Fabrication and characterization of hierarchical porous Ni2+ doped hydroxyapatite microspheres and their enhanced protein adsorption capacity
Yaling Li, Hao Ai, Liangzhi Qiao, Yinghong Wang, Kaifeng Du
Department of Pharmaceutical & Biological Engineering, School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Keywords:Hierarchical porous hydroxyapatite microspheres Templates Metal doping Proteins adsorption
ABSTRACT Hydroxyapatite (HAP) is a common bio-adsorbent, which performance depends heavily upon its morphology and microporous structure.In this study,a novel synthesis strategy was proposed for hierarchical porous HAP microspheres by a simple ‘‘one-pot” hydrothermal reaction.In the strategy, L-glutamic acid serves as soft template to modulate the morphology and inner crystalline of HAP.To evaluate the application potential,doping Ni2+on hierarchical porous HAP microspheres gives metal chelated affinity adsorbents.The prepared adsorbents show a perfect spherical shape,particles size of 96.6 μm,relatively specific surface area of 48.5 m2·g-1 and hierarchical pores(mesopores:4 nm and macropores:53 nm).By the adsorption evaluation, it reveals that the Ni2+-HAP adsorbents have high adsorption capacities of 275.11 and 97.55 m2·g-1 for hemoglobin and bovine serum albumin, respectively, which is comparable to other similar adsorbent.Therefore,this work provides a promising method for high-efficiency hydroxyapatite microspheres for proteins purification.
1.Introduction
Over the past decades, biopharmaceuticals, including proteins,monoclonal antibodies and nucleic acids have offered great highefficiency and flexibility in clinical treatments, such as insulin for diabetes, antibody protein for tumors and nucleic acid testing for virus.High purity of biopharmaceutical is a pre-requisite for the above applications.In order to meet the high-quality requirements,a series of downstream procedures have been necessarily applied including salting out, organic precipitant, dialysis, ultra-filtration and centrifugation,which account for 80%-90%of total production cost [1-3].Therefore, biotechnological and pharmaceutical industries have devoted great efforts to promote the downstream purification and separation techniques to obtain high-quality products[4,5].Among all available techniques,the column chromatography has been widely used for the separation and purification of biopharmaceuticals because of its high resolution, high separation efficiency and large adsorption capacity [6-8].To date, various chromatographic media have been developed according to different separation mechanisms, including size exclusion,hydrophobic interaction, ion-exchange, and affinity adsorption.Among these media, immobilized metal affinity chromatography (IMAC) has attracted considerable attention and has been applied most widely due to high resolution,high capacity,and high selectivity[9,10].In general, the IMAC media are composed of chelated metal ions(such as Cu2+, Ni2+, and Zn2+) and solid support (such as organic polymer, silica, agarose and cryogel) [11-13].IMAC coordinates with an exposed electron donor for high selectivity,especially histidine residues on surface of proteins.Moreover,IMAC exhibits the advantage of high recovery yield due to the use of mild, nondenaturing elution conditions by comparing it to other chromatographic methods[14-16].Despite these advantages as a chromatographic technique, current IMAC has low binding capacity due to the lower available surface area,which hinders its implementation in larger-scale production units.
Hydroxyapatite (Ca10(PO4)6(OH)2) is a biocompatible ceramic compound and is highly insoluble in water.Its unique crystal structure and chemical composition confer high crystal lattice flexibility and consequent capacity to exchange the OH-,PO43-and Ca2+ions for accommodating anions and cations with different sizes and charges [17].Considering the ability to incorporate heavy metal ions, hydroxyapatite has been extensively used as adsorbents for the chelation of heavy metals and further purification of proteins and nucleic acids in medicine field[18].Previous studies have been demonstrated that metal ion-loaded hydroxyapatite possessed high selectivity for target proteins, especially histidinerich proteins.Thus, hydroxyapatite-based adsorbents have been put forward and drawn great attention as chromatographic media for affinity purification [19,20].For example, Duet al.synthesized Cu2+chelated cellulose/magnetic hydroxyapatite particles hybrid beads, which possessed high specific adsorption capacity of 4533.1 mg·g-1for histidine-rich proteins[21].Chenet al.prepared an Ag-HAP nanoparticles (NPs) adsorbent, which showed high adsorption capacity of two blood plasma proteins, human serum albumin (HSA) and fibrinogen (Fib) [7].Dasguptaet al.incorporated hydroxyapatite nanoparticles with Zn2+and Mg2+for BSA uptake in phosphate buffer solution [22].However, recent reports suggested that hydroxyapatite adsorbents have low specific surface area and small pore size, which compromised the adsorption efficiency.It is well-known that porous structure at submicrometer scale can render macroscopic objects with unique properties, including similarity with biological interface, permeability and extremely large surface area for adsorption.From this view, it has attracted great effort for the synthesis of hydroxyapatite adsorbents.
