Insights into the cross-amyloid aggregation of Aβ40 and its N-terminal truncated peptide Aβ11-40 affected by epigallocatechin gallate
Yue Liang, Wenjuan Wang, Yan Sun, Xiaoyan Dong
Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering (Ministry of Education), School of Chemical Engineering and Technology, Tianjin University, Tianjin 300354, China
Keywords:Protein Aggregation Kinetics Nucleation Elongation Molecular interactions
ABSTRACT Inhibition of protein misfolding and aggregation is a great challenge in the field of biochemical and biopharmaceutical engineering.Alzheimer’s disease (AD) is a protein-misfolding disease, and the interactions between 40-amino-acid-residue β-amyloid peptide (Aβ40) and its N-terminal truncated peptide Aβ11-40 demonstrate that Aβ11-40 may play an important role in the pathological process of AD.However, the effect of inhibitors on Aβ11-40 aggregation and on the cross-amyloid aggregation (coassembly) between Aβ40 and Aβ11-40 has never been studied.Herein, coaggregation and seeding interactions between Aβ40 and Aβ11-40 as well as the effect of epigallocatechin gallate (EGCG), a small molecule inhibitor,on the cross-amyloid aggregation have been investigated by extensive analyses.It is found that Aβ11-40 participates in the aggregation of Aβ40 and leads to the formation of coaggregates that contain less β-sheet structures than pure Aβ40 aggregates.The aggregation kinetics along with morphologies and secondary structures of the coaggregates are also significantly affected by the Aβ40/Aβ11-40 ratio.EGCG accelerates the nucleation of Aβ40 but retards that of Aβ11-40 by affecting their elongation and secondary nucleation processes in solution and on solid surfaces.Meanwhile, EGCG makes the conformations of the seeding-induced Aβ aggregates more compact, especially for the homologous seedings.Isothermal titration calorimetry measurement indicates that hydrophobic interactions mainly contribute to the inhibition of the two Aβ isoforms by EGCG.The findings of this research have provided new insights into Aβ aggregation and the effect of an important inhibitor and the results would benefit in the development of potent inhibitors against co-assembly of different amyloid proteins.
1.Introduction
Proteins are unstable and prone to aggregation that has become a serious problem in biochemical engineering practice, such as recombinant protein expression, purification, and formulation,which lead to the significant decrease in product activity as well as productivity [1].Therefore, inhibition of protein aggregation is an important issue in efficient protein production and application.Besides, the misfolding and aggregation of some proteins also cause a variety of protein-misfolding diseasesin vivo, such as Alzheimer’s disease (AD) [2].The accumulation of β-amyloid (Aβ)aggregates in the brain of patients plays a critical role in the development of AD according to the amyloid hypothesis [3].Misfolded Aβ monomers undergo a preliminary aggregation process to form oligomers,which is the primary nucleation process(corresponding to the lag phase), and then the oligomers form protofibrils and mature fibrils in sequence after further aggregation and maturation processes(corresponding to the growth phase and the plateau phase).Fibrous amyloid accumulated in the brain results in the senile plaque eventually.In this process, protofibrils can act as‘‘seeds” to catalyze the aggregation of monomers in turn, which is known as secondary nucleation [4,5].
The most common isoforms of Aβ include Aβ40and Aβ42.However,several N-terminal and C-terminal truncated Aβ isoforms also exist in the brain of AD patients in addition to the full-length Aβ.It was found that the content of N-terminal truncated peptide Aβ11-40/42in cerebrospinal fluid is equivalent to Aβ42[6-8],accounting for about one-fifth of the senile plaque load[9,10].Barritt showed that Aβ11-40monomers could coaggregate with Aβ40[11].Hao also demonstrated that Aβ11-40seeds accelerated the fibrillation of Aβ40and induced the full-length peptides into more toxic aggregates [12].These results prove that one Aβ isoform can influence the amyloidogenesis kinetics and toxicity of another,suggesting the important role of truncated peptides in the pathological process of AD.
Some Aβ aggregates binding to cell membranes or blood vessel walls act as immobilized‘‘seeds”, which can catalyze the aggregation of monomersin vivo.This process will change the stability and permeability of the membranes [13], causing damage to the cell structure.Therefore, it is necessary to study the aggregation process of Aβ monomers on immobilized seeds.Quartz crystal microbalance with dissipation (QCM-D) is a sensor designed to measure mass and viscoelastic changes on the surface and has been used to study the growth process of amyloid as well as the influence of drugs on fiber growth [13-15].
A large number of studies have been conducted to reduce the level of Aβ in patients to treat and alleviate the development of AD [16].Although several types of inhibitors have entered clinical trials, the effects are not satisfactory due to their poor stability,biocompatibility and blood-brain barrier penetration [17].Moreover, the effects of these inhibitors on the truncated Aβ peptides are often ignored, which may be one of the key factors affecting their clinical results.
Epigallocatechin gallate (EGCG) with three benzene rings and multiple phenolic hydroxyl groups is a widely studied organic molecule inhibitor against full-length Aβ aggregation by hydrophobic interactions and hydrogen bonding [18].Recent studies have shown that EGCG can redirect full-length Aβ species into amorphous and non-toxic aggregates,thereby inhibiting Aβ aggregation or remodeling mature fibrils and reducing the cytotoxicity induced by Aβ aggregates [19,20].In addition, EGCG could chelate transition metal ions (such as Cu (II), Zn (II), and Fe (II)), scavenge radicals, affect cell signal transduction, and mitochondrial function[21-23], which are believed to contribute to alleviating the development of AD.However, the inhibitory effect of EGCG on Nterminal truncated peptide Aβ11-40and on the cross-amyloid aggregation between Aβ40and Aβ11-40in solution and on the surface are unknown.
Herein, different methods were used to explore the coaggregation and seeding interactions between Aβ40and its N-terminal truncated peptide Aβ11-40, as well as the effect of EGCG on these interactions.In this study, we first investigated the aggregation kinetics, morphology, and secondary structure of Aβ40/Aβ11-40aggregated in the absence or presence of EGCG using thioflavin T(ThT) fluorescence assay, atomic force microscopy (AFM), and far-UV circular dichroism spectroscopy (CD).Then the deposition rates and structural changes of Aβ40and Aβ11-40monomers on immobilized homogeneous/heterogeneous seeds on the solid surface were investigated by QCM-D.Finally, the thermodynamic interactions between Aβ40/Aβ11-40and EGCG were analyzed by isothermal titration calorimetry (ITC).This study would provide insights into the rational design of inhibitors that can simultaneously target multiple Aβ isoforms and their heterogeneous aggregates.
2.Materials and Methods
2.1.Materials
Aβ40and Aβ11-40(>95%, lyophilized powder) were obtained from GL Biochem (Shanghai, China).EGCG was obtained from Meilunbio (Liaoning, China).1,1,1,3,3,3-Hexafluoro-2-propanol(HFIP), thioflavin T (ThT) were from Sigma-Aldrich (St.Louis, MO,USA). 11-mercaptoundecanoic acid (MUA) andNhydroxysuccinimide (NHS) were obtained from Dingguo Biotech(Beijing, China).1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) was obtained from Alfa Aesar (Ward Hill,MA,USA).Other chemicals were from local sources with the highest purity.Deionized water was used for all solution preparations.
