Study on Quality Standard of Lujing Yiqi Shengxue Pills
LidaCHEN XianmeiXU YuchuanLI YuanfengYANG YulingLUO




Abstract[Objectives] This study was conducted to establish the quality standard for Lujing Yiqi Shengxue Pills to provide a basis for its quality control. [Methods]According to the quality requirements in the 2020 edition of Chinese Pharmacopoeia, the quality of Lujing Yiqi Shengxue Pills was evaluated from the aspects of properties, identification, inspection and content determination. Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma stir-fried with bran and Radix Paeoniae Alba in the preparation were identified and studied by thin layer chromatography (TLC). A high performance liquid chromatography (HPLC) method was established to determine the content of paeoniflorin in the preparation. [Results] The thin layer spots of Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma and Radix Paeoniae Alba were clear and round, and the negative preparations had no interference. The properties, moisture, weight difference, loading difference, dissolution time limit and microbial limit all met the requirements. The regression formula for the quantitative analysis of paeoniflorin in the concentration range of 4.86-194.40 μg/ml was: Y=12.024 1X+0.879 7, R=0.999 9; and the average recovery was 98.9%, and the RSD was 1.7%. [Conclusions]The method has good repeatability, and is stable, feasible, simple and convenient in operation and suitable for quality control of Lujing Yiqi Shengxue Pills.
Key wordsLujing Yiqi Shengxue pills; Quality evaluation; Thin layer chromatography; Paeoniflorin; Content determination
Lujing Yiqi Shengxue Pills is a compound traditional Chinese medicine preparation, which is composed of 14 traditional Chinese medicines such as Radix Astragali, Radix Paeoniae Alba, Radix Codonopsis, Radix Glycyrrhizae Preparata, and fried Ziziphi Spinosae Semen. In the prescription, Radix Astragali is sweet in taste, slightly warm in nature, attributive to the lung and spleen meridians, and has the functions of invigorating qi and lifting yang, and promoting the secretion of saliva or body fluid and nourishing blood[1], while Radix Paeoniae Alba is bitter and sour in taste, slightly cold in nature, attributive to the liver and spleen meridians, and has the effects of nourishing blood for regulating menstruation, retaining yin with astringent and relieving sweating[2], and the combination of the two is used as the monarch medicine for reinforcing qi and nourishing blood. Radix Codonopsis is sweet in taste, neutral in nature, and attributive to the spleen and lung meridians, and has the effects of strengthening spleen and tonifying lung, and nourishing blood and promoting the secretion of saliva or body fluid. Radix Glycyrrhizae Preparata is sweet in taste, neutral in nature, and attributive to the heart, lung, spleen, and stomach meridians, and has the effects of nourishing the spleen and harmonizing stomach, nourishing qi and restoring the pulse[4]. Fried Ziziphi Spinosae Semen is sweet and sour in taste, neutral in nature, and attributive to the liver, gallbladder and heart meridians, and has the effects of nourishing qi and tonifying liver, and calming the heart and tranquilizing the mind. The combined use of the three herbs not only assists the monarch herbs to reinforce qi and nourish blood, but also has the functions of nourishing the blood and soothing the nerves, and serves as the adjuvant medicine. Lujing Yiqi Shengxue Pills mainly treats the syndrome of qi deficiency and blood deficiency, and insufficiency of heart and spleen, and is suitable for iron-deficiency anemia.
The prescription is an agreed prescription of the Hematology Department of The Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine. It has a significant clinical effect in the treatment of iron-deficiency anemia. It is developed into water-bindered pills for the convenience of patients to take and carry, and it is convenient for clinical use. Water-bindered pills is one of the traditional dosage forms of traditional Chinese medicine. Water-bindered pills was recorded as early as in Fifty-two Diseases Prescriptions. Water-bindered pills are usually made from fine powder of medicinal materials with water or water-based liquid (wine, vinegar, decoction, etc.) as a binder, and are made by the general method[6]. Our research group conducted a thin layer and content study on Lujing Yiqi Shengxue Pills, in which four traditional Chinese medicines of Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma stir-fried with bran and Radix Paeoniae Alba were qualitatively identified by TLC, and the durability of the identification method was verified. HPLC was used to conduct methodological investigation on the determination of paeoniflorin, and meanwhile, moisture, weight difference, loading difference, dissolution time limit and microbial limit were checked. This study provides a basis for the quality methodology evaluation of Lujing Yiqi Shengxue Pills, as well as an experimental basis for improving the quality control of traditional Chinese medicine water-bindered pills, and lays a foundation for the application of hospital preparations.
