Co(Ⅱ)/Ni(Ⅱ) Coordination Polymer of Isomeric Terphenyl-2,2″,4,4″-tetracarboxylic Acids with a Single Water Bridge:Syntheses,Structures,and Magnetic Properties
SU Feng LI Shao-Dong HAN Chun WU Lin-Tao WANG Zhi-Jun
(Department of Chemistry,Changzhi University,Changzhi,Shanxi 046011,China)
Abstract:Two single water-bridged Co(Ⅱ)/Ni(Ⅱ) chains coordination polymers,namely[Co(m-H2tpta)(H2O)3]n(1)and{[Ni2(p-tpta)(H2O)6]·2H2O}n(2)were synthesized based on isomeric terphenyl-2,2″,4,4″-tetracarboxylic acid(m-H4tpta and p-H4tpta)ligands under hydrothermal conditions.They have been structurally characterized by FT-IR,elemental analysis,single-crystal,and powder X-ray diffraction analysis.Structurally,the central metal ions display slightly distorted octahedral geometries in 1 and 2,and are linked to 1D metal chains by single bridging water molecules.The isomeric H4tpta ligands coordinate metal ions in different μ1-η1∶η0∶η1∶η0and μ4-η1∶η1∶η1∶η1coordination modes,leading to the formation of 1D chain and 3D network structure.Complex 2 displays a 3D network with(4,4)-connected NbO net topology.Magnetically,complex 1 exhibited an antiferromagnetic interaction through μ2-H2O in the uniform Co(Ⅱ) chain model.Complex 2 also showed an antiferromagnetic coupling between Ni(Ⅱ) ions corresponding to magnetic coupling Ni—Ow—Ni angles.CCDC:1415021,1;1415020,2.
Keywords:coordination polymers;1,1′∶3′,1″-terphenyl-2,2″,4,4″-tetracarboxylic acid;1,1′∶4′,1″-terphenyl-2,2″,4,4″-tetracarboxylic acid;crystal structures;magnetism
0 Introduction
The studies of magnetochemistry have been of interest for decades in transition metal coordination compounds due to the diverse structures and magnetic properties of single-molecule magnets(SMMs)and single-chain magnets(SCMs)systems[1-5].A large number of coordination polymers based on metal chains were constructed by different bridging paths such asμ2-oxo,μ2-COO-,μ2-N3-,andμ2-X-(X=F,Cl),which exhibited intriguing structures and interesting magnetic properties[6-10].In general,the main influence factors of the magnetic behavior are paramagnetic metal ions and the nature of the bridging mediums[11-12].For example,a series of isostructural polymers[M(o,p-H2bpta)]n,(o,p-H4bpta=2,2′,4,4′-biphenyltetracarboxylic acid,M=Mn(Ⅱ),Fe(Ⅱ),Co(Ⅱ),Ni(Ⅱ),and Cu(Ⅱ))with[M(μ2-COO)2]nchains showing weak ferro-or anti-ferromagnetic interactions transmitted by doublesyn-anticarboxylate bridges[13].[Mn2(4,4′-bipy)(m,m-bpta)]n(m,m-H4bpta=3,3′,5,5′-biphenyltetracarboxylic acid)with 1D zigzag chains exhibited intrachain antiferromagnetic coupling between Mn(Ⅱ)ions related to raresyn-syncarboxylate bridges[14].Three isomorphous[M(L)(N3)]n·3nH2O(L-=1-(4-carboxylatobenzyl)pyridinium-4-carboxylate,M=Mn(Ⅱ),Co(Ⅱ),and Ni(Ⅱ))polymers with triple-bridged chains showed antiferromagnetic interaction in the Mn(Ⅱ)compound but ferromagnetic interactions in the Co(Ⅱ)and Ni(Ⅱ) analogs[15].Two water-bridged Co(Ⅱ) chains,[Co(H2O)3(2-na)2]nand{[Co(H2O)3(1-na)2]·2H2O}n,with isomeric naphthoate(na-)spacers exhibited metamagnetic transition and unusual single-chain magnetic behaviors mediated by single water bridges[16].Additionally,the magnitude of magnetic coupling can be correlated with the distances of M…M or the geometrical configurations of metal ions and the types of the bridging mediums.
