Micromotors Based on Ni-Mn Binary Oxide and Its Application for Effective Dye Adsorption
HUANG Jing CHEN Hui-Jun FENG Xiao-Miao
(Key Laboratory for Organic Electronics and Information Displays&Institute of Advanced Materials(IAM),School of Materials Science&Engineering,Nanjing University of Posts&Telecommunications,Nanjing 210023,China)
Abstract:A kind of binary Ni-Mn oxide(Ni6MnO8)micromotors were prepared by hydrothermal method followed by high-temperature annealing.The obtained binary Ni-Mn oxides showed obvious needle-punched shapes and hollow structures that can be used as micromotors.These binary Ni-Mn oxide micromotors displayed powerful propulsion at a low fuel level(H2O2mass fraction:1%),with a speed of over 83.75 μm·s-1and a long lifetime of up to 90 min.Even in an extremely low mass fraction of H2O2(0.4%),the micromotors could also perform excellent autonomous locomotion.Due to the presence of Ni oxide,the micromotors could perform the directional movement by magnetic manipulation.Benefiting from excellent catalytic and magnetic properties,the obtained Ni6MnO8micromotors could effectively remove methylene blue within 160 s by adsorption without secondary pollution.
Keywords:Ni-Mn bimetallic oxide;methylene blue;adsorption;micromotors,environmental remediation
0 Introduction
William Perkin discovered the first synthetic organic dye mauveine in 1865,which opened the prelude to th e research of synthetic organic dyes[1].Synthetic organic dyes have a wide range of applications due to their low cost,attractive colors,and stable ingredients,including textile and tannery[2],cosmetics indus-try[3],food and feed industry[4],biology[5],and other fields.However,with the large-scale application of synthetic organic dyes,the harm to the environment has gradually emerged:non-degradable colored wastewater,toxicity to aquatic organisms,pollution to soil and crops,etc.Traditional treatment processes,including coagulation/flocculation technology[6],membrane biotechnology[7],adsorption[8],advanced oxidation process[9],etc.,inevitably have high energy consumption or cause secondary pollution,and are usually difficult to completely remove synthetic organic dyes.Among them,activated carbon adsorption is one of the most effective methods for treating synthetic organic dye wastewater,but it still has a relatively high material and time cost,and requires mechanical mixing to enhance the mass transfer effect.These high inputs are a great challenge in economically underdeveloped areas.Therefore,developing a process with low-cost,low-energy,excellent environmental remediation ability is the main research focus in the treatment of synthetic organic dye wastewater in the world today.
The rapid development of nanotechnology has led to the exploration of the micromotor which is a microor nano-scale machine that can convert energy into motion to solve application problems.The research on micro/nanomotors(MNMs)originated in 2002.Whitesides′team first proposed a PDMS(polydimethylsiloxane)boat,which could convert other forms of energy into kinetic energy[10].Such research findings aroused great interest in researchers and laid the foundation for subsequent discoveries.Subsequently,the Au-Pt bimetallic rod micromotor was prepared by Sen and Mallouk in 2004[11].Rod-shaped particles could move autonomously in 2%-3% hydrogen peroxide(H2O2)solutions by catalyzing the formation of oxygen at the Pt end,and then the investigative prologue in micromotors was opened.Due to the different power sources of micromotors,they can be roughly divided into chemical-driven micromotors[12-13], magnetic-driven micromotors[14-15],ultrasonic-driven micromotors[16-17], electric-driven micromotors[18-19], and light-driven micromotors[20-22].The wide range of power sources makes them potentially useful in broad fields such as biomedicine[23]and environmental remediation[24]
In particular,MNMs have shown great interest as artificial active substances in the application of water purification.Their compact size facilitates them to cross obstacles and reach contaminants quickly.At the same time,the excellent autokinetic properties have been shown to enhance the mixing of the mass transfer and accelerate the diffusion process to significantly increase the ability to remove contaminants without additional agitation[25].Compared to chemical-driven MNMs,optically-driven,ultrasonic-driven,and electricdriven MNMs require higher external energy devices to reach the corresponding speed of motion.Chemicalriven MNMs have therefore become the most widely studied class of micromotors.