Materials and Methods
Experimental materials
Experimental medicinal materials and reagentsRadix Astragali reference medicinal material (National Institutes for Food and Drug Control, batch number: 121462-201705); Macrocephalae Rhizoma reference medicinal material (National Institutes for Food and Drug Control, batch number: 120925-202013); Radix Angelicae Sinensis reference medicinal material (National Institutes for Food and Drug Control, batch number: 121054-201306); Radix Paeoniae Alba reference material (National Institutes for Food and Drug Control, batch number: 120905-202011); paeoniflorin reference substance (National Institutes for Food and Drug Control, batch number: 110736-202044); ethyl acetate (Sichuan Xilong Scientific Co., Ltd., batch number: 201013); n-hexane (Xilong Scientific Co., Ltd., batch number: 210220); methanol (Sigma-Aldrich Chemical Reagent Co., Ltd., batch number: 67-56-1); ethanol (Chongqing Wansheng Chuandong Chemical Co., Ltd., batch number: 20200801); petroleum ether (Tianjin Zhiyuan Chemical Reagent Co., Ltd., batch number: 20201042); chloroform (Sichuan Xilong Scientific Co., Ltd., batch number: 210818); formic acid (Sinopharm Chemical Reagent Co., Ltd., batch number: T20111209); acetonitrile (Sigma-Aldrich Chemical Reagent Co., Ltd., batch number: 75-05-8); phosphoric acid (Sinopharm Chemical Reagent Co., Ltd., batch number: 20120425); silica G for thin layer chromatography (Qingdao Ocean Chemical Factory, batch number: 20211107); three batches of Lujing Yiqi Shengxue Pills (provided by the Preparation Department of The Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, batch number: 20220201/20220202/20220203).
Experimental instruments1260 high performance liquid chromatograph (Agilent Technologies); 101-2AB type electric heating blast drying oven (Tianjin Taisite Instrument Co., Ltd.); 2F-7 dark box three-purpose UV analyzer (Shanghai Jiapeng Technology Co., Ltd.); SK5210HP type ultrasonic cleaner (Shanghai Kedao Ultrasonic Instrument Co., Ltd.); AUW220D analytical balance (Shimadzu Corporation); BHS-6 digital constant temperature water bath (Ningbo Joan Lab Equipment Co., Ltd.).
Experimental method
TLC identification of Radix AstragaliFirst, 6 g of the product and 6 g of negative sample without Radix Astragali were weighed, respectively. Each sample was added with 30 ml of methanol, respectively, and then ultrasonically treated for 30 min and filtered, obtaining a filtrate, which was evaporated to dryness in a water bath. Next, each residue was added with 20 ml of water for dissolution, and the obtained solution was then extracted with 20 ml of ethyl acetate. The separated ethyl acetate parts were evaporated to dryness on a water bath, and the solutions obtained after dissolving the residues in 1 ml of methanol served as the test solution and the negative control solution, respectively. Also, 1 g of Radix Astragali reference medicinal material was taken, and the reference medicinal material solution was prepared by the same method. According to the thin-layer chromatography (general rule 0502) in the fourth volume of the 2020 edition of Chinese Pharmacopoeia, 5 μl of each of the above three solutions was drawn, and pointed on identical silica gel G thin-layer plates, and the silica gel G thin-layer plates were placed in developing cylinders containing chloroform-methanol (10∶1) as the developing solvent and saturated for 10 min. Then, the plates were taken out, dried in the air, and smoked in ammonia vapor for about 5 min. Finally, they were observed under a UV lamp (254 nm).
TLC identification of Radix Angelicae SinensisFirst, 10 g of the product and 10 g of negative sample without Radix Angelicae Sinensis were weighed, respectively. Each sample was added with 30 ml of petroleum ether, ultrasonically treated for 10 min, and filtered, and after the filtrates were evaporated to dryness in a water bath, the residues were dissolved in 1 ml of ethanol and used as the test solution and the negative control solution, respectively. Also, 1 g of Radix Angelicae Sinensis reference medicinal material was taken, and the reference medicinal material solution was prepared by the same method. According to the thin-layer chromatography (general rule 0502) in the fourth volume of the 2020 edition of Chinese Pharmacopoeia, 10 μl of each of the above three solutions was drawn, and pointed on identical silica gel G thin-layer plates, and the silica gel G thin-layer plates were placed in developing cylinders containing n-hexane-ethyl acetate (8∶1) as the developing solvent for development. Then, the plates were taken out, dried in the air, and observed under a UV lamp (365 nm).