This family ofμ-oxo systems has motivated interesting studies,aimed at gaining insight into magnetic phenomena and developing potential functional materials[17-19].Some experiments and theoretical research had given influence factors of magnetic coupling(geometric features such as the M—O—M angles and M—O bond lengths)[20-21].Whileμ-oxo bridges associated with other O-bridging moieties(hydroxo,carboxylato,carbonato,alkoxo,phenoxo,etc.)are also common in metal clusters and metal chain compounds[22-23].The most common feature of these compounds withμ-oxo bridges involves multiple bridges(double,or triple bridges).By contrast,the magnetic exchanges depending on the M—O—M angles could be justified and counterbalanced by other simultaneous bridging mediums[24].Hence,it is useful to clarify the relationship between structural features and the value of the intramolecular magnetic exchange interaction in the singlyμ-oxo bridged systems.Recently,we focus on the chain coordination polymers with a single water bridge that have shown a great diversity of intramolecular magnetic exchange phenomena.
Herein,we report two magnetic Co/Ni(Ⅱ)coordination polymers with single-water bridges,namely[Co(m-H2tpta)(H2O)3]n(1)(m-H4tpta=1,1′∶3′,1″-terphenyl-2,2″,4,4″-tetracarboxylic acid),and{[Ni2(p-tpta)(H2O)6]·2H2O}n(p-H4tpta=1,1′∶4′,1″-terphenyl-2,2″,4,4″-tetracarboxylic acid)(2).The complexes contain 1D uniform metal chains formed by single water bridging metal ions.Complex 1 is a supramolecular architecture with 1D metal chains,and complex 2 exhibits a 3D network with(4,4)-connected NbO nets topology.The variable-temperature magnetic susceptibility measurements reveal that single water bridges can effectively mediate magnetic interactions between the spin carriers.Complex 1 exhibited an antiferromagnetic behavior.Complex 2 showed an antiferromagnetic coupling between the intrachain Ni(Ⅱ)ions.
1 Experimental
1.1 Material and measurement
m-H4tpta andp-H4tpta were received from Jinan Camolai Trading Company,China.Other reagents and solvents were obtained from commercial sources and used without further purification.Powder X-ray diffraction(PXRD)data were collected on a Bruker D8-ADVANCE X-ray diffractometer with CuKαradiation(λ=0.154 18 nm,U=40 kV,I=25 mA)and 2θranging from 5°to 50°.The carbon,nitrogen,and hydrogen contents of the complexes were determined by CHNO-Rapid instrument.The FT-IR spectra were recorded from a pure solid sample in a range of 4 000-400 cm-1on a Bruker TENSOR27 spectrometer.Thermogravimetric(TG)studies were carried out on a Labsys Evo thermal analyzer with a temperature range of 298-1 073 K under nitrogen flow with a heating rate of 10 K·min-1.Magnetic susceptibility measurement data were performed by a SQUID magnetometer(Quantum MPMS)in a temperature range of 2-300 K by using an applied field of 1 000 Oe.Electron spin resonance(ESR)spectra were recorded with a Bruker EMXplus 10/12 spectrometer equipped with an ER4119 High-Q cylindrical cavity and Oxford ESR910 liquid helium continuous flow cryostat(Microwave power:1 mW)
1.2 Preparation of complexes 1 and 2
The pH value of a mixture ofm-H4tpta(0.041 g,0.10 mmol),CoCl2·6H2O(47.5 mg,0.20 mmol)in H2O(8 mL)was adjusted to about 6.5 with dilute KOH solution and then transferred to 13 mL Teflon-lined stainless steel reactor.The mixture was heated under autogenous pressure at 423 K for 72 h and then cooled to room temperature naturally.The pink block crystals of 1 were collected and washed with water.Yield:60%(based on Co). Elemental analysis Calcd. for C22H18O11Co(%):C 51.08,H 3.51.Found(%):C 50.96,H 3.68.IR(KBr,cm-1):3 274(s),1 945(w),1 690(s),1 606(m),1 555(s),1 432(s),1 371(m),1 220(s),1 123(m),899(w),780(m),685(m),511(w).
A mixture ofp-H4tpta(0.041 g,0.10 mmol),NiCl2·6H2O(71.3 mg,0.30 mmol)in H2O(6 mL)was placed in a 13 mL Teflon-lined stainless steel reactor.When the pH value was adjusted toca.7.5 by KOH solution,the mixture was sealed and heated at 423 K for 72 h.After the mixture was slowly cooled to room temperature,green block crystals of 2 were obtained.Yield:72%(based on Ni).Elemental analysis Calcd.for C22H26O16Ni2(%):C 39.81,H 3.95.Found(%):C 38.92,H 3.88.IR(cm-1):3 166(m),2 035(w),1 607(s),1 575(s),1 535(s),1 440(s),1 382(s),1 170(w),1 073(w),914(w),821(m),781(m),729(m),670(w),530(w).