In 2012,Wang′s team prepared a functional tubular micromotor and applied it to the dynamic removal of oil in water for the first time[26].Since then,the research of MNMs in the field of environmental remediation has continued to get rid of the stale and bring forth fresh[27].In 2015,bubble-propelled Janus micromotors consisting of Pt and activated carbon microspheres were developed[28].Using the high adsorption capacity of activated carbon and the high catalytic performance of Pt,the Janus micromotors couple rapid motion and corresponding fluid dynamics to achieve efficient dynamic removal of organic/inorganic substances.Orozco et al.presented a kind of SiO2@rGO-Pt Janus-based micromotor (rGO=reduced graphene oxide)that could effectively remove persistent organic pollutants[29].The decomposition of H2O2by Pt on the surface generates O2bubbles that can effectively drive the micromotor while adsorbing harmful compounds from the solution onto the rGO shell surface in a short time.Recently,Yang et al.reported a novelγ-Fe2O3@Ag-mSiO2-NH2magnetic Janus micromotor for the dynamic removal of heavy metal ions and organic pollutants from water[30].It is demonstrated that the micromotor is able to remove Cu2+and doxycycline much better than its static control sample due to the synergistic effect of self-propelled motion and amino-functionalized porous core-shell structure.
Despite the rapid development of micromotors in the field of environmental remediation,the choice of catalytic materials has still faced scientific challenges,which determine the speed of movement and recycling problem of the micromotors and the recycle.In previous studies,Pt and other precious metals were selected as the materials for micromotors because of their excellent catalytic performance.However,these metalsbased micromotors with high costs and short lifetimes limit their mass production.With the development of micromotors,new requirements for catalytic materials have begun to be put forward,and scientists start looking for more inexpensive,green,and environmentally friendly materials to replace precious metals.Among them,many metal oxides are favored for their unique advantages[31-35].
Transition metal oxides,represented by MnO2,have attracted great attention from many scholars as classical H2O2catalysts[36].For example,Fe2O3modified SiO2/MnO2micromotor can effectively catalyze H2O2to produce O2to propel the micromotors′motion and remove industrial organic pollutants and heavy metals in wastewater[37].Many other works about the application of metal oxides in catalyzing H2O2have been reported[38-40].However,although there have been many studies on the preparation of micromotors based on metal oxides,the research on the preparation of micromotors based on bimetallic oxides has been rarely reported.
Herein,binary Ni-Mn oxide was prepared by hydrothermal method and annealing using as micromotor,which not only had high catalytic activity but also made the micromotor have magnetic properties.The obtained binary Ni-Mn oxides had obvious needlepunched shapes and hollow structures.Scanning electron microscope(SEM),transmission electron microscope(TEM),X-ray diffraction(XRD),and Raman spectroscopy were used to analyze the morphology and the composition of the product.The effects of annealing temperature and mass fraction of H2O2on the speed and lifetime of micromotors were systematically investigated,along with the adsorption of methylene blue(MB)in water.The results showed that,compared with the previous work,the Ni-Mn oxides micromotors had higher moving speed and better performance in removing pollutants,and could be recycled by the magnetic field.Such binary Ni-Mn oxides micromotors possess great potential as an environment-friendly,recyclable,and mass-produced candidate for water remediation.
1 Experimental
1.1 Materials
Nickel chloride hexahydrate(NiCl2·6H2O),manganese chloride hexahydrate(MnCl2·6H2O),urea,ethanol,and MB were purchased from Sinopharm Chemical Reagent Co.,Ltd.H2O2(30%)and Triton X-100 were obtained from Alfa Aesar Chemical Reagent Co.,Ltd.
1.2 Preparation of binary Ni-Mn oxide
Ni-Mn oxide precursor was prepared by hydrothermal method[41].First,0.1 mol·L-1NiCl2·6H2O,0.33 mol·L-1urea,and 0.033 mol·L-1MnCl2·6H2O were dissolved in ultrapure water.The mixture was placed in a 100 mL Teflon-lined stainless autoclave and maintained at 160℃for 16 h.The obtained precursor was washed with ultrapure water and ethanol several times by centrifuge and then kept in an oven at 60℃for 18 h.The above-obtained product was placed in the porcelain boat and then put into a tube furnace,and it was annealed at 300,400,and 500℃for 5 h under a nitrogen atmosphere and then cooled to the room temperature naturally to obtain Ni-Mn oxides micromotors.At the same time,the precursors were annealed at 500℃for 3,5,and 7 h respectively to obtain Ni-Mn oxides micromotors.As a comparison,Mn oxide precursor was also prepared in the same way,replacing all of the Ni salts with Mn salts only.The precursor was subsequently annealed at 500℃for 5 h under a nitrogen atmosphere to obtain the Mn oxide micromotor.