2.2.Preparation of Aβ monomers
Aβ was treated as described in the literature [24].Aβ40and Aβ11-40were dissolved in HFIP at a concentration of 1.0 mg·ml-1,and left undisturbed for 2 h, followed by ultrasonication for 30 min to destroy the pre-existing Aβ aggregates.Then the vacuum freeze dryer(Labconco,MO)was used to remove HFIP to obtain the lyophilized Aβ which was stored at-20°C.Prior to use,the protein was dissolved in 20 mmol·L-1NaOH,sonicated for 20 min,and then centrifuged at 16,000gfor 30 min at 4°C.Finally,75%of the supernatant was carefully collected and the final concentration of Aβ was adjusted to 25 μmol·L-1by HEPES buffer (30 mmol·L-1HEPES,160 mmol·L-1NaCl, pH 7.4).
2.3.Preparation of Aβ seeds
Aβ40and Aβ11-40monomer solutions were incubated in an air bath with continuous shaking at 150 r·min-1for 48 h at 37 °C and then centrifuged at 16,000gfor 30 min at 4 °C.The concentration of the supernatant was measured with the BCA kit, and the actual content of Aβ aggregates in the precipitate was calculated by subtracting the content of Aβ monomers in the supernatant from that in the initial solutions.Finally,the Aβ aggregates were diluted with HEPES buffer to 10 μmol·L-1to obtain Aβ seeds suspensions.
2.4.Thioflavin T fluorescent assay
In thein situThT assays, the sample of 200 μl containing 25 μmol·L-1Aβ monomers,equimolar ThT and EGCG with or without Aβ seeds was mixed and added to a 96-well plate.Then ThT fluorescence intensities of these samples were monitored by a multifunctional microplate reader(Infinite M200 Pro,TECAN,Salzburg, Austria) at 37 °C with excitation and emission at 440 and 480 nm, respectively.The samples without Aβ monomers were used as blank control.Each set of experiments consisted of three parallel samples, and the data were averaged after measurement.The aggregation kinetic curves were fitted by Eq.(1)

whereyis the fluorescence intensity at timet,ymaxandy0are the maximum and minimum fluorescence intensities, respectively,kis the apparent first-order aggregation constant,andt1/2is the time required to reach half the maximum fluorescence intensity.From the fitting, the lag phase time (Tlag) can be calculated from Eq.(2)

In theex situThT assays, samples without ThT were incubated in an air bath with continuous shaking at 150 r·min-1at 37 °C.The concentrations of Aβ and EGCG were the same as those in the in situ ThT assays.After incubation for 72 h, the samples were then mixed with ThT solution (25 μmol·L-1) in a quartz cell.ThT fluorescence intensity was measured by a fluorescence spectrometer(LS-55,Perking Elmer,USA)with a slit width of 10 nm and excitation and emission at 440 and 480 nm, respectively.The samples without Aβ monomers were used as blank control,and the ThT fluorescence intensity was normalized with Aβ40alone.The data were the average of three repeats.
2.5.Atomic force microscopy
The morphologies of Aβ aggregates formed in the presence or absence of EGCG were observed by AFM (CSPM5500, Benyuan,China).Aβ samples were dripped onto the freshly cleaved mica substrate and then rinsed carefully with deionized water to remove impurities 5 min later.AFM was used to scan at least three independent areas of each sample to detect the morphologies of Aβ aggregates after drying overnight at room temperature.The coverage of immobilized seeds on the solid surface was calculated by ImageJ software [25].
2.6.Circular dichroism spectroscopy
The secondary structures of Aβ aggregates(25 μmol·L-1)formed in the presence or absence of EGCG (25 μmol·L-1) were detected using far-UV CD spectroscopy (JASCO).The samples were placed in a quartz cell with a path length of 1 mm, and the spectra between 260 and 190 nm were continuously recorded at a scanning speed of 100 nm·min-1with a 1 nm bandwidth.Each CD spectrum was a cumulative average of three scans.All spectra were corrected by subtracting the corresponding background (samples without Aβ monomers).
2.7.Quartz crystal microbalance with dissipation monitoring
QCM-D was used to monitor the aggregation processes of Aβ40and Aβ11-40on the sensor surface immobilized homologous/heterologous seeds in the presence or absence of EGCG as described in the literature [26].Firstly, the gold-covered AT-cut quartz chips were successively treated with piranha solution(98% H2SO4/ 30% H2O2at 3:1) for 10 min, 11-MUA / ultrapure ethanol solution (5 mmol·L-1) for 48 h, EDC (0.4 mol·L-1) and NHS(0.1 mol·L-1)mixed solution for 30 min to clean the chips,inoculate and activate the carboxyl groups,respectively.Then the chips were immersed in Aβ40or Aβ11-40seeds solution (10 μmol·L-1) for 12 h,and the remaining activated carboxyl groups were blocked with ethanolamine (1 mol·L-1, pH 8.0) for 1 h.During the measurement process,the chips were first placed in the QCM cell and rinsed with the HEPES buffer until a stable baseline was established.Then Aβ solutions (25 μmol·L-1) with or without EGCG (25 μmol·L-1) were pumped onto the chip surfaces for 30 min, and the resonance frequency (F) and dissipation (D) at the 3rd, 5th, 7th, 9th, and 11th overtone were recorded.The temperature was set at (37±0.05)°C and the flow rate was 50 μl·min-1.Three replicates were set and the data were averaged.In this study, QTools software was used to fit the frequency change (ΔF) and dissipation change(ΔD)at the 3rd,5th,and 7th overtone,and the Kelvin-Voigt model[27]was used to estimate the mass change(Δm)of the sensor surface, as described by Eqs.(3) and (4),
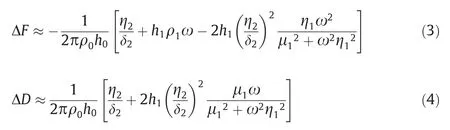
where ρ0is the density of quartz plate andh0is its thickness;ω is the angular frequency; μ1is the elastic shear modulus; η1is the shear viscosity;ρ1is the density;andh1is the thickness of the overlayer; δ2is the density; and η2is the viscosity of bulk fluid.Frequency of overtones 3, 5, and 7 along with dissipation were fitted pointwise using a least-squares algorithm, varying the thickness(h1), shear viscosity (η1), and the shear modulus (ρ1) of the adsorbed layer.These fitted viscoelastic parameters were subsequently recombined to yield the surface mass density Γ (h1ρ1).