TLC identification of Macrocephalae Rhizoma stir-fried with branFirst, 5 g of the product and 5 g of negative sample without Macrocephalae Rhizoma stir-fried with bran were weighed, respectively. The weighed product was added with 2 ml of n-hexane, ultrasonically treated for 15 min, and filtered to obtain the test solution. Also, 1 g of Macrocephalae Rhizoma reference medicinal material was taken, and the reference medicinal material solution was prepared by the same method. According to the thin-layer chromatography (general rule 0502) in the fourth volume of the 2020 edition of Chinese Pharmacopoeia, 10 μl of each of the above three solutions was drawn, and pointed on identical silica gel G thin-layer plates, and the silica gel G thin-layer plates were developed with petroleum ether (60-90 ℃)-ethyl acetate (100∶1) as the developing solvent. Next, the plates were taken out and dried in the air. Finally, they were sprayed with 5% vanillin sulfuric acid solution and heated until the spots were clear.
TLC identification of Radix Paeoniae AlbaFirst, 5 g of the product and 5 g of negative sample without Radix Paeoniae Alba were weighed, respectively. Each sample was added with 10 ml of ethanol, ultrasonically treated for 5 min, and filtered to obtain a filtrate, which was evaporated to dryness. The residues were dissolved with 1 ml of ethanol, and the solutions obtained served as the test solution and the negative control solution, respectively. Also, 2 g of Radix Paeoniae Alba decoction pieces was weighed and prepared in the same way as the reference medicinal material solution. According to the thin-layer chromatography (general rule 0502) in the fourth volume of the 2020 edition of Chinese Pharmacopoeia, 10 μl of each of the above three solutions was drawn, and pointed on identical silica gel G thin-layer plates, and the silica gel G thin-layer plates were developed with trichloromethane-ethyl acetate-methanol-formic acid (40∶5∶10∶0.2) as the developing solvent. Next, the plates were taken out and dried in the air. Finally, they were sprayed with 5% vanillin sulfuric acid solution and heated until the spots were clear.
Investigation on durability of the TLC identification methodTest solutions, negative sample control solutions and control medicinal material solutions were prepared according to the thin-layer identification methods of Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma stir-fried with bran and Radix Paeoniae Alba under the above-mentioned identification methods. The silica gel G plates after spotting were placed in different ambient temperatures (normal temperature, 4 ℃, 35 ℃) and different ambient humidity (RH%=23%, RH%=56%, RH%=85%) for development, after which the plates were taken out, air-dried and observed under the same conditions.
Comprehensive examinationAccording to references [7-11], the moisture, weight difference, loading difference, dissolution time limit and microbial limit were checked.
Content determination
Chromatographic conditions and system suitability testChromatographic conditions: octadecylsilane chemically bonded silica, used for packing the column used; mobile phase: acetonitrile-0.1% phosphoric acid solution (14∶86); column temperature: 30 ℃; flow rate: 1.0 ml/min; injection volume: 10 μl; detection wavelength: 230 nm. The number of theoretical plates should not be less than 2 000 according to the paeoniflorin peak.
Solution preparationAn appropriate amount of paeoniflorin reference substance was accurately weighed, and added with methanol to make a solution containing 50 μg per ml, that is, the reference substance solution. About 1 g of the test sample was accurately weighed and added in a 25 ml volumetric flask, into which 20 ml of dilute ethanol was added for ultrasonic treatment (240 W, frequency 45kHz) for 30 min. After cooling, the extraction system was added with dilute ethanol to the mark, shaken well, and filtered to obtain the subsequent filtrate, that is, the test solution was obtained. A negative control solution was prepared according to the proportions of the prescription while excluding Radix Paeoniae Alba.
Results and Analysis
Character observation
With the appearance and smell as indicators, the properties of the three batches of samples were checked, and it was determined that the product was brown, fragrant, and tasted slightly sweet. The results are shown in Table 1.
