Solubility determination and thermodynamic modeling of bis-2-hydroxyethyl terephthalate (BHET) in different solvents
Haoyu Yao, Dongxia Yan, Xingmei Lu,3,4,*, Qing Zhou,4,*, Yinan Bao, Junli Xu
1 Beijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2 School of Chemical and Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
3 Sino Danish College, University of Chinese Academy of Sciences, Beijing 100049, China
4 Innovation Academy for Green Manufacture, Chinese Academy of Sciences, Beijing 100190, China
5 College of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China
Keywords:Bis-2-hydroxyethyl terephthalate Purified Characterized Solubility Correlation model Thermodynamics properties
ABSTRACT Studies on the degradation process of waste polyethylene terephthalate (PET) have become increasingly mature,but there are relatively few studies on the separation of degradation products.The products contain many components and the separation of which is difficult.Therefore,the study on phase equilibrium thermodynamics of bis-2-hydroxyethyl terephthalate(BHET)is of great theoretical significance and practical value to provide basic data for the BHET crystallization separation.In this work,the degraded products were purified and characterized.The solubility of BHET in methanol,ethanol,ethylene glycol,water and the mixture of ethylene glycol+water were determined by static method.The experimental results were correlated with different models, such as ideal solution (IS) model, λh equation, Apelblat equation and NRTL model.Based on the van’t Hoff equation,the mixing Gibbs energy, enthalpy and entropy were calculated.From this work,the basic data which can be used to guide the crystallization process of BHET were obtained, including solubility data, correlation model and thermodynamic properties.
1.Introduction
As the consumption of polyethylene terephthalate (PET) products increases year by year,a large amount of waste PET is accumulated without effective recycling[1,2].In order to reduce the harm of waste PET products to the environment, lots of countries and organizations around the world have issued various policies to encourage the recycling of waste PET products.Researchers have developed different methods for PET recovery, such as physical recovery [3]pyrolysis [4]chemical degradation [1,5-7]and so on.Chemical degradation stands out for its advantages of mild reaction conditions and high value recovery of products.There are many kinds of chemical degradation products through different degradation methods, such as terephthalic acid can be obtained through hydrolysis [8]dimethyl terephthalate can be obtained through methanol alcoholysis [9], bis-2-hydroxyethyl terephthalate (BHET) (CAS No.959-26-2) can be obtained through ethylene glycol alcoholysis [10-12].
BHET is also an important intermediate for the synthesis of polyester polyols[13].At present,although studies on the degradation of PET are relatively mature,there are relatively few studies on the separation of components of degradation products.Because the product contains many components,which can not reach the purity requirement of repolymerization.Therefore, the study on crystallization thermodynamics of BHET can solve this problem well,and has very important theoretical significance and practical value.Crystallization has been regarded as an essential means of separation and purification in industry [14,15].It has a certain influence on the quality and quantity of the object.Further study of solubility can guide the crystallization process, reduce the doping of undesired compounds and reduce the loss of mother liquor.
In this work,the target product BHET was obtained by multiple means of glycolysis and recrystallization.Qualitative and quantitative analysis was carried out by various means.Since BHET obtained from PET degradation is usually dissolved in ethylene glycol, the solubility of BHET in ethylene glycol was studied in this work.However, BHET directly crystallized from ethylene glycol is often doped with a large number of solvents.In order to obtain higher purity of BHET,subsequent recrystallization must be carried out to purify it.Therefore,methanol,ethanol and water,which are cheap and easy to get, were selected for comparison.Different ratios of binary solvents of ethylene glycol and water were also selected for reference, considering the accumulation of ethylene glycol in the solvent during subsequent recrystallization operations.In various pure solvents (ethylene glycol, methanol, ethanol and water) and binary solvent systems with different proportions of ethylene glycol and water under atmospheric pressure at temperatures ranging from 283.15 K to 328.15 K,the solubility of BHET was measured.According to the experimental data,the correlation of solubility of BHET and thermodynamic analysis were studied.The experimental results were correlated with four thermodynamic models, including the ideal solution model, λhequation,Apelblat equation and NRTL model.In the end,the Van’t Hoff equation was used to calculate the mixing Gibbs energy, enthalpy and entropy.
2.Experimental
2.1.Materials
Ethylene glycol,methanol and ethanol in analytic reagent grade were purchased from Damao Chemical Co.Ltd., China.Ultrapure water is prepared by pure water mechanism in laboratory.All chemicals were used directly without further purification.BHET is prepared by the glycolysis of PET with ethylene glycol.The specific preparation process is shown in Section 2.2.
2.2.The preparation of BHET
The preparation of BHET was described in previous reports[16-19].Firstly, ethylene glycol, PET particles and zinc acetate (catalyst) were added to three fluses at a mass ratio of 20:5:0.05.At 197°C,the reaction continues until the system becomes clear.Secondly, the degradation liquid was cooled and added to a beaker with 500 ml water.The mixed solution was heated to 70°C and filtered to remove the impurities.BHET crystals were obtained by crystallization overnight at low temperature.Repeat the above operation for many times to obtain a relatively pure product and dry overnight at 70°C.
2.3.Solubility measurements
In this experiment, the solubility of BHET in different solvents were determined by a static method, and the BHET content in different saturated solutions was measured by HPLC.The experimental device is shown in Fig.1.