1.3 Characterization
The XRD pattern of the product was drawn by a Philip-X′Pert X-ray diffractometer with a CuKαX-ray source(λ=0.154 001 nm,2θ=15°-80°,step size of 0.01°,operating voltage of 40 kV,operating current of 40 mA).SEM(S-4800,acceleration voltage of 3 kV)was used to characterize the morphology of the micromotor.Energy dispersive X-ray spectra(EDX)were obtained using a TEM(FEI Talos F200X field emission transmission electron microscope)operating at 200 kV.Raman microscope(InVia,Renishaw,England)was excited by a laser at 633 nm to obtain the Raman spectrum.The UV-Vis absorption spectrum was recorded on a PerkinElmer ultraviolet-visible spectrometer Lambda 650S.Aperture and specific surface area measuring instruments were V-Sorb 2800P-Gold spectrum,and surface area was calculated based on the Brunauer-Emmett-Teller equation.Nitrogen adsorption-desorption isotherm was gained from an automated adsorption unit(Micromeritics ASAP 2020)at 77 K.The FT-IR spectroscopy (Bruker model VECTOR-22 Fourier transform spectrometer)was used to analyze the composition of the product.The video was obtained by capturing the motion of the micromotor with an inverted optical microscope(Nikon Instrument Inc.Ti-S/L100)and a 40×objective lens,and Nikon digital sight DS-Ri1.NIS-Elements software was used to calculate the speed of the micromotor.The video has been edited using Adobe Premiere software.Before the motion experiments,Ni-Mn oxide was coated with a 10 nm-gold layer by the E-1010 ion sputter.
2 Results and discussion
Binary Ni-Mn oxide with perfect morphology was prepared by hydrothermal method and then annealing.SEM and TEM images of Ni-Mn oxide are shown in Fig.1.Comparing the SEM and TEM images of the samples with different annealing temperatures,it can be found that with the annealing temperature increasing,the needle-like shape became more obvious and the inner microsphere became completely hollow.The needle-like structure could be seen in SEM images clearly.The diameter of the Ni-Mn microsphere was 2-5 μm,and the thickness of the shell layer was 0.6-0.8 μm.The needle-like shape on the surface of the microsphere could also be seen in TEM images,but the hollow structure was not observed because the thickness of the shell was relatively thick preventing the electron beam from penetrating the shell.Compared with the precursor(Fig.S1,Supporting information),the annealed Ni-Mn microspheres had a more pronounced needlepunched shape,a larger internal hollow space,and a relatively thin shell.In addition,due to the influence of external factors such as uneven heating,individual microspheres might have a phenomenon in which the inner core did not completely retreat,still,this situation could be effectively reduced by annealing.

Fig.1 SEM and TEM images of Ni-Mn oxide obtained by annealing at(a)300℃,(b)400℃,and(c)500℃
To understand the compositions and structures of the product and its precursor more clearly,XRD patterns and Raman spectra were used to characterize the samples.The XRD results(Fig.2a)are consistent with the characteristic peaks of NiO,Ni,and Ni6MnO8(PDF No.49-1295,70-0989,89-7131).The diffraction peaks at 35.785°,37.376°,43.45°,63.106°are attributed to Ni6MnO8,peaks at 44.604°,51.977°,76.588°are assigned to Ni,and peaks at 37.249°,43.289°,62.901°,75.385°,79.393°are ascribed to NiO.It is worth mentioning that the characteristic peaks became sharper with the annealing temperature increasing,showing the crystallinity became higher.Fig.2b shows the Raman spectra of Ni-Mn oxide obtained by different annealing temperatures and its precursor.It can be observed that the peaks between 400 and 700 cm-1are O—M—O and M—O(metal oxide)bending modes resulting from the vibration of oxygen atoms.The band at 900-1 200 cm-1of curve Ⅳ is attributed to CO32-ν1symmetric and ν3antisymmetric stretching vibration of carbonate anion.