2.8.Isothermal titration calorimetry
The thermodynamic interactions between EGCG and Aβ40/Aβ11-40were determined by ITC(TA Instruments,New Castle,DE, USA).The freshly prepared Aβ monomer solution and EGCG solution were degassed for 10 min.Then Aβ monomer solution was loaded to the sample pool and EGCG solution was loaded to the injector carefully.A volume of 2 μl EGCG solution was injected into Aβ solution 15 times at 25 °C with continuous stirring at 125 r·min-1.The dilution heat from the HEPES buffer titrated with EGCG was used as a background to correct all experimental results.The dissociation constant (Kd), binding enthalpy (ΔH), binding entropy (ΔS), and free energy change (ΔG) were obtained from titration data using an independent binding model by NanoAnalyze software.
3.Results and Discussion
3.1.Effects of EGCG on the coaggregation kinetics of Aβ40 and Aβ11-40
Coaggregation interactions of Aβ40with itsN-terminal truncated peptide Aβ11-40have been reported previously, but the research only conducted the aggregation study of an equimolar mixture of the two[11].Actually,the proportion of various Aβ isoform peptides may not be equalin vivo.To explore the aggregation characters of Aβ40and Aβ11-40at different ratios as well as the effect of EGCG on the aggregations, herein, ThT fluorescence assay was used to monitor the aggregation kinetics of mixtures of Aβ40and Aβ11-40in the presence or absence of EGCG (Fig.1).
As shown in Fig.1(A), the aggregation processes of Aβ11-40and its mixtures with Aβ40follow characteristic sigmoidal profiles like Aβ40, indicating that N-terminal truncated peptide Aβ11-40also undergoes a ‘‘nucleation - growth” process.However, Fig.1(A)shows that the lag phase time of pure Aβ11-40is significantly shorter than Aβ40, indicating that Aβ11-40is more aggregationprone than Aβ40.This might be attributed to the charge state and sequence length of Aβ11-40,as the contact frequency between peptides would increase with the decrease of their charge number and sequence length [28,29].Besides, ThT fluorescence intensity of Aβ11-40is much lower than that of Aβ40when the plateau phase is reached, proving that Aβ11-40aggregates contain less β-sheet structures than Aβ40aggregates,consistent with the literature[30].
The single-phasic sigmoidal curves of the mixtures (Fig.1(A))suggest the coaggregation interactions between Aβ40and Aβ11-40,which is consistent with the study of Barritt[11].If one Aβ isoform is in higher concentrations in the mixture (>50%), its aggregation process is similar to that of the high-content Aβ isoform.For example,lag phase time and final ThT fluorescence intensity of the system composed of 80% Aβ40and 20% Aβ11-40are similar to those of pure Aβ40,while the mixed system composed of 20%Aβ40and 80%Aβ11-40shows a trend comparable to pure Aβ11-40.It should be noted that when the molar ratio of Aβ40/Aβ11-40is 1/1,the mixture exhibited the longest lag phase time, implying the nucleation of heterogenous Aβ peptides is suppressed.
Several studies have also shown that the first step in coaggregation interactions between different kinds of amyloid is the formation of hetero-oligomers, such as Aβ40and Aβ42[31], Aβ and islet amyloid polypeptide (IAPP) [32,33], and IAPP and prion protein(PrP) [34].As the ratio of Aβ40and Aβ42could regulate the type of Aβ oligomers formed [35], it can be speculated that when the contents of Aβ40and truncated peptides Aβ11-40in the mixed system are equivalent, namely, when they are neither quantitatively dominant, Aβ40and Aβ11-40would also form hetero-oligomers at the initial stage of aggregation.Moreover, due to the structural incompatibility between Aβ40and Aβ11-40, the formation process of hetero-oligomers is quite slow, thereby resulting in the prolonged nucleation.
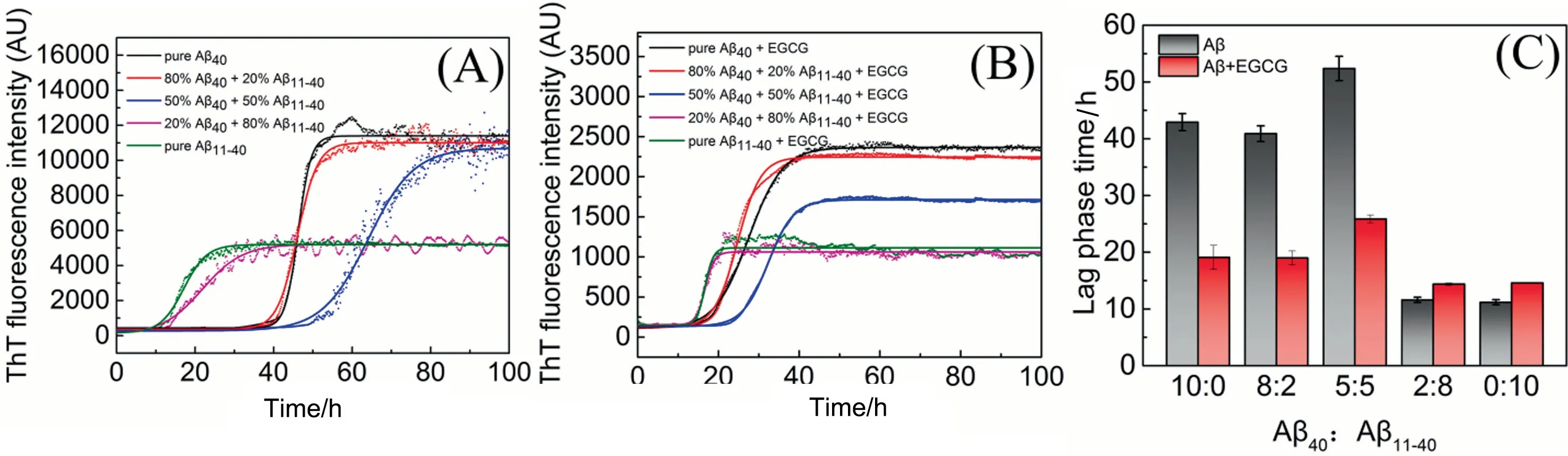
Fig.1. Co-aggregation kinetics of Aβ40 and Aβ11-40 in different ratios(total Aβ concentration is 25 μmol·L-1)incubated(A)without or(B)with 25 μmol·L-1 EGCG measured by ThT fluorescence assays.Kinetic traces of pure Aβ40(black),80%Aβ40 and 20%Aβ11-40(red),50%Aβ40 and 50%Aβ11-40(blue),20%Aβ40 and 80%Aβ11-40(pink)and pure Aβ11-40(green).(C) The lag phase time values of Aβ incubated without (gray) or with (red) 25 μmol·L-1 EGCG.