1.3 X-ray crystallography
Single-crystal X-ray diffraction data of complexes 1 and 2 were collected on a Bruker D8-Quest diffractometer equipped with a photon 100 detector by using a graphite monochromator utilizing MoKαradiation(λ=0.071 073 nm).Data integration and absorption correction were processed by the SAINT and SADABS programs.The structures were solved by intrinsic phasing with the SHELXS and refined by full-matrix leastsquares methods onF2by using the SHELXL-2018 program.H atoms bound to C atoms and carboxyl groups were placed in their expected positions accounting for the hybridization of the supporting atoms with C—H 0.093 nm and O—H 0.082 nm,and withUiso(H)=1.2Ueq(C).The H atoms of the water molecules were found from difference Fourier maps and fixed at their ideal positions according to hydrogen-bond geometries with O—H distances restraints of 0.082(1)nm andUiso(H)=1.5Ueq(O).For complex 2,the refinement of a twin processing improved the agreement between the structural model and the experimental data significantly.During the refinement,the reflection data were read via the HKLF 5 option in SHELXL and a parameter BASF was introduced,which was used to describe the fractional contribution of the twin domains.The fractional contributions of the two minor twin domains refined to 0.281 8(19)and 0.358 1(14).In addition,the water molecule(O8)of 2 was disordered over two positions with the site-occupation factors of 0.62(2)and 0.38(2)and treated anisotropically.A summary of the crystallographic data for complexes 1 and 2 is listed in Table 1.Selected bond lengths and angles for 1 and 2 are shown in Table S1(Supporting information).
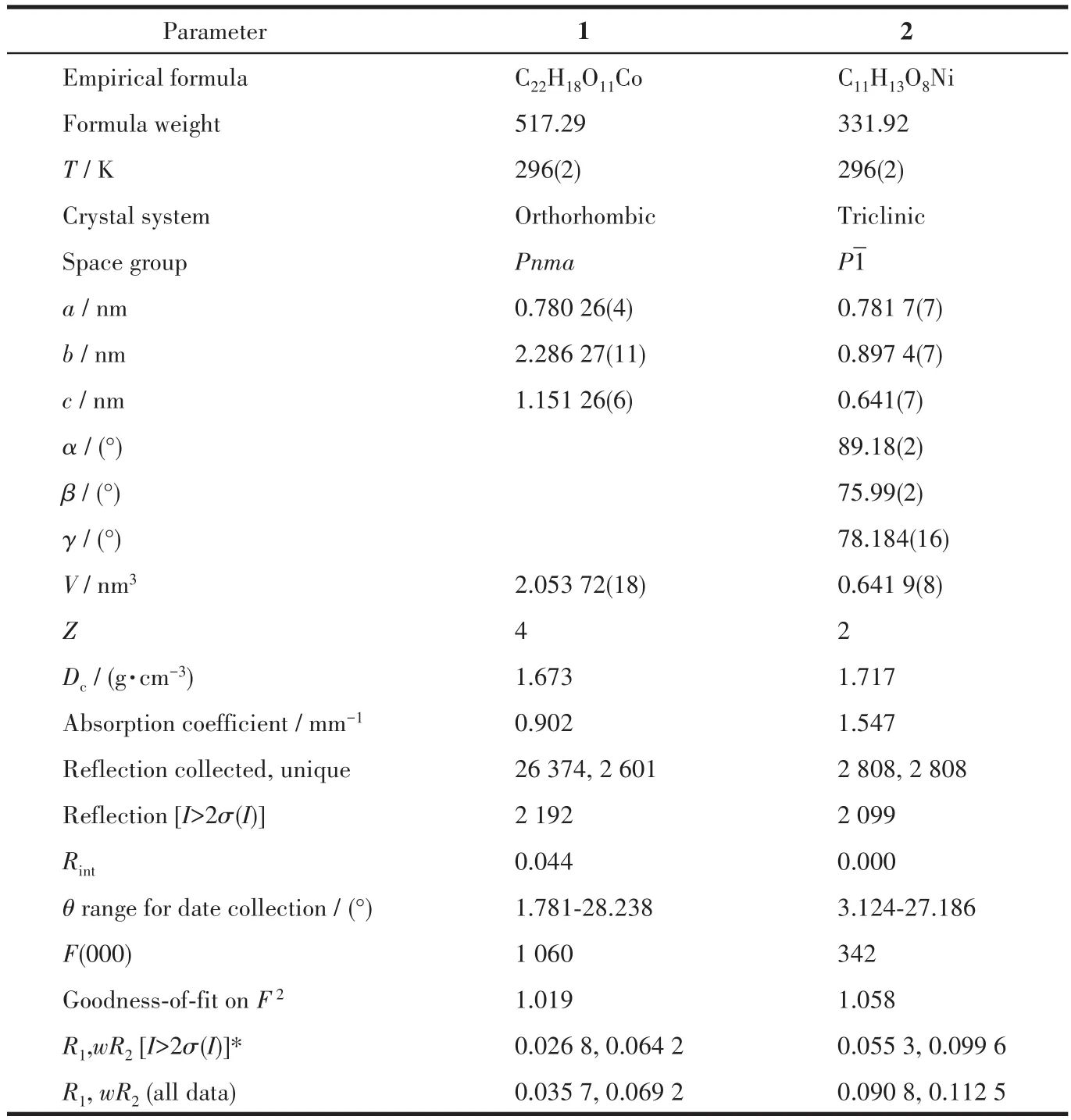
Table 1 Crystal data and structure refinement parameters for complexes 1 and 2
CCDC:1415021,1;1415020,2.