In this work, we introduced a simple ‘‘one-pot” hydrothermal synthesis to fabricate the novel HAP microspheres,which featured with macropores and mesopores and was suitable for protein purification.In preparation process, the L-glutamic acid (L-Glu)served as a key point in regulating the structural and surface property of final HAP microspheres and the added urea directly generated the mesopore structure to provide more adsorption sites for proteins adsorption.Following by doping Ni2+ions on HAP surface(HAP@Ni), the adsorption and desorption behavior of hemoglobin(Hb)and bovine serum albumin(BSA)were systematically investigated by comparison with pure HAP microspheres, which demonstrated that Ni2+doping on HAP microspheres efficiently improved the proteins adsorption capacity.The adsorption investigations proved that Ni2+doped HAP microspheres exhibited great potential in protein separation.
2.Experimental
2.1.Chemicals
L-Glu, urea(CO(NH2)2) and nickel sulfate hexahydrate (NiSO4-·6H2O) were purchased from Aladdin industrial corporation(Shanghai, China).Hb and BSA were obtained from Sigma (MO,USA).Calcium nitrate tetrahydrate(Ca(NO3)2·4H2O),diammonium hydrogen phosphate ((NH4)2HPO4) and nitric acid (HNO3) were obtained from Kelong Chemical (Chengdu, China).All chemical agents listed above were of analytical grade.
2.2.Preparation of porous hydroxyapatite microspheres
In a typical experiment, 1.5 g of Ca(NO3)2·4H2O and 0.6 g of(NH4)2HPO4were well dissolved in 40 ml of deionized water and then added 4 g of CO(NH2)2under magnetic stirring.Afterwards,the pH value of the resulting solution was adjusted by 5% (mass)HNO3and 3% (mass) L-Glu was added to the solution under magnetic stirring for 1 h.Subsequently,the obtained precursor solution was transferred to Teflon-lined stainless steel autoclave and heated to 190°C for 10 h.Finally,the products were cooled down to room temperature and washed by ethanol and deionized water for 3 times, and dried in a vacuum oven for 24 h.
2.3.Characterization
The microscopic morphology of HAP microspheres was investigated by scanning electron microscopic (SEM, XL30, Philips,Netherlands).The chemical property of microspheres was analyzed by Fourier transform infrared (FT-IR, Nicolet iS5, Thermo Fisher, USA) recorded with KBr pellets in the region of 4000-500 cm-1and resolution of 0.09 cm-1.The powder X-ray diffraction (XRD) patterns of the samples were analyzed with X-ray diffraction (D/max-2550 pc, Rigaku, Japan) with a scan range between 5° and 60°.The pore size distribution and specific surface area were determined by N2isotherm adsorption (ASAP 2460, Micromeritics, USA).The surface electronic state and surface atom of the samples were analyzed by X-ray photoelectron spectroscopy (XPS, AXIS Supra, Shimadzu Kratos, Japan).To evaluate the hydrodynamic property of the samples, the HAP microspheres were uploaded in chromatography column (Φ 4.6 mm×150 mm, Shimadzu, Japan) and the back pressure experiment was detected by HPLC system using water, ethanol and tetrahydrofuran (THF) as mobile phase.The hydraulic permeability was calculated by Darcy’s equation:

where Δpstands for back pressure,Pa;Lis the length of column,m;u, m·s-1and μ, Pa·s are the velocity and viscosity of the mobile phase, respectively.B0means the hydraulic permeability, m2.