EGCG had no interference with ThT fluorescence (Fig.S1, Supplementary Material), so in situ ThT assays were employed to investigate the effect of EGCG on the co-aggregation of Aβ40and Aβ11-40.The aggregation kinetics of Aβ incubated with EGCG(Fig.1(B)) show similar trends to those of Aβ without EGCG treatment, but the fluorescence intensities of all five systems remarkably decrease to about 20%.This indicates that the addition of EGCG significantly reduces the β-sheet content of the aggregates.Besides, EGCG exhibits similar inhibitory effects on the formation of β-sheet structures for Aβ40, Aβ11-40, and their mixtures (Fig.1(B)), illustrating that the first ten amino acid residues at the Nterminus of Aβ would not significantly affect the inhibitory effect of EGCG on Aβ aggregation.Similar results are also demonstrated by the ex-situ ThT assay(Fig.S2).It is intriguing to find that EGCG can shorten the lag phase time of Aβ40, but prolong that of Aβ11-40slightly,indicating that EGCG inhibits the aggregation processes of Aβ40and Aβ11-40in different ways (Fig.1(C)).
Since Aβ11-40retains most of the hydrophobic residues and the hydrophobic interactions are the driving force to form aggregates,EGCG shows similar inhibitory effects on the aggregation of Aβ11-40and Aβ40by blocking the hydrophobic interactions between Aβ monomers.The hydrophilic N-terminus of Aβ40, however, also plays a role in the interaction between EGCG and Aβ, thus leading to the different effects of EGCG on the initial nucleation of Aβ11-40and Aβ40.
3.2.Effects of EGCG on the morphology of Aβ40 and Aβ11-40 coaggregates
Fig.2(A1-A5) shows the morphologies of coaggregates of Aβ40and Aβ11-40at different ratios.The morphology of spherical and amorphous aggregates formed by Aβ11-40alone (Fig.2(A5)) is significantly different from the long fibers formed by Aβ40(Fig.2(A1)) [12].Due to the deletion of the N-terminal decapeptide,which is necessary to stabilize the N-terminal interactions and topology of Aβ [36], the electrostatic interactions between Aβ11-40are altered, leading to its different aggregation pathway and morphology.The distinct morphologies formed by Aβ40and Aβ11-40may be one of the reasons for the different effects of EGCG on Aβ aggregation kinetics(Fig.1(B) and 1(C)).In addition,with the proportion of Aβ11-40increased(Fig.2(A2-A4)),the amount of fibrillar aggregates gradually reduced and small amorphous aggregates were observed, indicating that the morphology of Aβ40-Aβ11-40coaggregates is significantly different from the two pure Aβ [11].
When EGCG is added to the systems (Fig.2(B), the fibrous aggregates formed by Aβ40become thinner (Fig.2(B1-B3)), and the granular aggregates formed by Aβ11-40become smaller (Fig.2(B4)and 2(B5)),indicating the aggregations of Aβ40and Aβ11-40are both inhibited by EGCG.
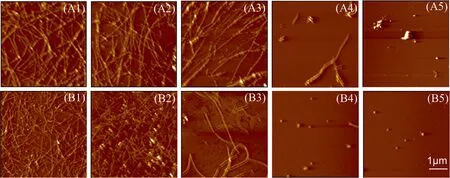
Fig.2. AFM images of Aβ40 and Aβ11-40 co-aggregates in different ratios(total Aβ concentration is 25 μmol·L-1)incubated(A)without or(B)with 25 μmol·L-1 EGCG.(A1,B1)pure Aβ40, (A2, B2) 80% Aβ40 and 20% Aβ11-40, (A3, B3) 50% Aβ40 and 50% Aβ11-40, (A4, B4) 20% Aβ40 and 80% Aβ11-40 and (A5, B5) pure Aβ11-40.
3.3.Effects of EGCG on the secondary structures of Aβ40 and Aβ11-40 coaggregates
Far-UV CD detection was performed to investigate the effect of EGCG on the secondary structures of Aβ40, Aβ11-40, and their mixtures (Fig.3).The CD spectrum of Aβ40alone presents one positive peak before 200 nm and one negative valley around 215 nm(Fig.3(A),black line),confirming the formation of a typical β-sheet-rich structure[37].While the content of β-sheet structures of Aβ11-40significantly reduces, as evidenced by the diminution of negative peak around 215 nm.In the mixed systems of Aβ40and Aβ11-40, the negative peak values around 215 nm decrease with increasing the proportion of Aβ11-40, indicating that the Aβ11-40monomers participate in the aggregation of Aβ40and lead to the formation of coaggregates that contain less β-sheet structures.
From Fig.3(B), it can be seen that EGCG decreases the negative peak values around 215 nm of all samples and reduces the β-sheet structure content of the aggregates,indicating the effective inhibition of EGCG on the aggregation of Aβ40, Aβ11-40, and their coaggregates.
3.4.Effects of EGCG on the seeding and cross-seeding aggregation kinetics of Aβ40/Aβ11-40
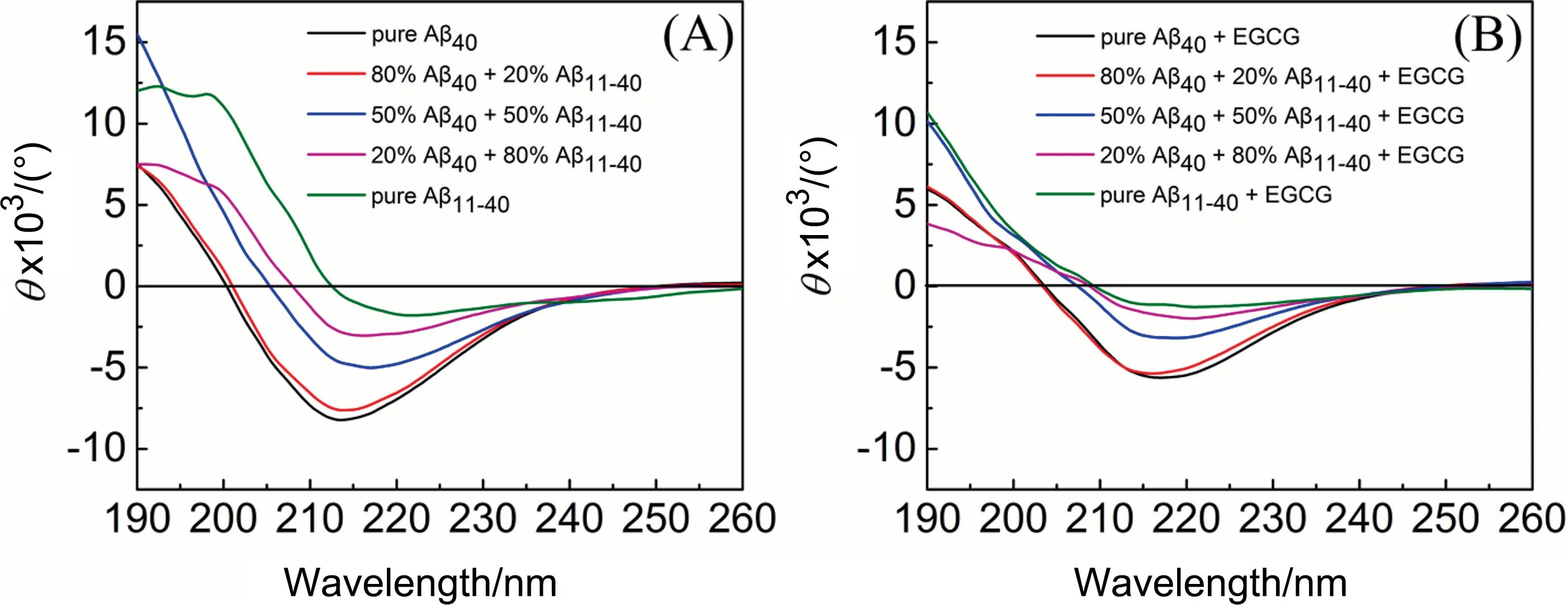
Fig.3. Far-UV circular dichroism spectra of Aβ40 and Aβ11-40 co-aggregates in different ratios (total Aβ concentration is 25 μmol·L-1) incubated (A) without or (B) with 25 μmol·L-1 EGCG.CD spectra of pure Aβ40(black),80%Aβ40 and 20%Aβ11-40(red),50%Aβ40 and 50%Aβ11-40(blue),20%Aβ40 and 80%Aβ11-40(pink)and pure Aβ11-40(green).