TLC identification results
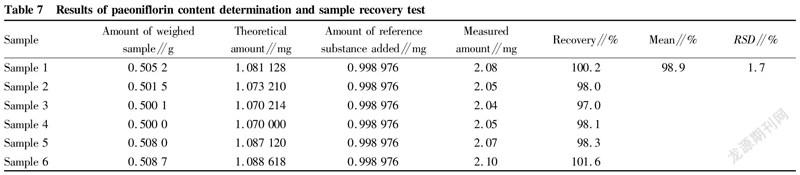
TLC identification results of Radix AstragaliAccording to the results of "TLC identification of Radix Astragali", the test solution and the reference medicinal material had spots of the same color at corresponding positions, and the negative sample had no interference. This method could identify Radix Astragali in the sample (Fig. 1).
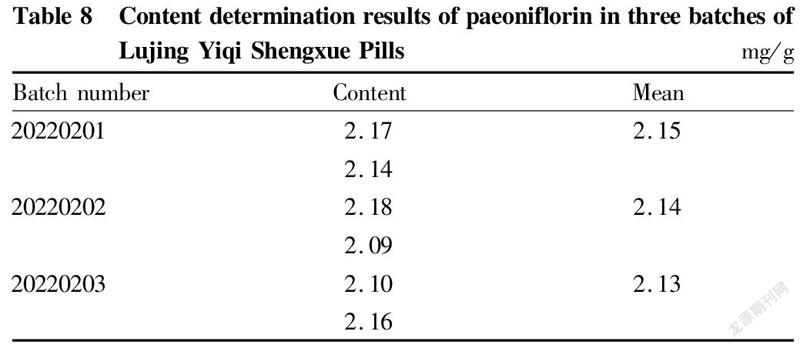
TLC identification results of Radix Angelicae SinensisAccording to the results of "TLC identification of Radix Angelicae Sinensis", the test solution and the reference medicinal material showed fluorescent spots of the same color at corresponding positions, and the negative sample had no interference. This method could identify Radix Angelicae Sinensis in the sample (Fig. 2).
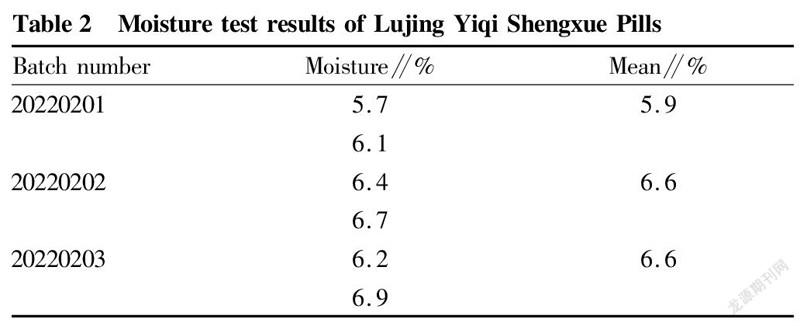
TLC identification results of Macrocephalae Rhizoma stir-fried with branAccording to the results of "TLC identification of Macrocephalae Rhizoma stir-fried with bran", in the chromatograms of the test products, there were spots of the same color at the position corresponding to the chromatogram of the control medicinal material, and the negative control had no interference (Fig. 3).
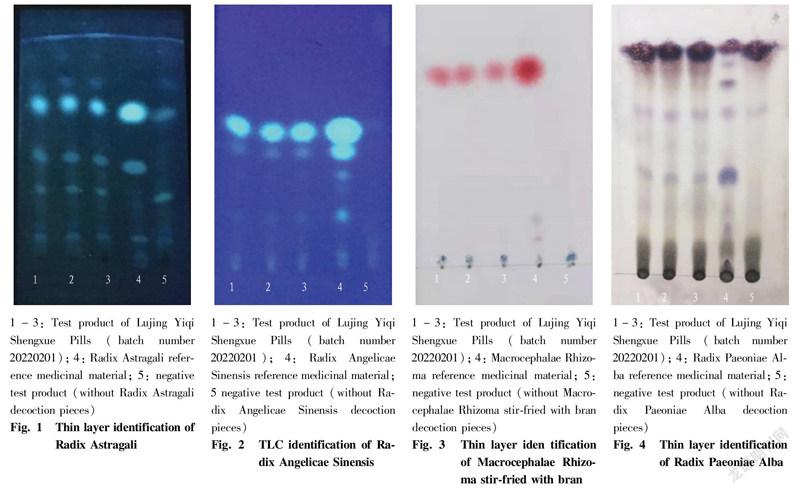
TLC identification results of Radix Paeoniae AlbaAccording to the identification result of "TLC identification of Radix Paeoniae Alba", in the chromatograms of the test products, the same blue-purple spots appeared at the position corresponding to the chromatogram of the reference medicinal material, and the negative control had no interference (Fig. 4).