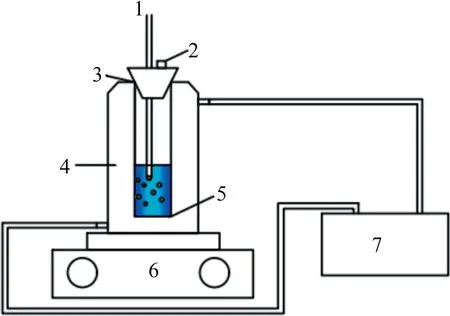
Fig.1. Schematic setup for the solubility determination.1—Thermometer; 2—Sampling port; 3—Rubber plug; 4—Glass jacket crystallizer; 5—Agitator; 6—Magnetic stirrer; 7—Thermostatic bath.
Firstly, the solvent system was prepared by weighing the pure solvent and the mixed solvent in different proportions by an analytical balance.During the preparation of the saturated solution,about 200 ml of the prepared solvent was added to the glassjacketed crystallizer, and a slightly excess of BHET solids was added to the container.The circulating fluid in the thermostatic tank is connected through a plastic tube to a glass-jacketed crystallizer and the solution system is kept at a specified temperature by a circulating water bath.After stirring for at least 3 h, turn off the magnetic agitator and allow the undissolved solute to settle in the lower layer of the container.The saturated solution is placed in the upper layer of the container.A small sample of the upper clarifier is extracted with a pre-heated syringe into a sample bottle(m0).After the vial was quickly capped,its mass(m1)was weighed again, and the sample vial was added with appropriate diluent(corresponding solvent phase) and shaken thoroughly until the clear solution without precipitation and weighed again (m2).The solubility is calculated by the following equations:

HereC0(mol·g-1)is the sample concentration obtained by HPLC,M(mol·g-1)is the molar mass of the solvent,andais the mass ratio of the mixed solvent.
2.4.Characterization
FT-IR spectra were carried out using a Nicolet 380 spectrometer(Thermo Fisher Scientific).1H NMR and13C NMR were carried out using Bruker ECA-600 instrument, while the solvent was d6-DMSO.Differential scanning calorimetry (DSC) scans of BHET was obtained using the DSC-1 STARe system by heating from 0°C to 600°C at a heating rate of 10°C·min-1in an atmosphere of nitrogen with a flow rate of 20 ml·min-1.
3.Results and Discussion
3.1.Characterization of the purified product
The purity of BHET was analyzed by HPLC as shown in Fig.2.The separation was carried out on a C18 column (5 μm,250 mm×4.6 mm) with a mobile phase of methanol and water(volume ratio 1:1, 0.2 ml·min-1) and column temperature of 303.15 K.As shown in Fig.2, BHET peaked at 23.5-24.5 min and contained almost no impurities.
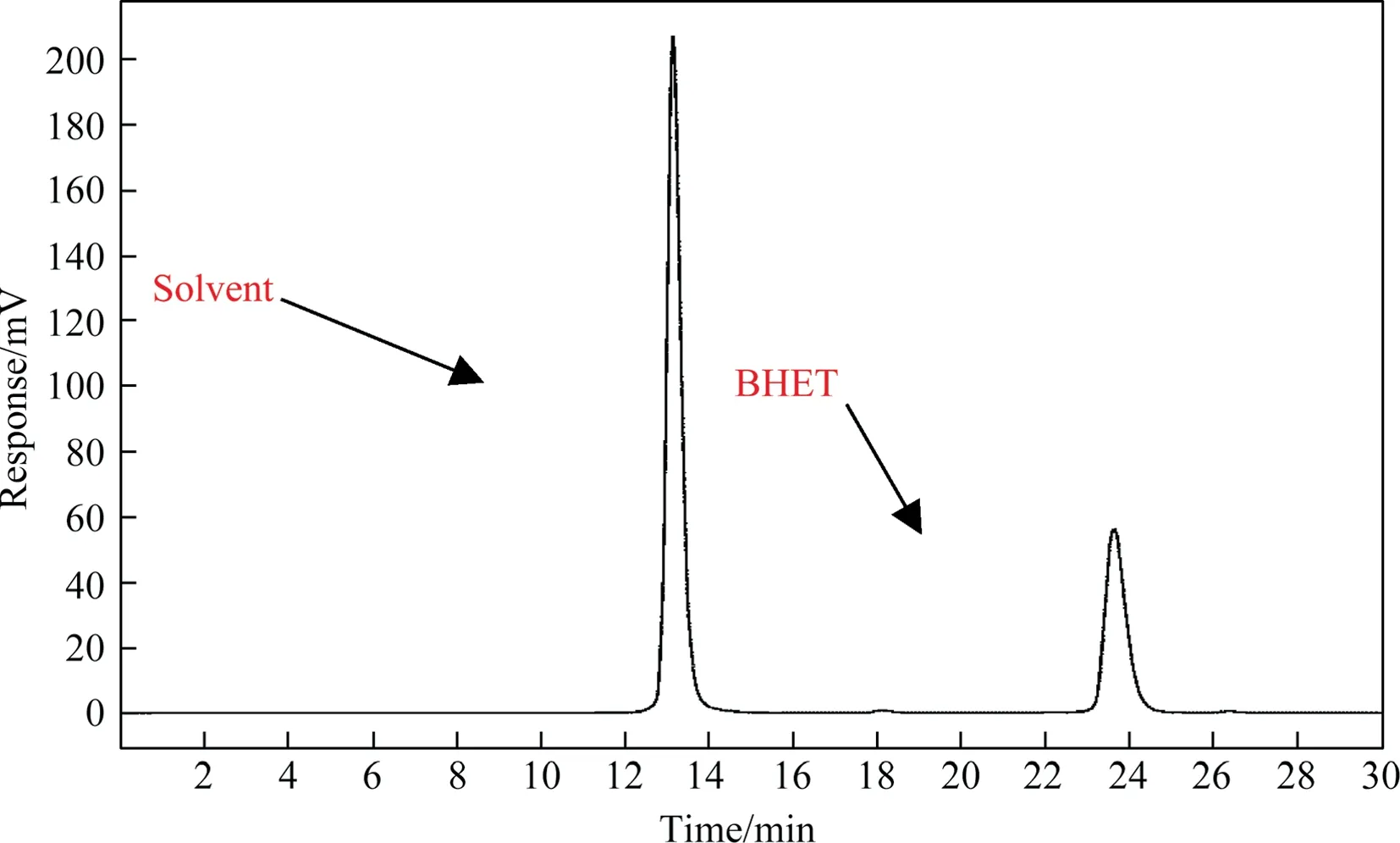
Fig.2. HPLC result of BHET.