2 Results and discussion
2.1 Crystal structure description
Single-crystal X-ray crystallographic analysis reveals that complex 1 crystallizes in the orthorhombic crystal system with thePnmaspace group.Complex 1 consists of one Co(Ⅱ)ion,a halfm-H2tpta2-anion,and one and a half coordinated water molecules in the asymmetric unit.As illustrated in Fig.1a,the Co(Ⅱ)ion exhibits octahedral geometry,which is composed of two carboxylate oxygen atoms(O1 and O1i,Symmetry code:ix,-y+3/2,z)from onem-H2tpta2-ligand and two termi-nal water molecules(O6 and O6i)in the equatorial positions.The axial sites are occupied by two water molecules(O5 and O5ii,Symmetry code:iix+1/2,y,-z+1/2).The Co—O bond lengths range from 0.204 9(2)to 0.216 3(1)nm,and the O—Co—O bond angles are in a range of 85.14(7)°-95.88(8)°(Table S1).

Fig.1 (a)Perspective view of the coordination of the Co(Ⅱ)ion for 1 with the thermal ellipsoids at a 30% probability level;(b)1D chain running along the a-axis(hydrogen atoms omitted for clarity);(c)A 2D sheet formed through the localized π-bonding interactions in the ac plane;(d)Packing hexagonal-shaped architecture viewed along the central projection,where hydrogen atoms are omitted for clarity
Herein,m-H4tpta is partly deprotonated and the 2,2″-carboxylate groups chelate one Co(Ⅱ) ion with an O1—Co—O1 angle of 85.14(7)°.The dihedral angle is 31.455(7)°between the planes of the two terminal aromatic rings.Adjacent Co(Ⅱ)ions are bridged by a water molecule with the Co…Co distance of 0.391 4(2)nm and the Co1—O5—Co1iangle of 130.59(8)°,resulting in the formation of a singly water-bridged Co(Ⅱ)chain along thea-axis(Fig.1b).The bridging water molecule assumes a radial-radial disposition with Co—O distances of 0.214 5(1)and 0.216 3(1)nm.
Thus,the coordination sites could be properly described ascis-arrangement concerning carboxylate groups and terminal water.In addition,intramolecular hydrogen bonds are observed between the carboxylate groups and coordinated water molecules,which form a compact structure.The intra-chain hydrogen bonds are formed between the water molecule(O6)and carboxylate(O6…O1 0.271 1(1)nm,Table S2),which produces a compact 1D chain.Additionally,weakp-πinteractions are established by C10/C12 and the phenyl rings(from C9 to C12 atoms)with the perpendicular distances of 0.308 6(2)and 0.317 0(2)nm.These chains are stacked through localizedπ-bonding interactions to form a 2D sheet in theacplane(Fig.1c),and further,give rise to a 3D supramolecular network by hydrogen bonds from carboxylate groups(O3…O2 0.274 9(2)nm,Fig.1d).