2.4.Adsorption experiments
Initially,the doping of Ni2+ions on HAP microspheres were performed under different pH values, adsorption times and different initial concentrations to confirm the maximum adsorption capacity.In brief, for the pH investigation, 50 mg of HAP microspheres were dispersed in 100 ml of 1000 mg·L-1NiSO4·6H2O by adjusting the solution pH values from 3.0 to 8.0 in centrifuge tubes, each experiment last for 4 h and the supernatant liquid was determined by atomic absorption spectrophotometer (AAS, AA6880,Shimadzu), the initial pH values were adjusted by diluted 0.1 mol·L-1HNO3and/or NaOH solution [23].For the analysis of adsorption equilibrium isotherm,50 mg of HAP microspheres were dispersed in 100 ml metal ions solutions in varied initial concentrations of 5-300 mg·L-1Ni2+and the adsorption time last for 4 h,the residual liquid was disposed by the method above.Subsequently, Langmuir (Eq.(2)) and Freundlich adsorption isotherms(Eq.(3)) were applied to analyze the experimental data.For the analysis of adsorption kinetics, 50 mg of HAP microspheres were dispersed in 100 ml of 1000 mg·L-1Ni2+solutions with time intervals ranging from 5 to 400 min and the experimental results were fitted by pseudo-first-order equation(Eq.(4))and pseudo-secondorder equation(Eq.(5))to research the dynamic adsorption behavior, respectively.
For the proteins adsorption experiments,100 ml of 4000 mg·L-1Hb and BSA solutions were dissolved in deionized water and the pH values were adjusted by adding 0.1 mol·L-1diluted HNO3ranged from 4.0 to 8.0, next, 50 mg of HAP@Ni and HAP adsorbents were separately added and the adsorption lasted for 120 min.For the adsorption isotherms,100 ml of different initial concentrations of protein solutions ranged from 0.5 to 4.0 mg·L-1of Hb and 0.2 to 1.6 mg·L-1of BSA were transferred to centrifugal tubes by adding 50 mg of HAP@Ni and HAP adsorbents and shook for 120 min respectively.For the adsorption kinetics, 100 ml of 1000 mg·L-1Hb and BSA solutions were added 50 mg of HAP@Ni and HAP adsorbents lasted for 20-160 min.The residual solutions were determined by Ultraviolet-visible spectrophotometer (UV-VIS/NIR, Alpha-1900S, LASPEC, China).
After the adsorption experiments, the adsorption capacity of Ni2+and proteins were calculated by the following (Eq.(6)).Each experiment was performed for three times and the mean value was taken for calculation in this study.

whereCe, mg·L-1andqe, mg·g-1represent the equilibrium concentration and saturated adsorption capacity at a certain initial concentration, respectively.qmrepresents the maximal adsorption capacity, mg·g-1;KLis Langmuir adsorption constant related to the affinity of binding sites,L·mg-1;KFis the characteristic constant related to the adsorption capacity, mg1-1/n·L1/n·g-1;nis a constant related to the adsorption intensity.
Pseudo-first-order equation:

Pseudo-second-order equation:

whereqeandqtrepresent the adsorption capacity at equilibrium and at a real time, mg·g-1;k1, min-1andk2, g·mg-1·min-1are the rate constants of adsorption.

whereqeis equilibrium adsorption capacity, mg·g-1;C0andCeare initial and equilibrium concentrations in solution, mg·L-1;Vis the volume of adsorbed solution, L;Mis the mass of dried HAP or HAP@Ni microspheres, g.