Durability of TLC identification methodThe tests were carried out according to the steps under “Investigation on durability of the TLC identification method”. The results showed that in the chromatograms of the prepared test solutions, spots of the same colors were displayed at positions corresponding to the chromatograms of the reference medicinal materials, and the negative controls had no interference, indicating that different ambient temperatures and ambient humidity had no significant effects on the identification of Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma stir-fried with bran and Radix Paeoniae Alba in Lujing Yiqi Shengxue Pills, and the identification method had good durability.
Results of comprehensive examination
MoistureThe moisture of the samples was measured by the moisture measurement method. The results showed that the moisture of the three batches of samples were 5.7%, 6.4% and 6.1%, respectively, all of which were less than 9%, which met the requirements for moisture under water-bindered pills (Table 2).Loading differenceAccording to the loading difference examination method, 10 bags of the test samples were weighed, respectively, and the weight of each bag was compared with the marked loading (15 g). Under the loading difference limit of ±4%, there should not be more than 2 bags exceeding the loading difference limit, and there should not be 1 bag exceeding the limit by 1 time. The results showed that the loading difference limits of the three batches of samples were in compliance with the regulations.
Dissolution time limitSix pills of the test product were checked according to the dissolution time limit detection method using a screen with an aperture of 1.0 mm. The product should be completely dissolved within 1 h. During the operation, if the samples to be tested adhered to the baffle and hindered the examination, another 6 pills should be taken and examined without the baffle. In the examination, the pills should all pass through the screen within specified time. If there were fine particles that failed to pass the screen, but they had softened and had no hard core, they could beconsidered as complying with the regulations. The dissolution time limits of the three batches of samples were 42, 40 and 39 min, respectively, and all of them were dissolved within 1 h, which was in line with the examination regulations of the dissolution time limit for water-bindered pills. The results are shown in Table 3.
Microbial limitAccording to the microbiological examination of non-sterile products: microbial enumeration tests and tests for specified microorganisms (general rule 1106) and the microbial limit standard for non-sterile drugs, the results of the microbial limit examination of the three batches of samples were in compliance with the regulations (Table 4).
Results of methodological investigation
Specificity testFrom each of the three solutions in “Solution preparation”, 10 μl was precisely pipetted, and injected and determined according to the proposed chromatographic conditions. In the chromatogram of the test solution, there was a corresponding chromatographic peak at the retention time corresponding to the chromatogram of the reference substance solution, while the negative control had no corresponding chromatographic peak, indicating that the negative control did not interfere with the sample determination (Fig. 5). The resolution between the target peak and the adjacent chromatographic peaks was >1.5.
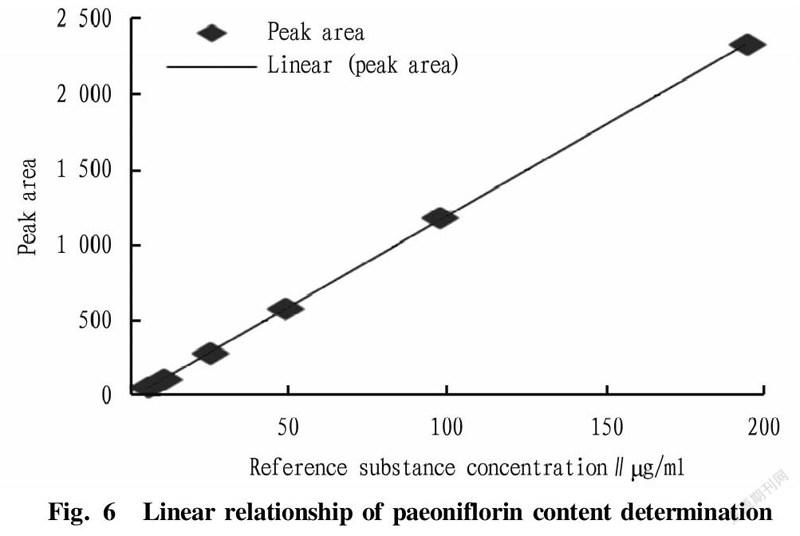
Examination of linear relationshipThe above-mentioned reference substance solution was precisely drawn to prepare 6 parts of reference solutions of different concentrations, which were injected to a liquid chromatograph with an injection volume of 10 μl and measured for peak area. With the reference substance concentration (X) as the abscissa and the peak area (Y) as the ordinate, linear regression was performed to obtain the regression equation: Y=12.024 1X+0.879 7, R=0.999 9 (n=6). The results showed that the concentration of paeoniflorin had a good linear relationship with the peak area in the range of 4.86-194.40 μg/ml (Fig. 6).