The reaction product was purified by recrystallization and the crystal was analyzed qualitatively by FT-IR.The resulting spectrogram is shown in Fig.3(a).The types of chemical bonds and functional groups were determined according to the position of absorption peaks, and then the types of compounds were determined.As shown in the figure, absorption peaks of the product appeared at 3460, 2960, 1710, 1690, 1500 and 1280 cm-1, respectively being the stretching vibration peaks of O-H,C-H,C=O,benzene ring and C-O [6].These characteristic peaks cover all the groups of BHET.1H NMR and13C NMR were respectively obtained by nuclear magnetic resonance characterization of the purified BHET, as shown in Fig.3(b) and (c).From the1H NMR, we obtain the characteristic peaks of several functional groups, that is, the chemical shift of 8.07 is the characteristic peak of the hydrogen on benzene ring, the chemical shift of 7.19 is the characteristic peak of deuterated chloroform (CDCl3) solvent peak position, the chemical shift of 4.44 is the characteristic peak of the hydrogen on methylene(CH2-),the chemical shift of 3.92 is the characteristic peak of the hydrogen on methylene (-CH2-) which directly connected with ester (-COO-), and the chemical shift of 1.18 is the characteristic peak of the hydrogen on hydroxyl (OH).At the same time, other impurity peaks are rare and almost negligible.In the same way, the13C NMR was analyzed.The chemical shift of 165.01 is the characteristic peak of carbon atom on ester, the chemical shift of 132.85 and 128.69 are the characteristic peaks of the carbon on aromatic ring, the chemical shift of 75.99 is the characteristic peak of solvent, the chemical shift of 66.03 is the characteristic peak of the carbon on methylene, the chemical shift of 60.30 is the characteristic peak of the carbon on methylene which attached directly to the hydroxy, and the chemical shift of 28.68 is the characteristic peak of secondary carbon atom.In conclusion, more pure BHET can be obtained by multiple recrystallization.
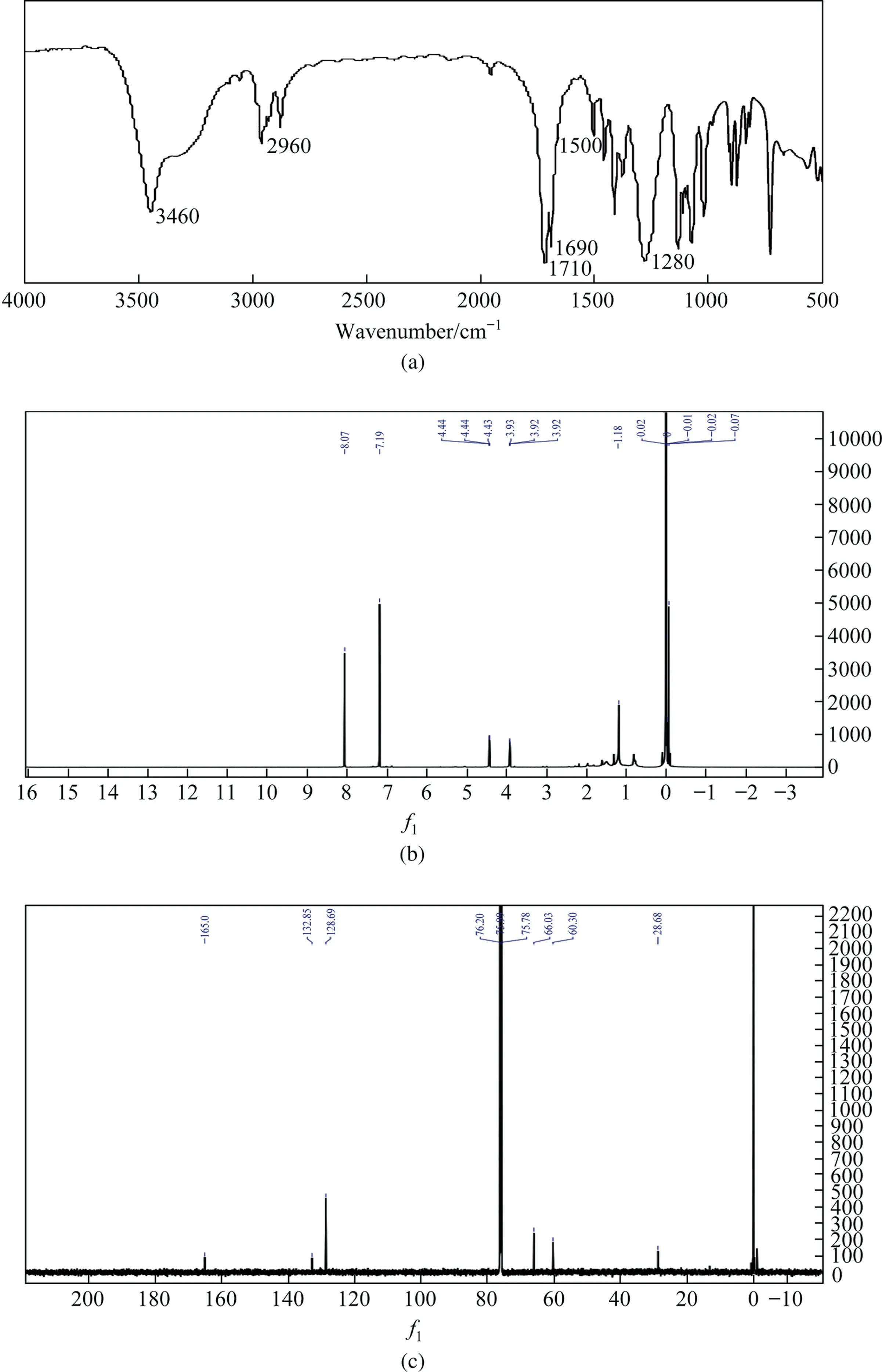
Fig.3. Characterization of BHET.(a) FT-IR; (b) 1H NMR; (c) 13C NMR.