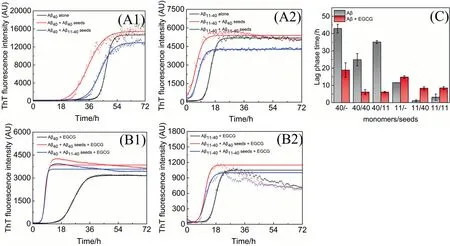
Fig.4. Aggregation kinetics of Aβ incubated without or with 0.5 μmol·L-1 seeds in the(A)absence or(B)presence of 25 μmol·L-1 EGCG measured by ThT fluorescence assays.(A1,B1)Kinetic traces of Aβ40 in the absence(black)or presence of Aβ40 seeds(red),Aβ11-40 seeds(blue).(A2,B2)Kinetic traces of Aβ11-40 in the absence(black)or presence of Aβ40 seeds(red),Aβ11-40 seeds(blue).(C)The lag phase time values of Aβ incubated without(gray)or with(red)EGCG.In the abscissa of(C),‘‘11”stands for Aβ11-40,‘‘40”for Aβ40.The notation 40/11, for example, denotes the lag phase time value of Aβ40 monomers aggregated with Aβ11-40 seeds.
To further explore the influence of EGCG on the aggregation kinetics of Aβ40/Aβ11-40induced by seeding and cross-seeding,ThT fluorescence assay was used to investigate the aggregation kinetics (Fig.4).Fig.4(A1) and (A2) show that Aβ40/Aβ11-40seeds accelerate the aggregation processes of both homologous and heterologous Aβ monomers, and Aβ40seeds exhibit a more efficient influence on accelerating nucleation of both Aβ monomers [12].Moreover,when EGCG is added to the systems,the final ThT fluorescence intensities are all reduced to about 20% (Fig.4(B), indicating that EGCG could not only inhibit the aggregation of Aβ40/Aβ11-40monomers (Fig.1(B), but also inhibit the aggregation of Aβ40/Aβ11-40induced by homologous or heterologous seeds.Similar results are also demonstrated by theex-situThT assay (Fig.S3).Consistent with the results of aggregation kinetics without seeds(Fig.1(C)), EGCG shortens the lag phase time of Aβ40induced by homologous/heterologous seeds, but prolongs that of Aβ11-40(Fig.4(C),indicating that EGCG also inhibits the seeding-induced aggregation of Aβ40/Aβ11-40in different manners.
3.5.Effects of EGCG on the morphology of seeding-induced Aβ40/Aβ11-40 aggregates
Fig.5 shows the morphologies of seeding-induced Aβ40/Aβ11-40aggregates in the absence(Fig.5(A)or presence(Fig.5(B))of EGCG.It was found that both seeds show no significant effects on the morphologies of their homologous Aβ aggregates (Fig.5(A1 and A4).In addition, Aβ40seeds have a higher cross-seeding efficiency than Aβ11-40, because Aβ40seeds alter the appearance of Aβ11-40aggregates into some fibrillar aggregates (Fig.5(A3)), while the Aβ40aggregates induced by Aβ11-40seeds are still fibrous structures(Fig.5(A2)).
It is obvious that EGCG changes the morphologies of the original aggregates, making the fibers finer or the particles smaller (Fig.5(B1-B4)).The results further prove that EGCG not only changes the morphologies of Aβ40and Aβ11-40aggregates (Fig.2(B)), but also shows similar effects on Aβ40/Aβ11-40aggregates induced by homologous or heterologous seeds.
3.6.Effects of EGCG on the secondary structure of seeding-induced Aβ40/Aβ11-40 aggregates
CD spectra of Aβ40and Aβ11-40aggregates induced by homologous or heterologous seeds are shown in Fig.6(A).Negative peak values of seeding-induced Aβ40aggregates around 215 nm are larger than those of Aβ11-40, indicating that the β-sheet structures of Aβ40aggregates formed in the presence of seeds are more than that of Aβ11-40aggregates, which is consistent with the results of ThT fluorescence (Fig.4(A)).In addition, Aβ40aggregates induced by heterologous seeds(Aβ11-40seeds)(Fig.6(A),blue line)contain less β-sheet than pure Aβ40aggregates(Fig.6(A),black line),suggesting the introduction of Aβ11-40seeds altered the structure of Aβ40aggregates and led to the formation of aggregates with less β-sheet.However, after addition of EGCG (Fig.6(B)), negative peak values near 215 nm of all systems significantly decreased, demonstrating that EGCG could inhibit the formation of β-sheet structures of both Aβ40/Aβ11-40formed by seeding and cross-seeding.