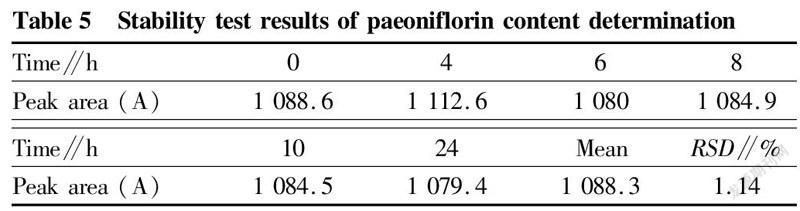
Stability testFrom the above-mentioned test solution, 10 μl was accurately drawn and injected at 0, 4, 6, 8, 10 and 24 h, respectively, and the paeoniflorin peak areas were recorded, and the RSD was1.14%, indicating that the stability of the test solution was good, as shown in Table 5.
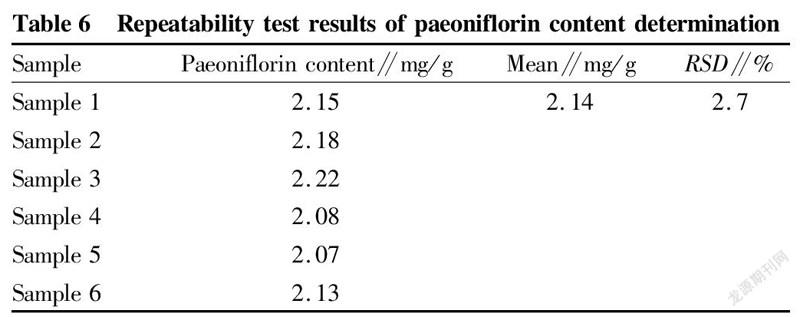
Repeatability testAn appropriate amount of samples from the same batch (batch number: 20220201), about 1 g, 6 parts in total, were ground finely, and accurately weighed. Each part was added into a 25 ml volumetric flask, and ultrasonically treated (240 W, 45 kHz) in 20 ml of diluted ethanol for 30 min. After cooling, dilute ethanol was added to the mark, and the extraction systems were shaken well and filtered, obtaining filtrates, which were determined according to the above chromatographic conditions. The results showed that the average content was 2.14 mg/g, and the RSD was 2.7% (n=6), indicating that the method had good repeatability, as shown in Table 6.
Recovery testSix samples of Lujing Yiqi Shengxue Pills with known paeoniflorin content were added with a certain amount of reference substance with known paeoniflorin content, respectively. Then, the samples were prepared according to the preparation method of the test solution, and the test solutions obtained were determined according to the above chromatographic conditions. The recovery of paeoniflorin was calculated, and the results showed that the recovery of paeoniflorin was 98.9%, RSD=1.7%, indicating that the recovery test results were good, as shown in Table 7.
Durability test resultsAn appropriate amount of Lujing Yiqi Shengxue Pills (batch number: 20220202) was prepared to a test solution according to the method under "Solution preparation". With the change of column temperature at ±2 ℃ and the change of flow rate at ±0.2 ml/min, the tolerance of the chromatographic column to different chromatographic conditions were investigated. The results showed that the target peak and the adjacent chromatographic peaks had good resolution and the durability under various investigation conditions was good.
Determination of the three batches of samples
Three batches of samples of Lujing Yiqi Shengxue Pills (20220201, 20220202, 20220203) were taken, and test solutions were prepared according to the method under "Solution preparation", and determined by HPLC. The results are shown in Table 8.