Differential scanning calorimetry(DSC)analysis results of BHET shows in Fig.4.The melting temperature of BHET can be obtained from the figure whereTmis 385.20 K.The melting enthalpy(ΔfusH)of BHET is 6.56 kJ·mol-1by integrating the peak area.The melting entropy ΔfusScan be calculated by the following formula.The melting entropy is 17.03 J·mol-1·K-1.

3.2.Study on dissolution of BHET in pure solvent
In this experiment, constant temperature and static method was used to determine the solubility of BHET in pure solvents,such as methanol, ethanol, ethylene glycol and water.The solubility of BHET are shown in Table 1.With the increase of temperature,the solubility in four pure solvents increases.The solubility curve is smooth without inflection point.At low temperature, the solubility of BHET in methanol, ethanol, ethylene glycol and waterchanges little with temperature.When the temperature is higher,the solubility of BHET increases, and the slope of the solubility curve increases,indicating that the solubility changes greatly with the increase of temperature, and the influence of temperature on the solubility of BHET increases greatly.Compared with other three pure solvents, it is less sensitive to temperature in water.The solubility of BHET in water is the smallest in the measured temperature range, while that in methanol and ethanol is the largest.Within the experimental range, the order of solubility of BHET in different solvents is:methanol >ethanol >ethylene glycol >water.It can be seen from the solubility results that the solubility of BHET in organic system is higher than that in inorganic system.When dissolved in the organic system, the high molecular polarity of the solvent is conducive to the dissolution of BHET.
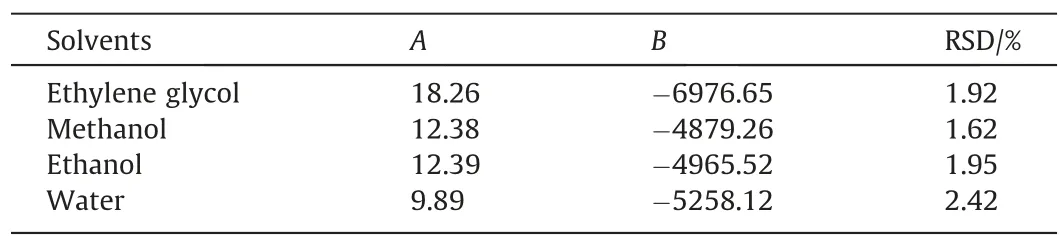
Table 2The fitting parameter value and RSD of IS equation
3.2.1.Correlation of the experimental solubility data
(1) Correlation with the ideal solution (IS) model The IS model has a simple form and is often used in the study of solid-liquid equilibrium.The activity coefficient is 1 in the ideal solution model and the equation is as follows;

whereAandBare model parameters.RSD was used to represent the fitting relative standard deviation of IS model.Parameter fitting results and RSD of the equation are shown in Table 2.It can be summarized from the calculation results that for the solubility of BHET in four pure solvents,the IS model is applicable within the range of temperature in this experiment,and RSD is less than 2.42%.The solubility were fitted with the above parameters,and the results were shown in Fig.5.It can be seen that the experimental values are in good agreement with the model.