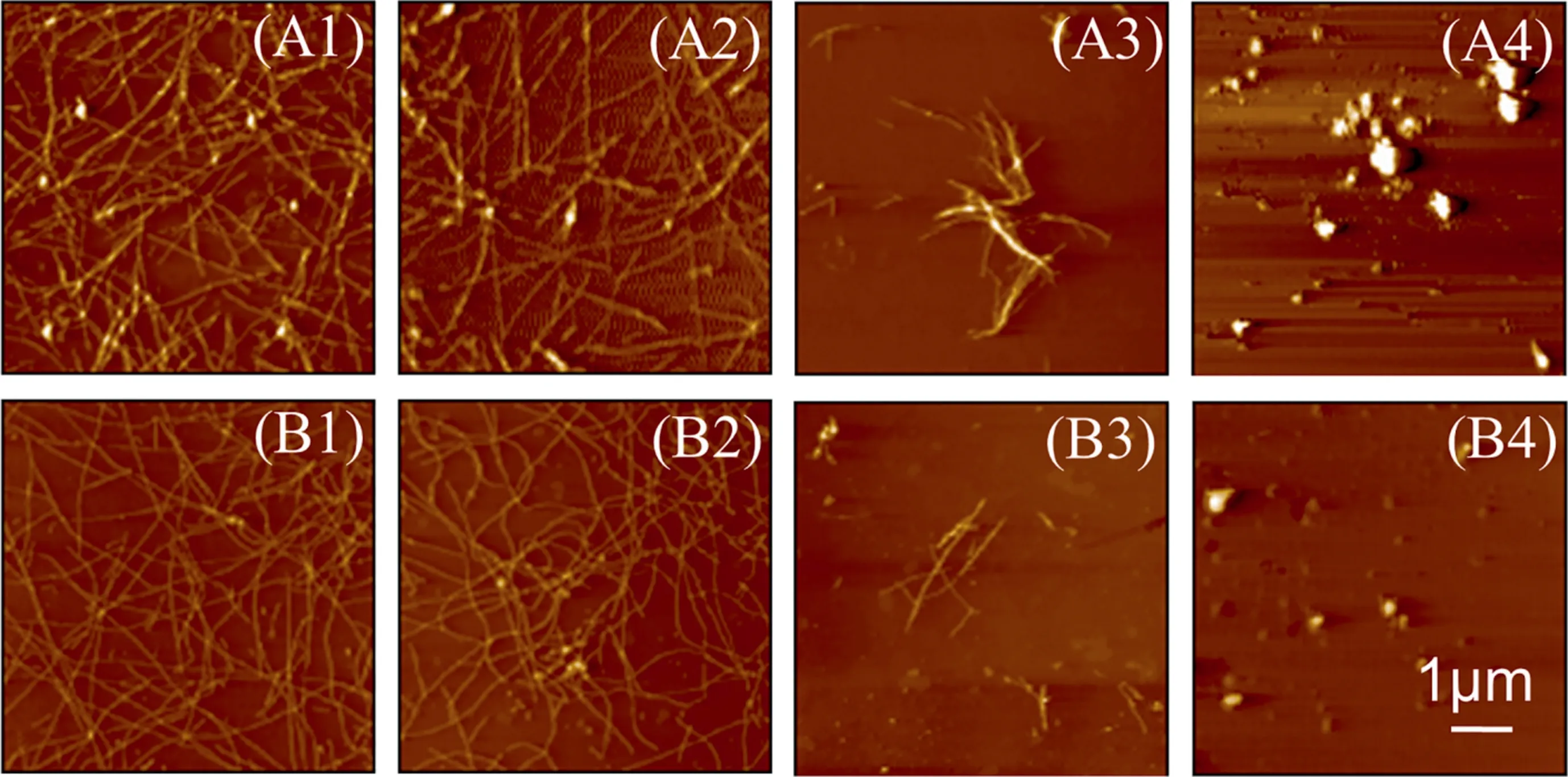
Fig.5. AFM images of Aβ aggregates incubated without or with 0.5 μmol·L-1 seeds in the(A)absence or(B)presence of 25 μmol·L-1 EGCG.(A1,B1)Aβ40 with Aβ40 seeds,(A2,B2) Aβ40 with Aβ11-40 seeds, (A3, B3) Aβ11-40 with Aβ40 seeds, (A4, B4) Aβ11-40 with Aβ11-40 seeds.

Fig.6. Far-UV circular dichroism spectra of Aβ aggregates incubated without or with 0.5 μmol·L-1 seeds in the(A)absence or(B)presence of 25 μmol·L-1 EGCG.CD spectra of Aβ40 alone (black), Aβ40 with Aβ40 seeds (red), Aβ40 with Aβ11-40 seeds (blue), Aβ11-40 alone (pink), Aβ11-40 with Aβ40 seeds (green), Aβ11-40 with Aβ11-40 seeds (purple).
3.7.Effects of EGCG on the deposition of Aβ40/Aβ11-40 monomers on immobilized seeds
The structure of amyloid deposited on immobilized seeds is significantly different from that formed in the solution [14,38].Herein, QCM-D was used to explore the seeding and crossseeding of Aβ40/Aβ11-40in the presence or absence of EGCG on the solid surface.
The morphology of the chip surface modified with Aβ40seeds or Aβ11-40seeds was observed before the QCM assays.As depicted in Fig.S4, fibrous Aβ40seeds (Fig.S4(A) and granular Aβ11-40seeds(Fig.S4(B) are successfully immobilized on the chips.Coverage of immobilized seeds on the solid surface was about 13.8% for Aβ40and 2.3% for Aβ11-40(Fig.S4(C)).To exclude the effect of nonspecific adsorption, frequency variations (ΔF) for Aβ monomers flowing through the surface of untreated chips are shown in Fig.S5 (black line).Then the deposition of Aβ40/Aβ11-40monomers on the fixed seeds was investigated by recording the ΔFand dissipation variation (ΔD).The deposited mass variation during the measurement is further estimated according to the Kelvin-Voigt model [27].In Fig.7, continuous mass increases on the chip surfaces are detected under all experimental conditions, indicating that seeding and cross-seeding interactions of Aβ40/Aβ11-40have occurred on the chip surfaces in the presence or absence of EGCG.The average binding rates of Aβ monomers(with or without EGCG)to Aβ seeds were obtained by calculating the deposition amount of Aβ monomers per unit area per unit time within 30 min operation(Fig.7(C)).It can be seen that the average binding rates of both Aβ40and Aβ11-40monomers on Aβ40seeds are significantly higher than those on Aβ11-40seeds, illustrating that Aβ40seeds exhibit higher efficiency in promoting Aβ40/Aβ11-40aggregation processes,which is consistent with the result of ThT fluorescence assay(Fig.4(A)).
Moreover,EGCG raises the average binding rates of Aβ40monomers on both homologous and heterologous seeds, but brings out opposite effects on Aβ11-40monomers,suggesting that EGCG accelerates the deposition of Aβ40monomers on homologous/heterologous seeds, but inhibits that of Aβ11-40monomers.These findings are consistent with the aggregation kinetics assay (Fig.4(B)),namely, both in the solution and on the solid surface, EGCG promotes the seeding and cross-seeding processes of Aβ40but inhibits the processes of Aβ11-40at the initial nucleation.It is reported that microscopic processes of primary nucleation, elongation, and secondary nucleation affect the lag phase time of amyloid aggregation[39].QCM experiment involves the interactions between Aβ monomers and seeds, which corresponds to the processes of elongation and secondary nucleation.So it is speculated that EGCG could change the lag phase time of Aβ40/Aβ11-40by affecting their elongation and secondary nucleation microscopic processes.
The structural characteristics of the biomacromolecules on the chip surface can be speculated according to the ratio of the energy dissipation variation to the oscillation frequency variation (ΔD/ΔF).A low ΔD/ΔFvalue means no significant viscoelasticity increases during the adsorption process, leading to the compact molecular layer structure; on the contrary, a higher ΔD/ΔFvalue represents a softer and looser structure[26].Fig.8 shows the structural changes on the chip surfaces during binding incidents of Aβ40/Aβ11-40monomers with homologous/heterologous seeds in the presence or absence of EGCG.From the result, Aβ aggregates formed in the presence of EGCG display smaller slopes than those without EGCG,implying the formation of more compact conformation in the four systems [40,41], especially for the homologous seeds-induced Aβ aggregates (Fig.8A and D).These results also correspond to the finding that EGCG makes the fibrous structure of Aβ40thinner and the granular structure of Aβ11-40smaller(Figs.3 and 6).The formation of compact Aβ structures with the EGCG treatment may be the internal mechanism for the antiamyloidogenic reactivity of EGCG towards Aβ [41].