Complex 2 features a chain-based 3D network constituted by thep-tpta4-ligands and bridging water molecules.It crystallizes in the triclinicP1 space group with the Ni(Ⅱ)ions located in the inversion centers.The asymmetric unit consists of one crystallographically independent Ni(Ⅱ)ions,halfp-tpta4-anions,three coordinated water molecules,and one lattice water molecule.As depicted in Fig.2a,the Ni(Ⅱ)ions adopt slightly distorted octahedral geometries defined by four water molecules and two carboxylate oxygen atoms from twop-tpta4-ligands.The Ni—O bond lengths are 0.202 8(5)-0.211 5(4)nm(Table S1),similar to those reported for other Ni(Ⅱ)multicarboxylates[25].The completely deprotonatedp-tpta4-ligand serves as a tetradentate ligand to bind four Ni(Ⅱ)ions withμ4-η1∶η1∶η1∶η1coordination mode.The dihedral angle formed by the planes of the two adjacent aromatic rings is 47.939(7)°.Thep-tpta4-ligand is linked to four Ni(Ⅱ)ions in monodentate mode,forming a 2D sheet in theabplane(Fig.2b).Adjacent Ni(Ⅱ)ions are bridged by water molecules to produce a water-bridged metal chain with Ni…Ni 0.387 1(1)nm and Ni—O7—Ni 133.3(2)°along thea-axis(Fig.2c).The octahedral geometries of Ni1 and Ni2 centers are interlinked to each other in a vertex-sharing fashion.Such neighboring 1D chains are further cross-linked via the organic backbonep-tpta4-ligands,generating a 3D pillaredlayered structure with channels along thea-axis(Fig.2d).The lattice water molecules(O8)are located in voids and fixed by coordinated water molecules by hydrogen bonds with O8…O3 0.278 2(9)nm and O5…O8 0.284 9(10)nm.In addition,the intra-chain hydro-gen bonds are generated between water molecules and carboxylate groups(Table S2),forming a compact network structure.From the topological point of view,thep-tpta4-ligand acts as a 4-connector linking four Ni(Ⅱ)ions and each Ni(Ⅱ)ion is a 4-connected node,the network can be simplified as a(4,4)-connected NbO topology[26]with the point symbol{64.82}(Fig.2e).

Fig.2 (a)Perspective view of the coordination of the Ni(Ⅱ)ion for 2 with the thermal ellipsoids at 45% probability level;(b)2D layered structure formed by p-tpta4-ligands linking to Ni(Ⅱ)ions in the ab plane;(c)1D chain formed by water-bridged Ni(Ⅱ)ions running along[100]direction;(d)Packing of the 3D rhombus structure of 2 with the water molecules residing in channels(hydrogen atoms omitted for clarity);(e)NdO net topology with the point symbol{64.82}for 2
2.2 PXRD analyses and thermal stability
PXRD analyses of complexes 1 and 2 had been further performed at room temperature.The experimental patterns were in good agreement with the calculated patterns obtained from the crystal structures,indicating that the single-crystal structures are representative of the bulk materials(Fig.3).

Fig.3 PXRD patterns of complexes 1(a)and 2(b)
To investigate the thermal stability of complexes 1 and 2,thermal analyses were performed(Fig.4).For 1,the first weight loss was 10.02% at 398 K,corresponding to the sharp exothermic peak in the DSC(differential scanning calorimetry)curve,which is attributable to the loss of three coordinated water molecules(Calcd.10.43%).Framework decomposition of 1 occurred at 600 K,corresponding to the endothermic peaks of the DSC curve.The TG curve of 2 exhibited two continuous weight loss stages in a range of 333-434 K(16.35%),corresponding to the loss of two lattice water molecules(Calcd.5.42%)and four coordinated water molecules(Calcd.10.85%),respectively.Further,a weight loss of 5.30% is ascribed to the loss of two coordinated water molecules(Calcd.5.42%)in a range of 463-505 K.Finally,the decomposition of organic groups occurred at 723 K.The observed endothermic peaks of the DSC curve were approximate consistency with the TG results.
2.3 Magnetic properties
Magnetic measurements of complexes 1 and 2 were performed on powder samples.Magnetic susceptibility data were collected in the 2-300 K range with an applied magnetic field of 1 000 Oe.The magnetic couplings between metal ions with 3d7or 3d8electronic configurations could be effectively transmitted by a single atom bridge.According to the structural features,the complexes can exhibit antiferromagnetic interactions due to the larger bonding angles of M(Ⅱ)—O—M(Ⅱ).
For complex 1,the temperature dependences of the molar susceptibilitiesχMand its productχMTare depicted in Fig.5.TheχMTvalue was 2.89 cm3·mol-1·K at 300 K,and largely exceeded that expected for the spin-only case(1.875 cm3·mol-1·K)withS=3/2 andg=2.0,indicating that an orbital contribution is involved.Upon cooling,theχMTcurve exhibited a continuous decrease with a minimum of 0.059 cm3·mol-1·K at 2 K,which is indicative of the antiferromagnetic coupling between the Co (Ⅱ) ions.While theχMvalue first increased to a broad maximum at 25 K,then decreased until 6 K,and finally increased again.The increasing trend can be attributed to the trace of paramagnetic impurities in the low-temperature range.Moreover,the presence of a broad maximum in theχMcurve at 25 K shows an antiferromagnetic ordering(Fig.5a).The magnetic susceptibility above 25 K was fitted by the Curie-Weiss law and the parameter(θ=-74.80(2)K,Fig.5b,Inset)can be the combined effect of the orbital contribution and possible antiferromagnetic coupling between the high-spin Co(Ⅱ)ions.The field-dependent magnetization at 2 K further confirms the antiferromagnetic interaction at low temperature,which had a value of 0.16μBat 7 T without saturation(Fig.5b).The hysteresis loop of 1 measured at 2 K exhibited no obvious opening,consistent with the antiferromagnetic phase of 1 under low fields.