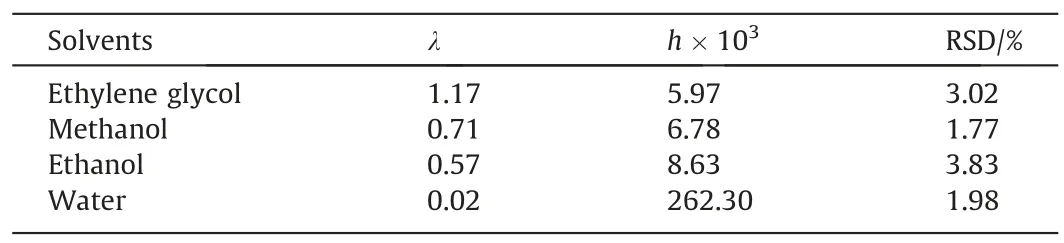
Table 3The fitting parameter values and RSD of λh model
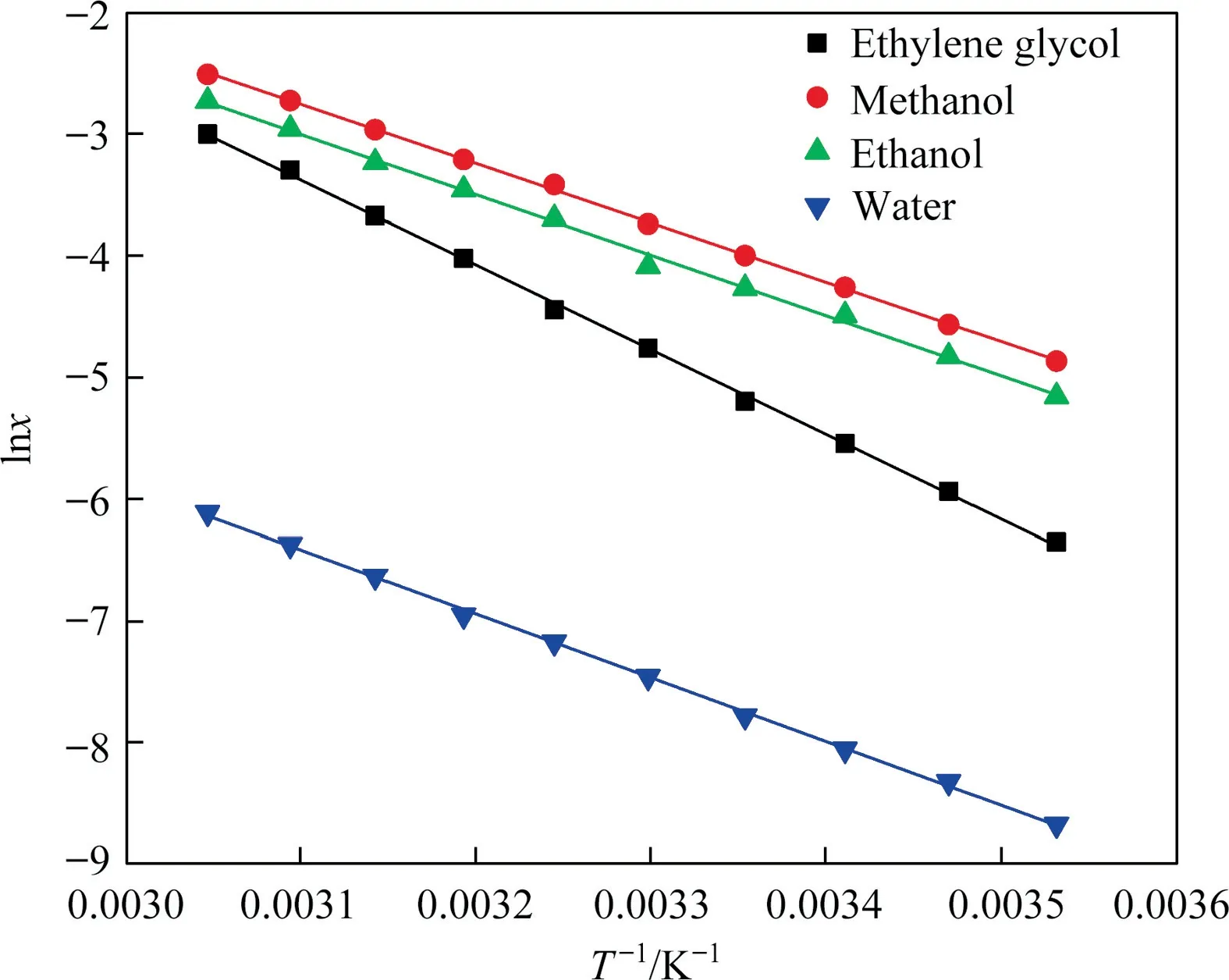
Fig.5. Solubility of BHET in pure solvent in relation to temperature.
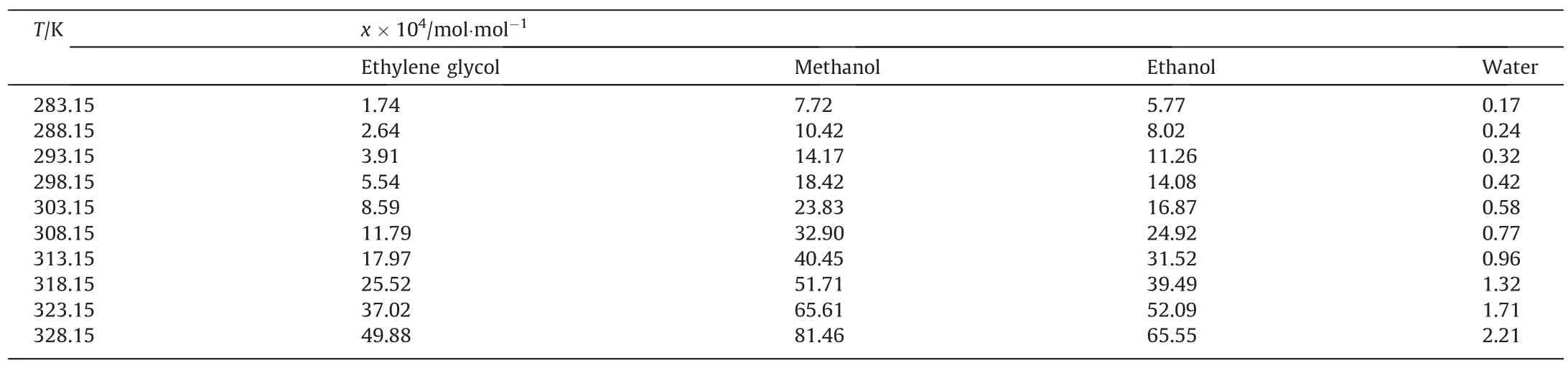
Table 1The solubilities of BHET in methanol, ethanol, ethylene glycol and water
(2) Correlation with λhmodel
Buchowski [20]first proposed the λhmodel, as follows:
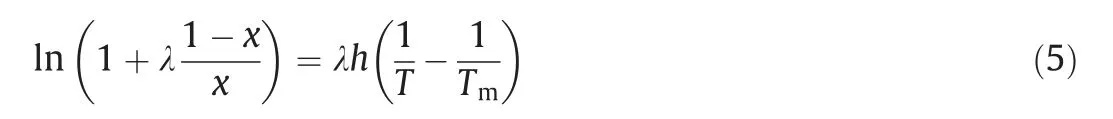
whereTmis the melting point of solute;xis the mole fraction of solute; λ andhare the fitting parameters of the equation.λ andhhave thermodynamic meanings.The value of λ can reflect the non-ideal solution system.The enthalpy of the solution can be predicted by the value ofh.