AFM was used to observe the morphological changes after pumping Aβ40/Aβ11-40monomers onto the surface of chips for 30 min(Fig.9).The elongation and the increase in content of fibers are observed on the chips immobilized with Aβ40seeds(Fig.9(A1)and (A3)); uniform filamentous fibers are found around partially immobilized Aβ11-40seeds (Fig.9(A2) and (A4)), corresponding to the growth processes of Aβ40/Aβ11-40monomers on immobilized homologous/heterologous seeds.
Although injections of Aβ monomers could change the morphologies of immobilized seeds (Fig.9(A)), EGCG exhibits little effect on the morphologies of the aggregates formed by Aβ monomers flowing through immobilized seeds(Fig.9(B)),it may be due to the inability of AFM to observe the nanogram-scale-mass variation in the process of QCM assays [42].
3.8.Thermodynamic interactions between EGCG and Aβ40/Aβ11-40
To further determine the driving forces between EGCG and Aβ,the thermodynamic parameters for the interactions between EGCG and Aβ40/Aβ11-40were measured by ITC,a biophysical technique for studying intermolecular interactions (Fig.S6) [43].The selfaggregation of Aβ42had been demonstrated to have little effect on the thermodynamic studies during ITC experiments[18],so it is speculated that the self-aggregation of neither Aβ40nor Aβ11-40whose aggregation tendency is weaker than Aβ42could significantly affect the thermodynamic intereactions between EGCG and Aβ in ITC assays.
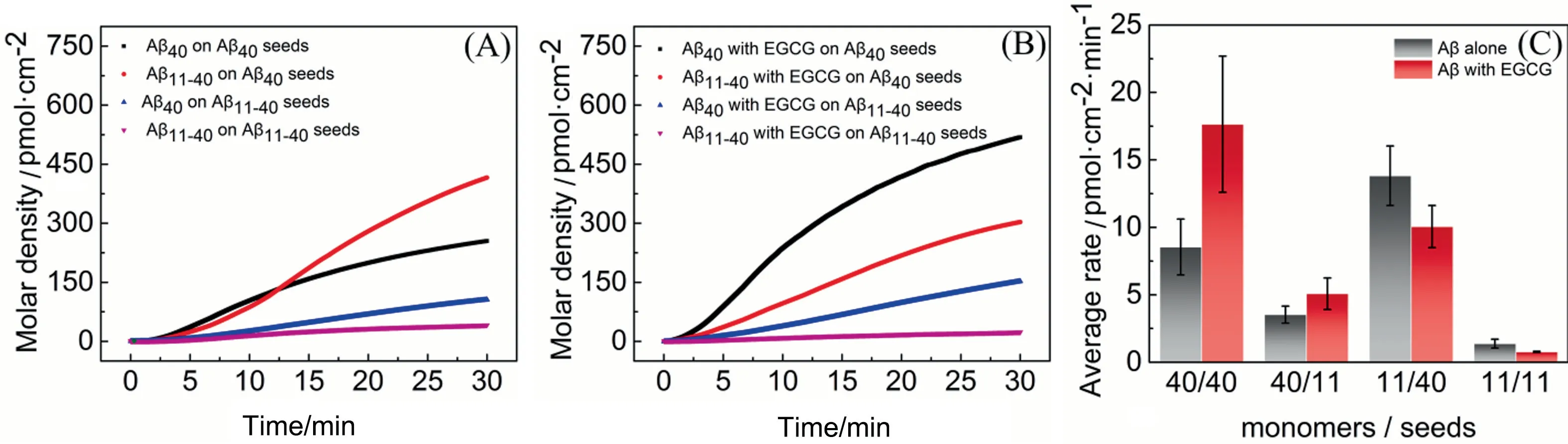
Fig.7. Growth of 25 μmol·L-1 Aβ monomers onto immobilized seeds in the(A)absence or(B)presence of EGCG(25 μmol·L-1).Change of molar density during the adsorption of Aβ40 monomers(black),Aβ11-40 monomers(red)onto immobilized Aβ40 seeds,and Aβ40 monomers(blue),Aβ11-40 monomers(pink)onto immobilized Aβ11-40 seeds.(C)The average adsorption rate during the adsorption of Aβ monomers onto the seeds(black)compared with the conditions in the presence of EGCG(red).In the abscissa of(C),‘‘11”stands for Aβ11-40, ‘‘40” for Aβ40.The notation 40/11, for example, denotes the deposition of Aβ40 monomers onto Aβ11-40 seeds.
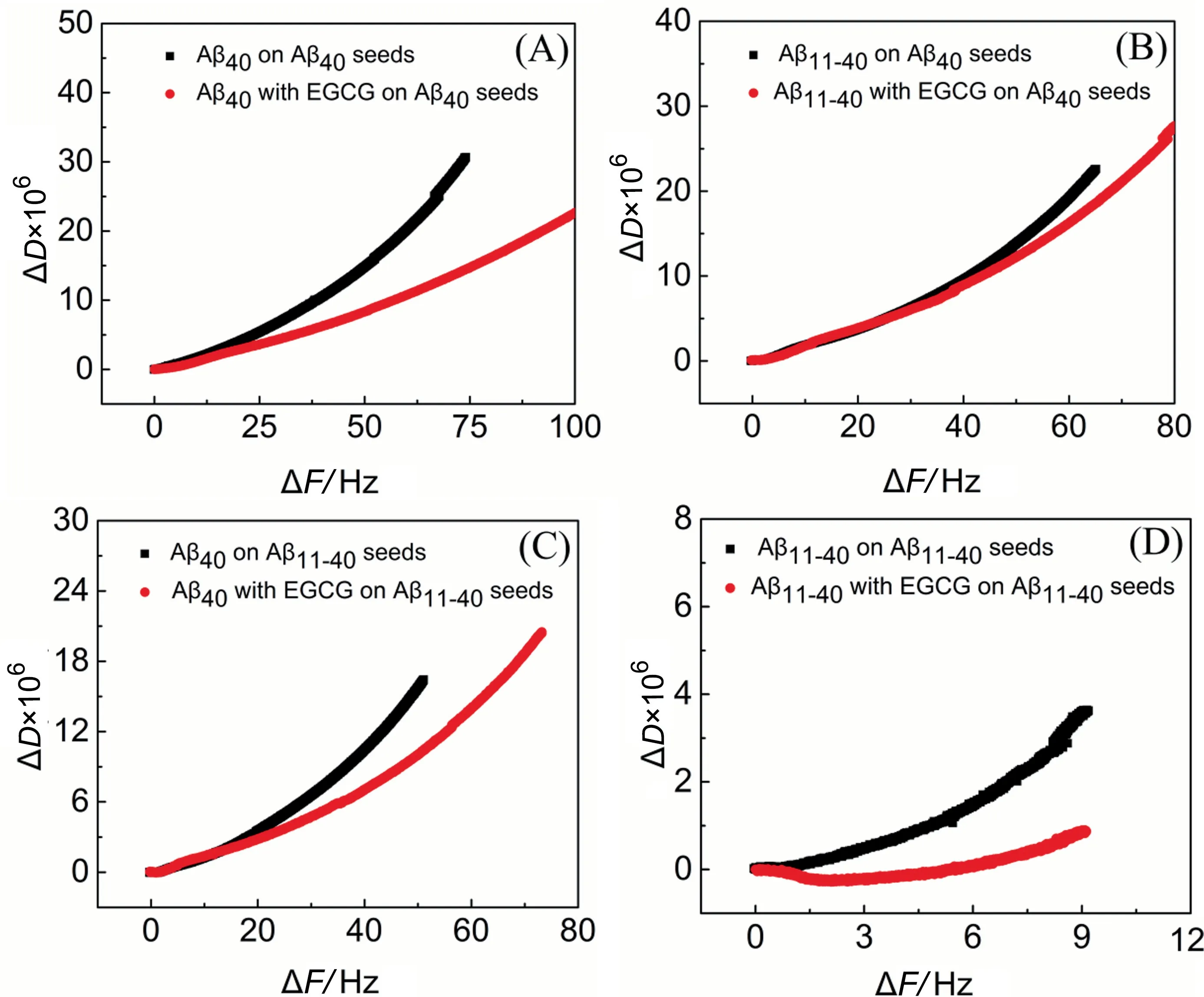
Fig.8. Change of dissipation(ΔD)versus frequency change(ΔF)during the adsorption of Aβ monomers(25 μmol·L-1)without(black)or with(red)25 μmol·L-1 EGCG on Aβ seeds.(A) Aβ40 monomers on immobilized Aβ40 seeds.(B) Aβ11-40 monomers on immobilized Aβ40 seeds.(C) Aβ40 monomers on immobilized Aβ11-40 seeds.(D) Aβ11-40 monomers on immobilized Aβ11-40 seeds.

Fig.9. AFM images of the resultant structure after adsorption of Aβ40 monomers on the chips immobilized with(A1)Aβ40 seeds,(A2)Aβ11-40 seeds,and Aβ11-40 monomers on the chips immobilized with(A3)Aβ40 seeds,(A4)Aβ11-40 seeds;AFM images of the resultant structure after adsorption of Aβ40 monomers with EGCG on the chips immobilized with (B1) Aβ40 seeds, (B2) Aβ11-40 seeds, and Aβ11-40 monomers with EGCG on the chips immobilized with (B3) Aβ40 seeds, (B4) Aβ11-40 seeds.