Fig.5(a)Temperature dependence of χMand χMT curves of complex 1;(b)Field dependent magnetization of 1 at 2 K
For Co(Ⅱ)systems with the spin-orbit coupling contribution,it is difficult to find a precise expression to explain the magnetic properties of polymeric chains.This is due to the strong orbital contribution to the magnetic moment and thus to a strong magnetic anisotropy.The lines model is only valid for the ideal octahedral geometries of the Co(Ⅱ)ions with theOhsymmetry.However,a small deviation of the octahedron does not exert a significant influence on the magnetic property of the 1D infinite Co(Ⅱ)chain.Firstly,we have attempted to reproduce theoretically the experimental susceptibility by using the classical spin Heisenberg chain model[27]through the Hamiltonian(H→ =-J∑S→iS→i+1,J<0),in whichJis the nearest neighbor magnetic exchange constant andS→iis the total spin operator on sitei.Thus,the magnetic data of 1 was fitted by Eq.1:
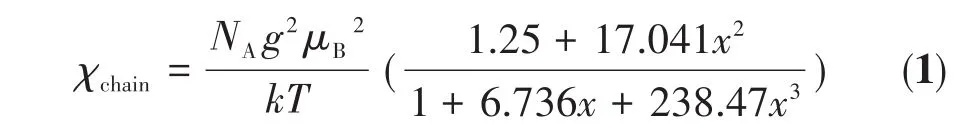
whereNA,g,μB,k,andTare the Avogadro constant,gfactor,the Bohr magneton,the Boltzmann constant,and the temperature of magnetic coupling,respectively.In addition,x=|J|/(kT),whereJis the intrachain spinexchange parameter between the adjacent Co(Ⅱ)ions.Some correction terms for a proportionρof paramagnetic impurity and temperature-independent paramagnetism(TIP)are included as appropriate.Srepresents the spin quantum number associated with mononuclear high spin Co(Ⅱ)ions.The total magnetic susceptibility is Eq.2:
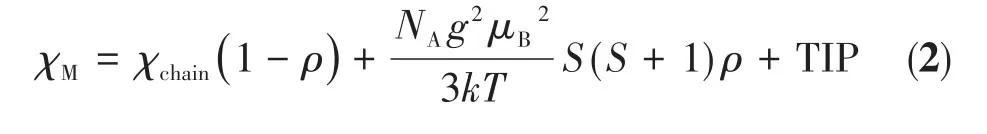
The best fits to the experimental data wereg=2.41(3),J=-9.94(3)cm-1,ρ=0.002 2(1),TIP=1.41(2)×10-4cm3·mol-1,andR=9.82×10-5for 1 in the total temperature range.A negativeJvalue indicates an antiferromagnetic coupling occurring between the Co(Ⅱ)ions.To determine thegfactor of the antiferromagnetic interaction,the ESR of the crystal powder sample was recorded.The ESR spectra of complex 1 had broad signals at 2 and 100 K and thegvalue obtained was 2.376(Fig.6).We did not try to assign other spectra since complicated transitions between spectral terms of the octahedral Co(Ⅱ)system.