As can be seen from Table 3,when fitting the solubility of BHET in these four selected pure solvents using the λhmodel, the RSD with relative standard deviation ranges from 1.77% to 3.02%, indicating that the solubility data of BHET fitted by the λh model in the selected pure solvents is not well fitting.
(3) Correlation with the Apelblat model
Apelblatet al.[21]deduced the Apelblat model from the Calusius-Clapeyron equation on the premise that solute molecules and solvent molecules formed complexes, which is a semiempirical equation.

whereTis the absolute temperature;xis the molar fraction solubility atTtemperature;A,B, andCare empirical parameters.The values ofAandBrepresent the change in the activity coefficient of the solution, while the values ofCreflect the effect of temperature on the enthalpy of melting.
The solubility data of BHET in the selected pure solvents were correlated with apelblat equation.The fitting error of Apelblatequation is still represented by RSD, and the parameter fitting results and RSD of the equation are shown in Table 4.

Table 4The fitting parameter values and RSD of Apelblat equation
It can be summarized from Table 4 that when the solubility value of BHET in the selected pure solvent is fitted by Apelblat equation, its relative standard deviation is large and the fitting effect is not ideal.
(4) Correlation with the Wilson equation
In order to better represent the dependent relationship between activity coefficient and temperature,the binary interaction parameter in Wilson equation is expressed as a function of temperature.[22]

The values of parametersa1,b1,a2andb2in the formula and the RSD are shown in Table 5.
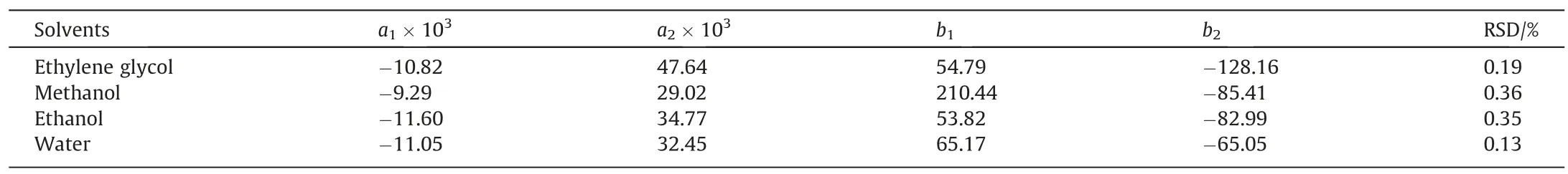
Table 5The fitting parameter values and RSD of Wilson equation
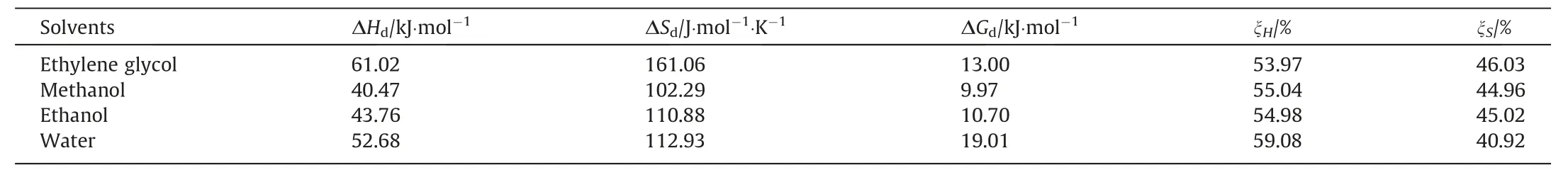
Table 6The dissolution entropy, dissolution enthalpy and gibbs energy of BHET in these four pure solvents (323.15 K)
It can be seen from the fitting results that the calculated results of the equation are in good agreement with the experimental values,and the relative standard deviation of all pure solvent systems is less than 0.4%, indicating that this model can be well applied to the correlation fitting of BHET in pure solvent systems.
3.2.2.Thermodynamic properties
The van’t Hoff equation[23]describes the relationship between the molar fraction solubility of solute and the temperature of the actual solution by considering the solvent effect, which is expressed as:

whereTis the absolute temperature;x1is the mole fraction of solute;ΔHdis the enthalpy of dissolution;ΔSdis the entropy of dissolution;Ris the gas constant.