3.Results and Discussion
3.1.Characterization of HAP microspheres
Fig.1 shows the whole preparation process of HAP microspheres (HAP) with hierarchical multi pores by a simple one-step hydrothermal method.Firstly, adequate amounts of Ca(NO3)2·4H2O and(NH4)2HPO4are mixed and followed by adding a certain quantity of L-Glu and urea as the templates and alkali source,respectively.For a further interpretation, a local highly negative saturated region is initially formed by the electrostatic interaction between L-Glu and phosphate anions, which is beneficial to decrease the nuclear crystalline energy and promote the growth of crystal.Furthermore, the phosphate ions reveal a regular tetrahedron structure, which resembles to the amino groups and primarily endows the phosphate anions more available to gather around.After that, the ACP, the precursor of HAP, is generated when solution pH value is below 6.0.Through the decomposition of urea,the ACP gradually transforms into HAP crystals as solution pH value approaches 8.0 along theC-axis.Meanwhile, the mesopores are formed by releasing of CO2,which provides more adsorption sites in this step.Subsequently,the HAP crystals crystallize out as the solution reaches the critical concentration before ACP exhausts.Finally, the growth of HAP microspheres terminate after concentration of ACP decreases below the critical concentration,by which the macropores are formed by the accumulation of HAP crystals [24-26].
Fig.2 shows the external morphology of HAP microspheres at various stages.In the preparing process, both L-Glu content and reaction time were investigated to explore the optimum experimental conditions.Among them, L-Glu content mainly affects the crystallinity and morphology and crystal time directly regulates the growth of HAP microspheres.In detail, the morphology of HAP microspheres ranges gradually from an irregular HAP product(0%(mass),Fig.2(a))to regular spherical microspheres(3%(mass),Fig.2(c)), which implies a more favorable crystal growth.It found that the HAP crystals mainly alongC-axis without the addition of L-Glu.When adequate