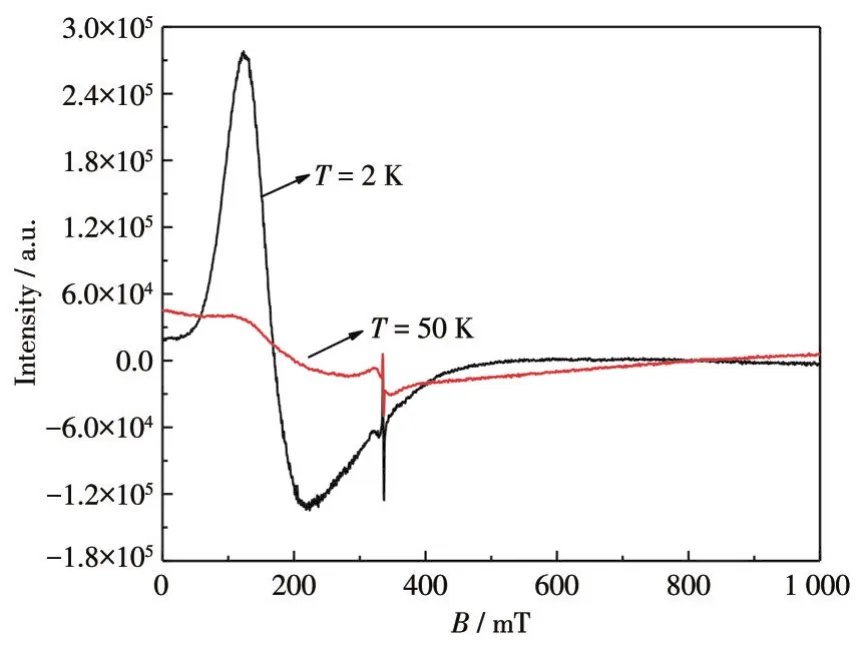
Fig.6 ESR spectra of the powder sample of complex 1 at 2 and 100 K
Additionally,theχMTcurve can be fitted by using an expression derived from Rueff et al[28-29].According to the above description of theχMvsTcurve,the contribution of the paramagnetic Co(Ⅱ) ions(ρ)was added.The expression has been modified to be the following Eq.3,which can estimate the antiferromagnetic interactions of low-dimensional Co(Ⅱ)systems and adequately describe the spin-orbit coupling.Some reasonable results for magnetic coupling and spin-orbit interaction have been reported for 1D and,even,for 2D cobalt(Ⅱ)complexes.
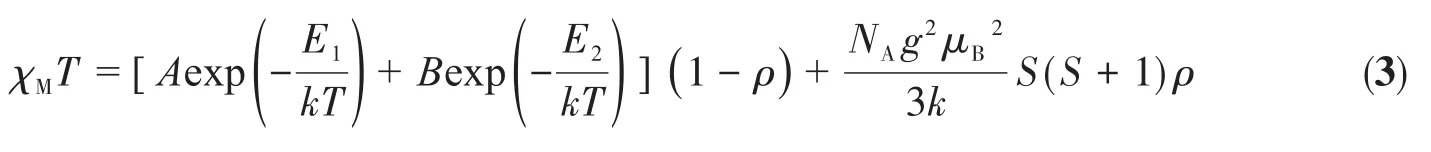
Here,the sum ofAandBis Curie constant(2.8-3.4 cm3·mol-1·K)for octahedral Co(Ⅱ) ions,andE1andE2represent the“activation energies”corresponding to the spin-orbit coupling and the antiferromagnetic exchange interaction,respectively.The best fit to the experimental data wasA=1.86(7)cm3·mol-1·K,E1/k=54.08(1)K,B=1.61(8)cm3·mol-1·K,E2/k=14.99(7)K,and the paramagnetic impurityρ=0.047(Fig.5a).The value found forA+B(3.47 cm3·mol-1·K)perfectly agreed with the Curie constant.Likewise,E1/k=55.72(2)K was consistent with those given by Rueff et al.for both the effects of spin-orbit coupling and site distortion in various Co(Ⅱ) complexes.E2> 0 indicates the antiferromagnetic interaction within the chain.
For complex 2,the temperature dependences of the molar susceptibilities are shown in Fig.7a.TheχMTvalue was around 1.67(2)cm3·mol-1·K for 2 at 300 K,larger than the spin-only value(1.00 cm3·mol-1·K)expected for an isolated high-spin Ni(Ⅱ)ion(g=2.0 andS=1).Upon cooling,theχMTvalue gradually decreased to a minimum of 0.030(2)cm3·mol-1·K at 2 K,indicating the presence of an antiferromagnetic coupling between the Ni(Ⅱ) ions.The value ofχMcontinuously increased to a peak maximum(0.011 cm3·mol-1)at about 35 K,and then increased rapidly at 8 K,which shows the appearance of the trace of paramagnetic impurities in the low-temperature range.The magnetic susceptibility in a range of 50-300 K followed the Curie-Weiss law withC=2.39(2)cm3·mol-1·K andθ=-148.73(2)K(Fig.7b,Inset)for 2.The significantly negativeθvalue further indicates antiferromagnetic interaction between the Ni(Ⅱ)ions.