Conclusions and Discussion
Our research group carried out TLC identification of Radix Astragali, Radix Angelicae Sinensis, Macrocephalae Rhizoma stir-fried with bran and Radix Paeoniae Alba in Lujing Yiqi Shengxue Pill, and investigated the durability of the identification method. The results met the requirements, proving that the identification method is convenient, simple and stable. The quantitative determination of paeoniflorin was carried out by HPLC. The method had good repeatability, and the specificity, linearity, repeatability, stability and sample recovery all met the requirements, and the content of paeoniflorin could be determined efficiently and accurately. The three batches of samples were tested for moisture, weight difference, loaidng difference, dissolution time limit, microbial limit, and paeoniflorin content. All the results met the requirements, which proved that the quality control method is simple and feasible. This study systematically evaluated the quality control of Lujing Yiqi Shengxue Pills. The method is accurate and perfect, which provides an experimental basis for the establishment of the quality standards of Lujing Yiqi Shengxue Pills, and lays a research foundation for the later filing of hospital preparations.
In the identification of Radix Astragali by TLC, during the treatment of the test product, when using ethyl acetate for extraction, it is necessary to pay attention not to shake too vigorously to prevent emulsification; and after the thin-layer plate of Radix Astragali was developed, it was dried in the air, smoked in ammonia vapor for about 5 min and then observed under an ultraviolet lamp (254 nm). The spots were clear and round, and the development effect was better.
Radix Astragali and Radix Paeoniae Alba are the monarchs in Lujing Yiqi Shengxue Pills, and the amount of Radix Astragali is relatively large, but in the method for detecting the content of astragaloside IV, the sample processing and determination methods are relatively complicated, and the detection is difficult and cost high, so astragaloside IV should not be used as a quality control indicator for large-scale production of the preparation. Therefore, paeoniflorin was selected as the research index of content determination methodology. Furthermore, paeoniflorin has a relatively large content in Radix Paeoniae Alba and is stable, so it is suitable for the establishment of a content determination method.
References
[1] GU ZR, GE B, XU AX, et al. Efficacy and indications of Radix Astragali and the excavation of drug contraindications based on the textual research of Chinese herbal medicine[J]. Chinese Traditional Patent Medicine, 2018, 40(11): 2524-2530. (in Chinese).
[2] CHEN Y, JIA B, LI J, et al. Analysis on the medication characteristics of medicinal herb Radix Paeoniae Alba in Bianzheng Lu[J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2020, 26(4): 531-533. (in Chinese).
[3] LYU LM, GAO J, LI DW, et al. Changes of component content of Dangshen (Radix Codonopsis) before and after steaming and its effect on effectiveness[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacology, 2020, 26(9): 45-48. (in Chinese).
[4] CHEN LM, FU YL. Exploration on the tonic effect of Radix Glycyrrhizae Preparata from its use by Zhang Zhongjing[J]. Global Traditional Chinese Medicine, 2013, 6(8): 608-610. (in Chinese).
[5] LI ZY, WEI M, SUN DM, et al. Study on HPLC-ELSD fingerprint of raw materials Suanzaoren (Ziziphi Spinosi Semen) and its processed products[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacology, 2020, 26(4): 19-23. (in Chinese).
[6] GAO Y. Study on preparation technology and quality standard of Rumo Bawei Jiegu Pill[D]. Lanzhou: Lanzhou University, 2017. (in Chinese).
[7] LI CY. Research and development for Zhuyuan Jianpi Zhitong Wan of preparations in medical institutions[D]. Qingdao: Qingdao University, 2017. (in Chinese).
[8] CHEN P, MI WW, YING YY, et al. Study on the quality control of compound Jianshen granules [J]. China Modern Doctor, 2019, 57(36): 37-43, 169. (in Chinese).
[9] WU Y. Study on the preparation technology and quality standard of Tang ganjian concentrated Pill[D]. Wuhan: Hubei University of Chinese Medicine, 2017. (in Chinese).
[10] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia (fourth volume)[M]. Beijing: China Medical Science Press, 2020. (in Chinese).
[11] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia (first volume)[M]. Beijing: China Medical Science Press, 2020. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Report on the Breeding of Dahen 799 Broilers
- Evaluation and Analysis for Survey of Tea Production Quality Safety Management and Control
- Study on the Preparation of "Oil-tea" Instant Tea from the Compound Extract of Green Tea and Ginger
- Research Progress on Chemical Constituents and Pharmacological Effects of Zhuang Medicine Cocculus laurifolius DC.
- Research on the Development of Guangxi Zhuang and Yao Ethnic Medicine Industry
- Study on Leaching Conditions of Miao Medicine Xiange Zuyu Powder