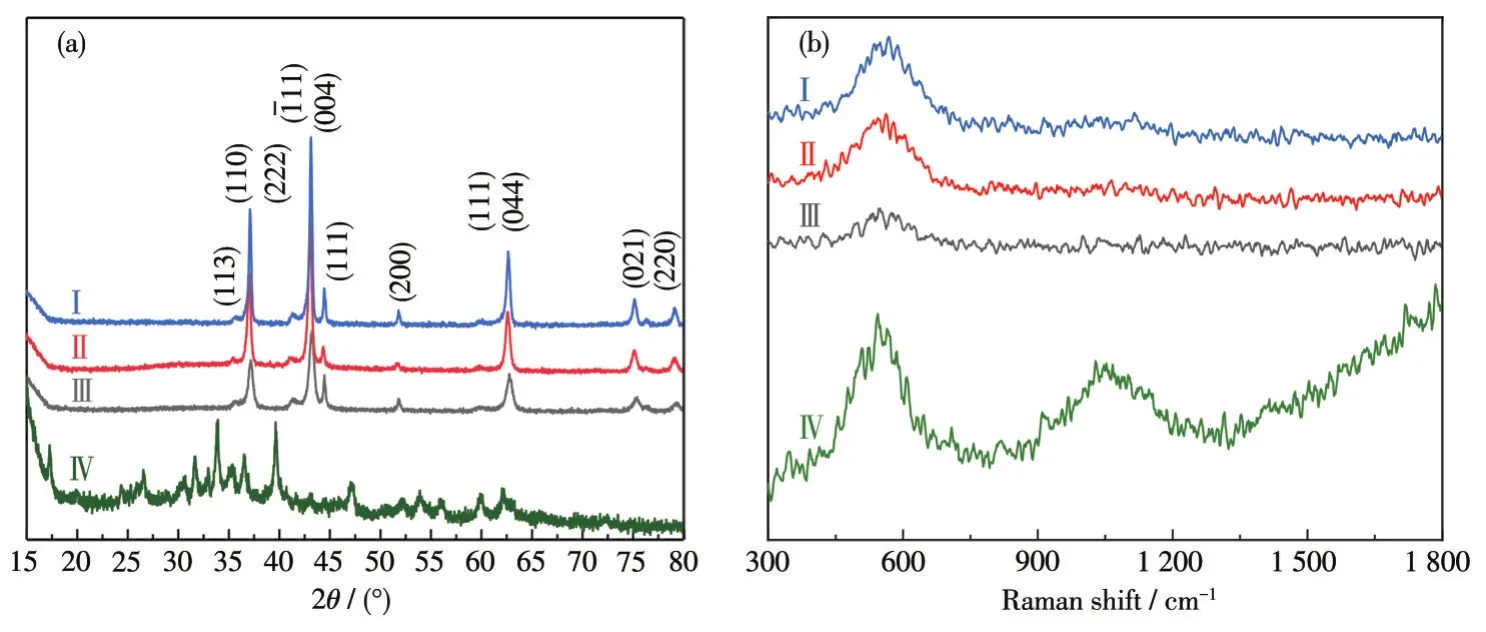
Fig.2 XRD patterns(a)and Raman spectra(b)of binary Ni-Mn oxide with different annealing temperatures
Fig.3 shows the nitrogen adsorption-desorption isotherms of the Ni-Mn oxide micromotor annealed at 500℃.The surface area,adsorption cumulative volume of pores,and adsorption average pore diameter were 195 m2·g-1,0.704 925 cm3·g-1,and 15.465 6 nm,respectively.Based on the characteristics of the mesoporous material present in the IUPAC classification,it can be concluded that the adsorption curve belongs to the type Ⅳ isotherm.The pore size distribution also verifies that the material had a high number of mesopores and microporous structures.The large specific surface area and the porous structure are necessary for the efficient adsorption of organic pollutants by the micromotor.