According to the magnetic structure of 2,the magnetic coupling model can be handled as 1D uniform spin chains,whereas the interchain magnetic interaction should be ignored due to the longer distance of the ligand(Ni…Ni 0.898 4(1)nm).Thus,the magnetic exchange of 2 is only transmitted by a single waterbridged pathway with the Ni…Ni distance of 0.387 1(1)nm and Ni—Ow—Ni bonding angle of 133.34°,respectively.To estimate the intrachain interaction,we employed an isotropic Heisenberg chain to simulate the intra-chain antiferromagnetic coupling.The magnetic susceptibility was simulated within the classical approach according to Eq.4[30-31]:

wherex=|J|/(kT),Jis the intrachain spin-exchange parameter of the adjacent Ni(Ⅱ)ions.According to the above description of theχMvsTcurve,the contribution of the paramagnetic Ni(Ⅱ) ions(ρ)and the TIP for the Ni(Ⅱ)complex were added.Thus,the fitting equation of total magnetic susceptibilities of 2 can be modified to Eq.5:

The lines model can be successfully used to treat the magnetic data of the Ni(Ⅱ)compound.The best fits to the experimental data wereg=2.19(2),J=-31.31(4)cm-1,ρ=0.011(3)and TIP=4.74(2)×10-4cm3·mol-1,and
R=1.62×10-5for 2 over the whole temperature range.Thegvalue is corresponding to the expectation for the reported Ni(Ⅱ) complexes[15,32].NegativeθandJvalues indicate antiferromagnetic interaction exchanged by the single water bridge between the Ni(Ⅱ)ions.The magnetization at 70 kOe(about 0.14μB)was far from the saturation value for one octahedral Ni(Ⅱ)ion at 2 K(Fig.7b),indicating antiferromagnetic coupling between the Ni(Ⅱ)ions.The hysteresis loop measured at 2 K exhibited no obvious opening,consistent with the antiferromagnetic phase under low fields.Compared with the Ni(Ⅱ) complexes involvingμ-oxo bridges(Table 2),one of the influence factors of magnetic exchange depends on the Ni—O—Ni bonding angles.The relationship is found between theJvalues and the bridging angles,for which a limit value of 97°,the magnetic interaction being antiferromagnetic for larger values of this angle.The most common feature of complexes containingμ-oxo bridges is several multiplicity bridges(double or triple bridges).The magnetic exchange could be mediated and counterbalanced depending on the Ni—O—Ni angles.However,magneto-structural studies on similar Ni(Ⅱ)complexes with a single-water bridge are scarce.The magnetic behavior of 2 agrees with the view that an anti-ferromagnetic interaction occurs by the larger Ni—O—Ni bonding angles between adjacent Ni(Ⅱ)ions.The coupling interaction is transmitted by a singleμ-oxo bridge,avoiding other effects from magnetic exchange mediums.It is worthwhile to the analysis of magnetic exchange relying on simultaneously multiple bridges.

Table 2 Structural and magnetic parameters with the μ-oxo-bridged unit
3 Conclusions
In summary,two water-bridged Co(Ⅱ) and Ni(Ⅱ)complexes based on isomericm-H4tpta andp-H4tpta ligands have been successfully synthesized and structurally characterized.Complex 1 shows a 1D chain structure and is further extended to a 3D supramolecular architecture by intermolecular interactions.Complex 2 displays a(4,4)-connected 3D network with a single water-bridged Ni(Ⅱ)metal chain.Magnetic studies indicate that complex 1 shows antiferromagnetic behavior(E2/k=14.99(7)K)in the uniform chain model.Complex 2 exhibits an antiferromagnetic interaction(J=-31.31(4)cm-1)between intrachain Ni(Ⅱ)ions corresponding to larger Ni—Ow—Ni angles.Magnetic exchange interactions involvingμ-oxo bridges are com-mon in the coordination compounds.However,magnetic interactions transmitted via a single water-bridged mode are scarce,especially in Co(Ⅱ)/Ni(Ⅱ) coordination polymers.Single water-bridged Co(Ⅱ)/Ni(Ⅱ) chain complexes are rich sources for magnetic models,which provide an option for the analysis of multiple-bridged magnetic interaction involvingμ-oxo bridge.
Conflicts of interest:The authors declare no competing financial interest.
Supporting information is available at http://www.wjhxxb.cn
- 无机化学学报的其它文章
- La-Doped BaSnO3/Multi-walled Carbon Nanotube Modified Separator:Synthesis and Application in Lithium-Sulfur Battery
- Micromotors Based on Ni-Mn Binary Oxide and Its Application for Effective Dye Adsorption
- Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol by Mg-Doped Ceria Monolithic Catalyst
- Hydrogen Storage Capabilities of the Low-Lying Ca2B4Clusters
- 盘状镝簇合物的合成及缓慢磁弛豫
- 内放射状中空TiN颗粒作为锂硫电池正极的性能