The above equation relates the logarithm of the molar fraction of solute (lnx) to the reciprocal of the absolute temperature (1/T)through a linear function.From the slope and intercept of the curve,the ΔHdand ΔSdof BHET can be obtained.Gibbs molar dissolution can be calculated by the Gibbs-Helmholtz equation:

The relative contribution of enthalpy and entropy to standard Gibbs energy were represent by ξHand ξSrespectively:


The dissolution entropy, dissolution enthalpy and gibbs energy obtained in these four pure solvents are shown in Table 6.From the results, it can be seen that in all pure solvents, the dissolution of BHET is an endothermic process, which is caused by entropy change rather than spontaneous change.In addition, from the experiment it also found ΔHdsignificantly increased with the solubility of BHET decreased.It means that the solid-liquid phase equilibrium system requires more energy to overcome the cohesion of the solute.The correlation of the van’t Hoff equation in the water system is low,and the obtained thermodynamic parameters will be less accurate than other ones.This may be due to the relatively low solubility of BHET in water.These results are helpful to further understand and optimize the dissolution and crystallization process of BHET.What’s more,the molar Gibbs energy of dissolution is main depending on entropy.
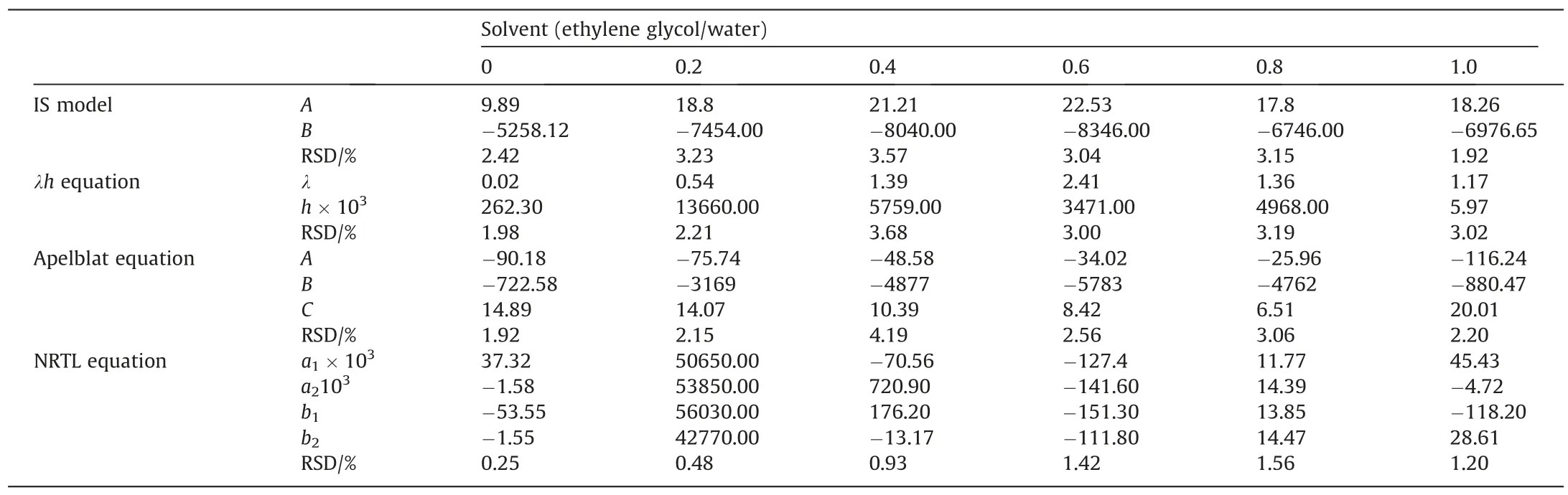
Table 7Fitting parameters of BHET solubility in binary solvent
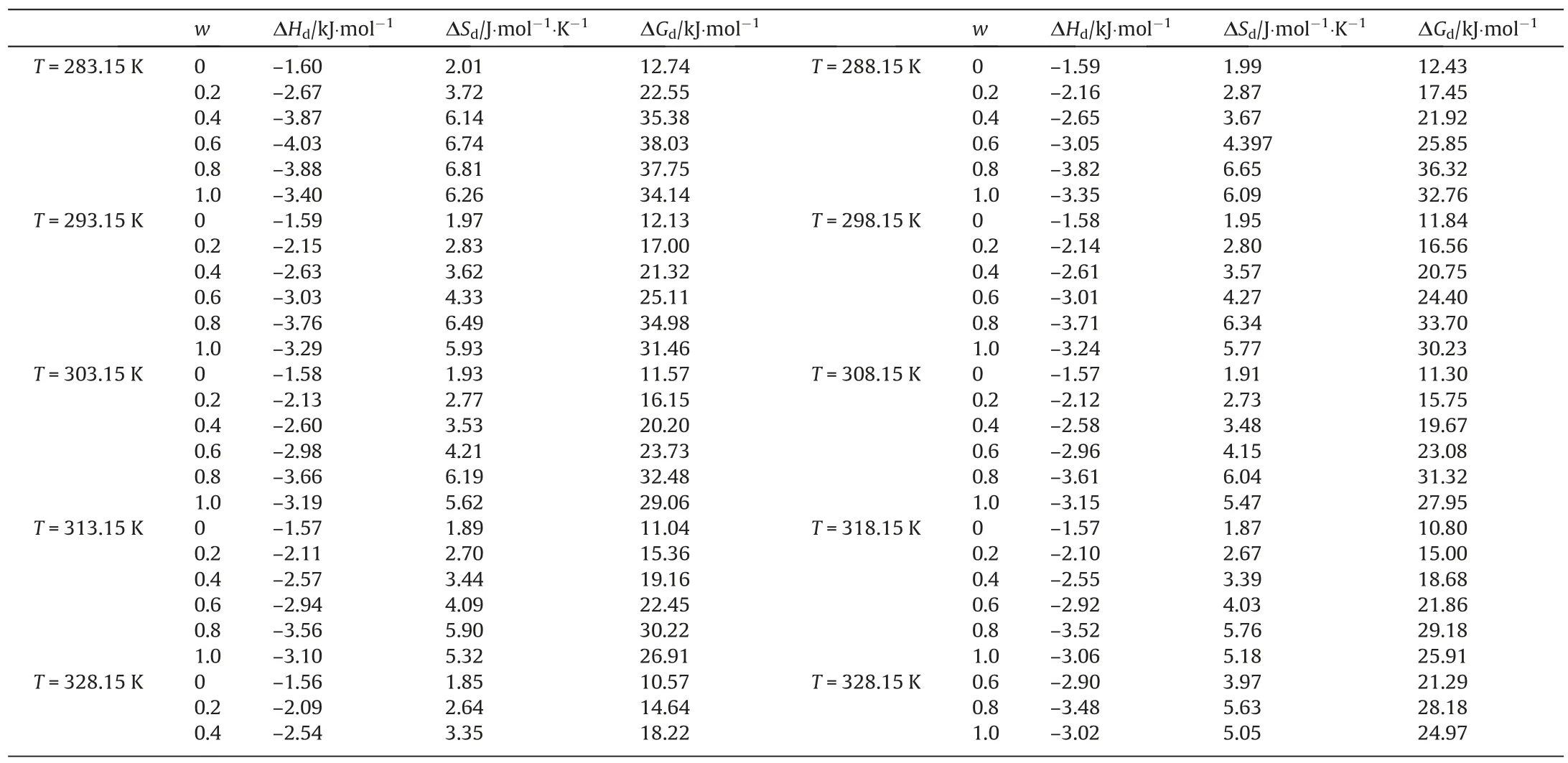
Table 8The dissolution entropy, dissolution enthalpy and gibbs energy of BHET in binary solvents
3.3.Study on solubility of BHET in binary solvent
After PET degradation, crystallization separation is usually carried out in the binary solvent of ethylene glycol and water.Therefore, in this part, we measured the solubility data of BHET in the binary system (ethylene glycol+water) by the constant temperature and static method.The results are shown in the Fig.6.The solubility data of BHET in glycol+water binary solvent showed a positive correlation with temperature.With the increase of temperature, the solubility of BHET in binary system of different proportions also increased.The results suggeste that the solubility of BHET increased with the increase of the mass fraction of ethylene glycol(w=0-0.8).In addition, when the mass fraction of ethylene glycol component exceeds 0.8, the solubility decreases slightly with the increase of mass fraction.This phenomenon may be caused by intermolecular interactions and steric hindrance between binary solvents.

Fig.6. Three-dimensional diagram of solubility of BHET in glycol+water binary system Note: w is the mass ratio of ethylene glycol to water; x is the solubility of BHET.