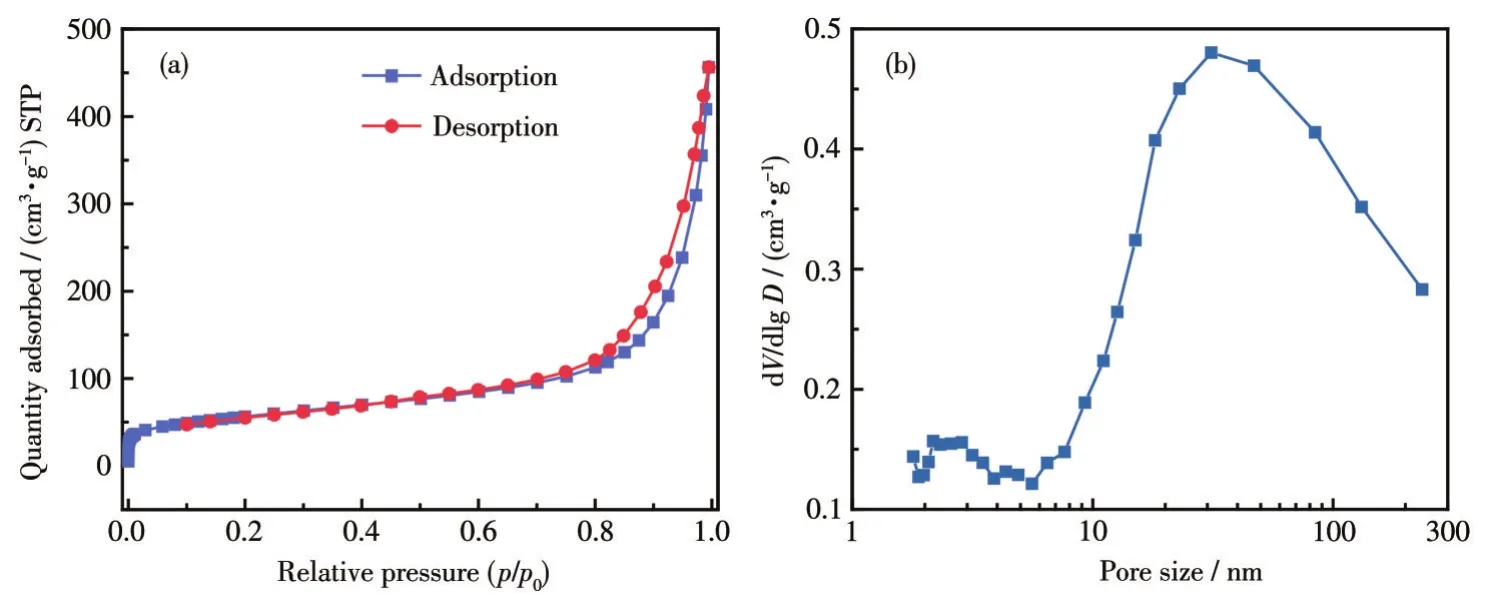
Fig.3 Nitrogen adsorption-desorption isotherms(a)and pore size distribution(b)of Ni-Mn oxide micromotor annealed at 500℃
The Ni-Mn oxide micromotor with excellent catalytic properties could move efficiently by decomposing H2O2to produce O2bubbles.The left panel of Fig.4 shows the time-lapse images of the Ni-Mn oxide micromotor obtained at different annealing temperatures taken from Video S1(Supporting information)in a 0.25 s period when the H2O2mass fraction was 1%.These images illustrate a tail of O2bubbles generated and released from the Ni-Mn oxide surface.Video S1 shows that the Ni-Mn oxide micromotors displayed the autonomous locomotion at different speeds when the annealing temperature was different.As can be seen from the right panel of Fig.4,the speed of the micromotor was 52.08,68.35,and 83.75 μm·s-1when the annealing temperature was 300,400,and 500℃,respectively.It is obvious that the speed was increased with the increasing of annealing temperature,which may be due to that higher crystallinity formed at higher annealing temperature leads to better catalytic ability.The motion performances of the samples were also compared at different annealing times at 500℃.Video S2 shows that when the annealing time of the micromotor was only 3 h,the movement speed of the micromotor was significantly slower than that of 5 h.This might be due to the incomplete conversion of the precursor to oxide,which leads to weak catalytic performance.In contrast,when the annealing time was 5 h,the motion speed of the micromotor was significantly increased.However,when the annealing time was extended to 7 h,the movement speed of the micromotor was as much as that of 5 h.So,Ni-Mn oxide micromotor obtained at an annealing temperature of 500℃with an annealing time of 5 h was taken as the sample for the following research.