The fitting results of thermodynamic experiments are shown in Table 1.The negative ΔGaccounts for spontaneous binding between EGCG and Aβ40/Aβ11-40, however, the smaller values ofKdand ΔGof EGCG binding to Aβ11-40indicate that EGCG has a higher binding affinity to Aβ11-40than Aβ40.According to thereports, hydrogen bonding leads to negative ΔHand negative ΔS,while hydrophobic interactions lead to positive ΔHand positive ΔS[44,45].The negative ΔHand positiveTΔS(Table 1) indicate that EGCG binds to Aβ40and Aβ11-40with favorable enthalpy and entropy contributions.That is, both hydrogen bonding and hydrophobic interactions contributed to the binding events.In detail, it can be speculated that hydrophobic interactions are the predominant contributors to the binding of EGCG-Aβ by comparing the values of ΔHandTΔS, and hydrophobic interactions play a more important role in the binding of EGCG to Aβ11-40than Aβ40,further proving the importance of hydrophobic interactions for the binding of EGCG to Aβ [46,47].
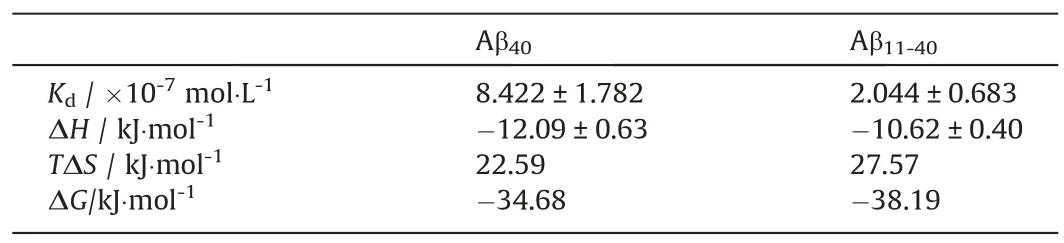
Table 1Thermodynamic parameters for binding of EGCG to Aβ40 and Aβ11-40
4.Conclusions
The coaggregation and seeding interactions between Aβ40and Aβ11-40as well as the effect of EGCG on the cross-amyloid aggregation were investigated in this study.The results demonstrate that Aβ11-40monomers could coaggregate with Aβ40, and reduce the β-sheet structures of the coaggregates in a dose-dependent manner, altering the original aggregation pathway of Aβ40.The aggregation kinetics and β-sheet structure content of the coaggregates are similar to those of the dominated Aβ isoform.When the contents of two Aβ isoforms are equivalent,the aggregation lag phase time is significantly prolonged because of the structural incompatibility between Aβ40and Aβ11-40at the early stage of nucleation.
EGCG shortens the aggregation lag phase time of Aβ40by promoting the elongation and secondary nucleation processes, but prolongs that of Aβ11-40by inhibiting its elongation and secondary nucleation both in solution and on the solid surface.Besides, the conformations of Aβ40/Aβ11-40aggregates induced by seeds (especially for homologous seeds)are more compact with the treatment of EGCG than those of EGCG-untreated Aβ species.Moreover,EGCG shows a higher binding affinity to Aβ11-40and exhibits stronger hydrophobic interactions with Aβ11-40than Aβ40, which probably leads to different behaviors of EGCG towards Aβ40and Aβ11-40.These findings may provide clues for the design of inhibitors against amyloid aggregation and give insights into the modulation of other protein folding and aggregation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by the National Natural Science Foundation of China (21978207 and 21621004) and the Natural Science Foundation of Tianjin from Tianjin Municipal Science and Technology Commission (19JCZDJC36800).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.04.018.
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Notes for Contributors
- Solubility determination and thermodynamic modeling of bis-2-hydroxyethyl terephthalate (BHET) in different solvents
- Numerical study on hydrodynamic characteristics of spherical bubble contaminated by surfactants under higher Reynolds numbers
- Spray-drying assisted layer-structured H2TiO3 ion sieve synthesis and lithium adsorption performance
- Innovative hydrophobic/hydrophilic perfluoropolyether (PFPE)/polyvinylidene fluoride (PVDF) composite membrane for vacuum membrane distillation
- Fabrication and characterization of hierarchical porous Ni2+ doped hydroxyapatite microspheres and their enhanced protein adsorption capacity