3.3.1.Correlation of the experimental solubility data
The results are fitted by the model in Section 3.2.1 above.Fitting parameters of BHET solubility in binary solvent system with different models are shown in Table 7.The order of average relative standard deviation after fitting of various models is IS >λh>Apelblat >NRTL.In other words,NRTL equation can better demonstrate the solubility law of BHET in binary solvent.
3.3.2.Thermodynamic properties of BHET dissolution in binary systems
Similarly, the Van’t Hoff equation was used to calculate the thermodynamic properties of BHET dissolution in binary solvent.The corresponding calculated values of standard enthalpy, standard entropy and standard Gibbs free energy are listed in Table 8.For binary solvents, these thermodynamic properties can provide useful information about molecular events.
4.Conclusions
The solubility of BHET in four pure solvents(methanol,ethanol,ethylene glycol and water)and four binary solvents(ethylene glycol/water: 0.2, 0.4, 0.6 and 0.8) at temperatures from 283.15 to 328.15 K were determined in this work.The solubility has a positive correlation with the temperature.The experimental solubility values were correlated by IS model,λhequation,Apelblat equationand NRTL model.By comparing the mean standard deviation of different models,it is found that NRTL model has the best correlation with the solubility data of BHET in all solvent.Through Van’t Hoff model, the dissolution enthalpy, entropy and gibbs free energy of BHET in all solvents were also determined.The dissolution process of BHET in all solvents were endothermic.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFC1908204),the National Natural Scientific Fund of China (No.21878292,21776289, 21908232, and 21978291), Innovation Academy for Green Manufacture, Chinese Academy of Sciences (No.IAGM2020C12, IAGM2020C21 and IAGM-2019-A06), and K.C.Wong Education Foundation (No.GJTD-2018-04).
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Notes for Contributors
- Insights into the cross-amyloid aggregation of Aβ40 and its N-terminal truncated peptide Aβ11-40 affected by epigallocatechin gallate
- Numerical study on hydrodynamic characteristics of spherical bubble contaminated by surfactants under higher Reynolds numbers
- Spray-drying assisted layer-structured H2TiO3 ion sieve synthesis and lithium adsorption performance
- Innovative hydrophobic/hydrophilic perfluoropolyether (PFPE)/polyvinylidene fluoride (PVDF) composite membrane for vacuum membrane distillation
- Fabrication and characterization of hierarchical porous Ni2+ doped hydroxyapatite microspheres and their enhanced protein adsorption capacity