The influence of H2O2mass fractions on the micromotor′s motion was studied.As shown in Fig.5a-5c taken from Video S3,the time-lapse images illustrate the movement trajectories of the Ni-Mn oxide micromotor.When the H2O2mass fraction was increased from 0.4% to 1%,the micromotor′s speed became fast clearly.As given in Fig.5d,we can see that the micromotor′s speed correspondingly increased from 83.75 to 203.35 μm·s-1,when the H2O2mass fraction was increased from 1% to 7%.The effect of the H2O2mass fraction on the micromotor′s motion was very strong.In addition,the lifetime was also an important factor in micromotors′performance.This data of Ni-Mn oxide micromotor was tested in 1% H2O2.It is worth mentioning that the micromotor could still move although its speed became slow after 2.5 h.This advantage is in favor of realizing its practical applications in different fields.
The Ni-Mn oxide micromotor with good magnetic properties is easy to recycle,environmentally friendly and controllable in motion.The time-lapse images of Fig.6 taken from Video S4 depict the directional movement of the micromotor under the applied magnetic field indicated by the red arrow.It could follow the predetermined trajectories of magnetic field direction and become free autonomous motion when the external magnetic field was removed.This proves that the mag-netism of the micromotor can also be used as a second driving force.We can take advantage by enabling the micromotors to cross obstacles and reach the contaminants quickly,which is difficult to achieve with normal water treatment technology.As a comparison,the pure Mn oxides micromotors showed regular hexahedral morphology,but the sizes were relatively uneven,ranging from 500 nm to 1 μm(Fig.S2).Video S5 shows that although the Mn oxide micromotor exhibited catalytic activity towards H2O2,it moved slowly and couldn′t make directional movements under a magnetic field.

Fig.6 Time-lapse images depicting the directional movement of the Ni-Mn oxide micromotor in 1% H2O2solution under the applied magnetic field
UV-Vis absorption spectra were used to investigate the application for water remediation of the Ni-Mn oxide micromotor by using MB as the organic model dye.As shown in Fig.7a,the static adsorption process of MB using 0.5 mg·mL-1of the Ni-Mn oxide without H2O2was not obvious after 20 min.However,MB could be adsorbed quickly and the absorbance decreased significantly when after adding 1% H2O2,and it can be adsorbed completely within 160 s(Fig.7b).The result proves that the dynamic adsorption efficiency was much higher than the static adsorption efficiency.The effects of the amount of H2O2and the Ni-Mn oxide micromotor on the adsorption of MB were also studied.Fig.7c shows that the absorbance of MB decreased significantly with the increase of H2O2mass fraction from 0% to 3%,and the maximum removal capacity was 94.78% after 150 s.It indicates that removal efficiency can be enhanced by a fast motion of the micromotors.It can be clearly stated here that the Ni-Mn oxide micromotor has a higher moving speed and better performance in removing pollutants compared with the previous work[42-45](Table 1).At the same mass fraction of H2O2,the absorbance decreased with the amount of the micromotors increasing(Fig.7d).There was no change in the absorbance with 1% H2O2without the micromotors showing that H2O2did not affect the adsorption of MB.In addition,the Ni-Mn oxide micromotors could be easily recycled under an external magnetic field due to their magnetic property.The magnetism of the micromotor allows us to recycle the micromotor in time for the end of the water treatment,thus avoiding secondary contamination.

