purposes in phytoremediation strategies. Our data indicated that Aohan could be used to remove Zn from substrate to the aboveground tissues. Based on these findings, Aohan may be considered as a suitable candidate plant for the phytoremediation of metal-contaminated sites in the future research.
Conclusions
In this paper, we investigated zinc distribution across alfalfa plant parts, zinc distribution in different tissues, zinc uptake and accumulation in four alfalfa species. This study clearly showed that relationships between zinc toxicity and the uptake and accumulation were species specifically related to zinc distribution and storage. Different alfalfa species had evolved different strategies in response to high zinc accumulation. Our study indicated that the zinc bioavailability between the closely related species is different. Therefore, the present study opened new research avenues to understand the regulation of phytoremediation and restoration of land contaminated by toxic metals.
References
[1] MORILLO J, USERO J, GRACIA I. Heavy metal distribution in marine sediments from the southwest coast of Spain[J]. Chemosphere, 2004(55): 431-442.
[2] DOUMETT S, LAMPERI L, CHECCHINI L, et al. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: influence of different complexing agents[J]. Chemosphere, 2008(72): 1481-1490.
[3] FAN KC, HIS HC, CHEN CW, et al. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications[J]. J Environ Manage, 2011(92): 2818-2822.
[4] CUTILLAS-BARREIRO L, ANSIAS-MANSO L, FERNNDEZ-CALVIÑO D, et al. Pine bark as bio-adsorbent for Cd, Cu, Ni, Pb and Zn: Batch-type and stirred flow chamber experiments[J]. J Environ Manage, 2014(144): 258-264.
[5] LAHIVE E, O'CALLAGHAN MJ, JANSEN MA, et al. Uptake and partitioning of zinc in Lemnaceae[J]. Ecotoxicology, 2011(20): 1992-2002.
[6] LAHIVE E, O'HALLORAN J, JANSEN MAK. Differential sensitivity of four Lemnaceae species to zinc sulphate[J]. Environ Exp Bot, 2011, 71(1): 25-33.
[7] NUNES B, CAPELA RC, SÉRGIO T, et al. Effects of chronic exposure to lead, copper, zinc, and cadmium on biomarkers of the European eel, Anguilla anguilla[J]. Environ Sci Pollut Res, 2014(21): 5689-5700.
[8] JEYAKUMAR P, LOGANATHAN P, ANDERSON CWN, et al. Comparative tolerance of Pinus radiata and microbial activity to copper and zinc in a soil treated with metal-amended biosolids[J]. Environ Sci Pollut Res, 2014(21): 3254-3263.
[9] DAI HP, SHAN CJ, ZHAO H, et al. The difference in antioxidant capacity of four alfalfa species in response to Zn[J]. Ecotox Environ Safe, 2015(111): 312-317.
[10] HASSAN Z, AARTS MGM. Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants[J]. Environ Exp Bot, 2011(72): 53-63.
[11] TODESCHINI V, LINGUA G, D'AGOSTINO G, et al. Effects of high zinc concentration on poplar leaves: a morphological and biochemical study[J]. Environ Exp Bot, 2011(71): 50-56.
[12] CAMBROLLE J, MANCILLA-LEYTON JM, MUÑOZ-VALLES S, et al. Evaluation of zinc tolerance and accumulation potential of the coastal shrub Limoniastrum monopetalum (L.) Boiss[J]. Environ Exp Bot, 2013(85): 50-57.
[13] DAI HP, ZHAO H, WU SQ, et al. Zinc detoxification and tolerance in alfalfa[J]. J Food Agric Environ, 2014, 12(2): 1097-1099.
[14] CHANEY RL, ANGLE JS, BROADHURST CL, et al. Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies[J]. J Environ Qual, 2007(36): 1429-1443.
[15] LIANG HM, LIN TH, CHIOU JM, et al. Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators[J]. Environ Pollut, 2009(157): 1945-1952.
[16] DARY M, CHAMBER-PÉREZ MA, PALOMARES AJ, et al. "In situ" phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria[J]. J Hazard Mater, 2010(177): 323-330.
[17] ZHANG XF, XIA HP, LI ZA, et al. Potential of four forage grasses in remediation of Cd and Zn contaminated soils[J]. Bioresour Technol, 2010(101): 2063-2066.
[18] LIN WJ, XIAO TF, WU YY, et al. Hyperaccumulation of zinc by Corydalis davidii in Zn-polluted soils[J]. Chemosphere, 2012(86): 837-842.
[19] SURESH B, RAVISHANKAR GA. Phytoremediation: A novel and promising approach for environmental clean-up[J]. Crit Rev Biotechnol, 2004(24): 97-124.
[20] SUNAYANA G, SUCHISMITA D. Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance[J]. Ecotoxicology and Environmental Safety, 2016(126): 211-218
[21] SHI GR, LIU CF, CAI QS, et al. Cadmium accumulation and tolerance of wwo safflower cultivars in relation to photosynthesis and antioxidantive enzymes[J]. Bull Environ Contam Toxicol, 2010(85): 256-263.
[22] DISANTE KB, FUENTES D, CORTINA J. Sensitivity to zinc of Mediterranean woody species important for restoration[J]. Sci Total Environ, 2010(408): 2216-2225.
[23] KACHOUT SS, MANSOURA AB, MECHERGUI R, et al. Accumulation of Cu, Pb, Ni and Zn in the halophyte plant Atriplex grown on polluted soil[J]. J Sci Food Agric, 2012(92): 336-342.
[24] HAN YL, YUAN HY, HUANG SZ, et al. Cadmium tolerance and accumulation by two species of Iris[J]. Ecotoxicology, 2007(16): 557-563.
[25] REEVES RD, BAKER AJM. Metal accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment[J]. Wiley, New York, 2000.
[26] YOON J, CAO X, ZHOU Q, et al. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site[J]. Sci Total Environ, 2006(368): 456-464.
[27] CORNARA L, ROCCOTIELLO E, MINGANTI V, et al. Level of trace elements in plants growing on serpentine and metalliferous soils[J]. J Plant Nutr Soil Sci, 2007(170): 781-787.
[28] RASCIO N, NAVARI-IZZO F. Heavy metal hyperaccumulating plants: how and why do they do it and what makes them so Interesting[J]. Plant Sci, 2011(180): 169-181.
[29] ISLAM F, YASMEEN T, RIAZ M, et al. Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants[J]. Ecotox Environ Safe, 2014(110): 143-152.
[30] KASHEM MA, SINGH BR, KUBOTA H, et al. Zinc tolerance and uptake by Arabidopsis halleri ssp. gemmifer a grown in nutrient solution[J]. Environ Sci Pollut Res, 2010(17): 1174-1176.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Report on the Breeding of Dahen 799 Broilers
- Evaluation and Analysis for Survey of Tea Production Quality Safety Management and Control
- Study on the Preparation of "Oil-tea" Instant Tea from the Compound Extract of Green Tea and Ginger
- Research Progress on Chemical Constituents and Pharmacological Effects of Zhuang Medicine Cocculus laurifolius DC.
- Study on Quality Standard of Lujing Yiqi Shengxue Pills
- Research on the Development of Guangxi Zhuang and Yao Ethnic Medicine Industry