Fig.7 (a)UV-Vis spectra of MB solution in the presence of the Ni-Mn oxide micromotor at different times(0,5,10,15,and 20 min);(b)UV-Vis spectra of MB solution in the presence of the Ni-Mn oxide micromotor at different times(0,30,90,120,and 160 s)in 1% H2O2,where the inset shows the picture of MB(blue)being adsorbed after 160 s;(c)Effect of the H2O2mass fractions(0%,0.5%,1%,2%,and 3%)on the adsorption of MB by Ni-Mn oxide micromotor;(d)Effect of the amount of the Ni-Mn oxide micromotor(0,0.5,0.8,1,and 2 mg·mL-1)on the adsorption of MB in 1% H2O2

Table 1 Selected micromotors in dye removal
Fig.8 shows the FT-IR spectra before and after adsorption to confirm whether MB is adsorbed on the Ni-Mn oxide micromotor.The small peak at 514 cm-1in curve Ⅰ is attributed to the coupling oscillation of Ni—O—Mn.The absorption peak located at 495 cm-1is a result of the stretching vibration of the Ni—O[46].The characteristic absorption peaks of MB(Inset)at 1 599,1 489,and 1 492 cm-1in curve Ⅱ are ascribed to the C=C,C=S+,—CH vibrations respectively,indicating that MB was successfully adsorbed to the surface of Ni-Mn oxide.This could be explained that the—OH in the water molecules on the surface of the Ni-Mn oxide plays an important role in the adsorption of MB[47].
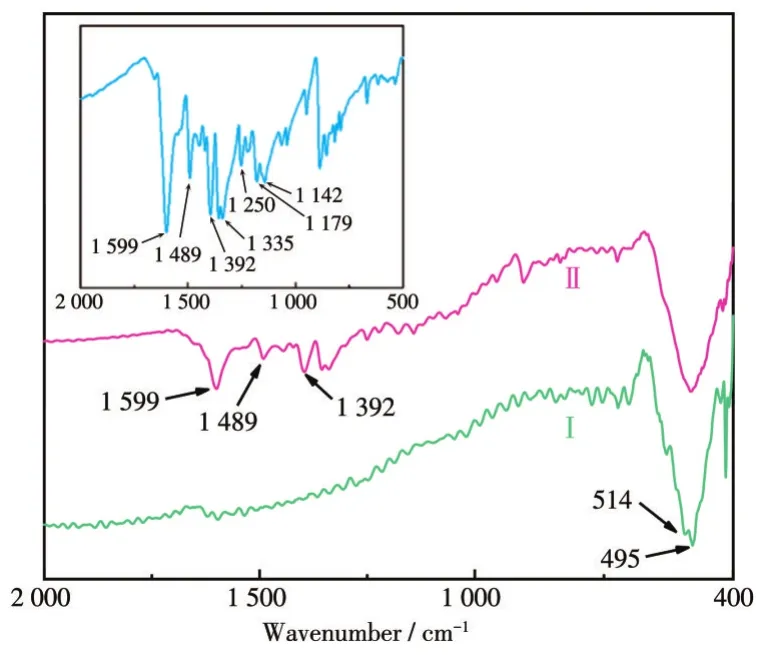
Fig.8 FT-IR spectra of Ni-Mn oxide micromotor(Ⅰ)before and(Ⅱ)after MB adsorption
3 Conclusions
In general,we proposed green and environmentfriendly binary Ni-Mn oxide micromotors with perfect morphologies prepared by a simple hydrothermal and heat treatment process.The Ni-Mn oxide micromotors could effectively catalyze H2O2to generate bubbles to achieve autonomous movement.At the same time,the motion direction could be accurately controlled by an external magnetic field.The application of dye removal in the water of this micromotor was investigated by using the organic dye MB as a model.The experiment results showed MB could be effectively removed by the Ni-Mn oxide micromotors through adsorption without secondary pollution.Such micromotors with long lifetimes and environment-friendly nature have broad application prospects in sustainable environmental remediation.
Supporting information is available at http://www.wjhxxb.cn
- 无机化学学报的其它文章
- La-Doped BaSnO3/Multi-walled Carbon Nanotube Modified Separator:Synthesis and Application in Lithium-Sulfur Battery
- Co(Ⅱ)/Ni(Ⅱ) Coordination Polymer of Isomeric Terphenyl-2,2″,4,4″-tetracarboxylic Acids with a Single Water Bridge:Syntheses,Structures,and Magnetic Properties
- Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol by Mg-Doped Ceria Monolithic Catalyst
- Hydrogen Storage Capabilities of the Low-Lying Ca2B4Clusters
- 盘状镝簇合物的合成及缓慢磁弛豫
- 内放射状中空TiN颗粒作为锂硫电池正极的性能