amounts of L-Glu templates are added, the crystal growth tendency along withC-axis is weakened which is ascribed to the lower crystalline nuclear energy around amino groups, which results in higher crystal efficiency by lamellar selfassembly.Finally, the SEM image reveals that the obtained HAP microspheres exhibit porous structure and excellent sphericity with an average diameter of about 96.6 μm.Moreover, the porous structure of HAP is easily observed with the diameter of 1-2 μm,which is beneficial to reduce the mass transfer limitation in adsorption.In addition, as the reaction is carried out in different time,the morphology of HAP microspheres ranged from a petaloid surface (Fig.2(d)) to an accumulated surface (Fig.2(f)) with the diameter increased from 20.0 to 96.6 μm.These figures indicated that the conversion of ACP to HAP has terminated after 10 h and the fabricated HAP microspheres have good sphericity in diameter.
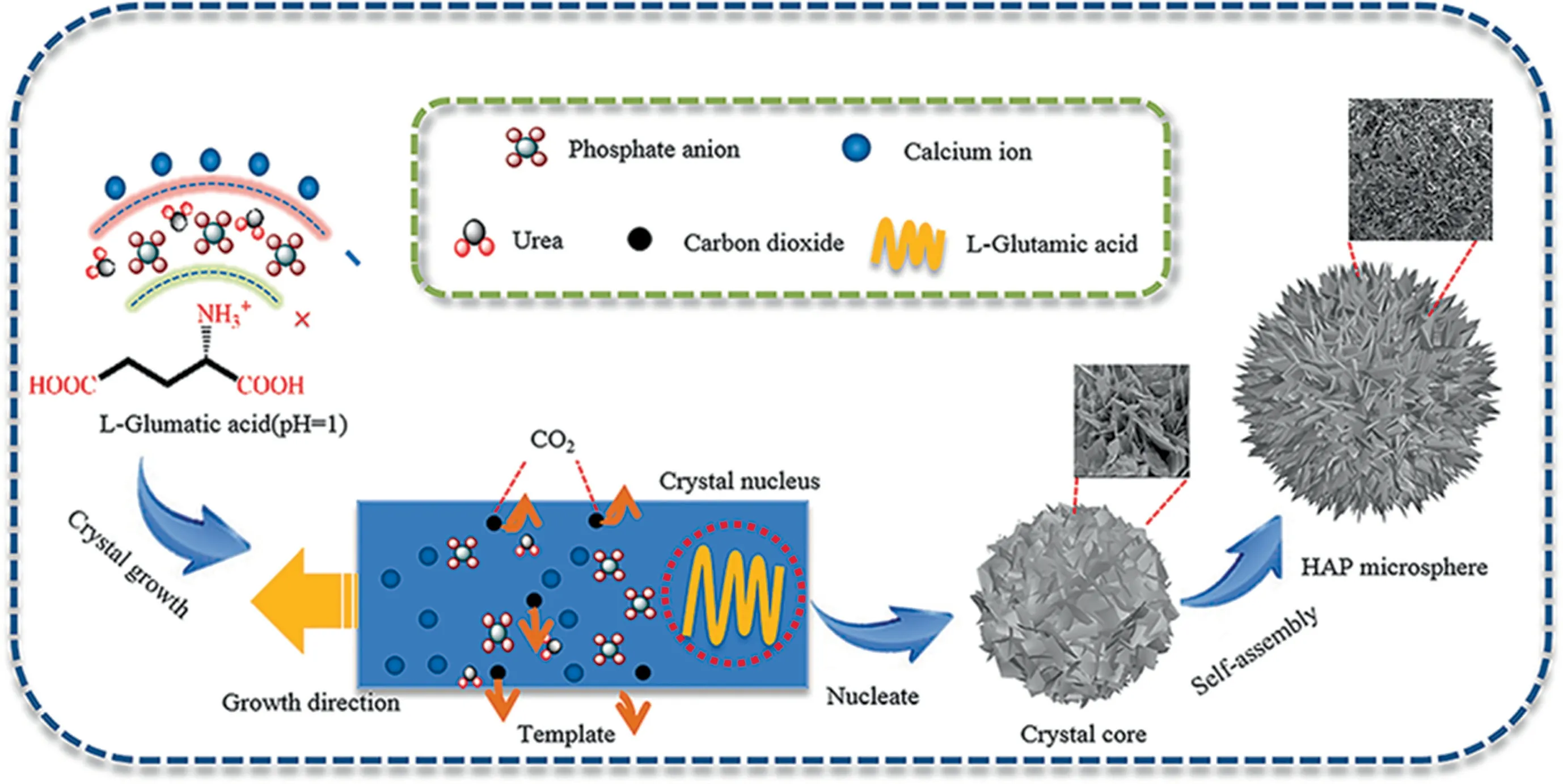
Fig.1. Schematic illustration of the growth of HAP microspheres.
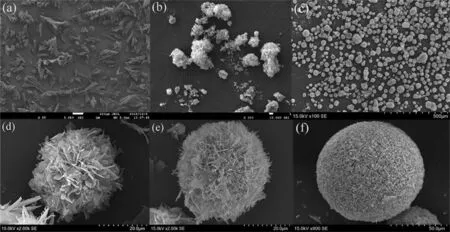
Fig.2. SEM images of HAP microspheres fabricated by varied quantities of L-Glu 0% (mass) (a), 1% (mass) (b), 3% (mass) (c) and ripening time (6 h (d), 8 h (e), 10 h (f)).
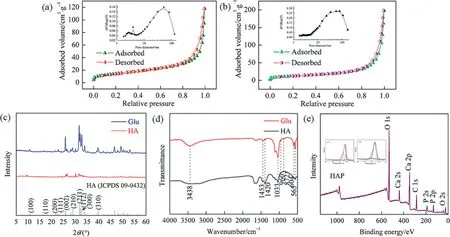
Fig.3. Physical characterizations of HAP microspheres: N2 adsorption isotherms with L-Glu (a), no template (b), XRD spectrum (c), FT-IR spectra (d) and XPS analysis (e).
Fig.3 shows the chemical and physical properties of HAP microspheres.In the Fig.3(a), N2adsorption experiments were carried out to determine the specific surface area and pore size distribution of the fabricated HAP microspheres and the corresponding parameters were shown in Table1.It found that the HAP microspheres exhibit a typical type IV isotherm with an H1 type hysteresis loop according to the IUPAC classification,and the insert image reveals that the HAP microspheres contain a hierarchical porous structure of mesopore (4 nm) and macropore (53 nm).The meso-pores are generated due to the releasing of CO2of the decomposition of urea in the hydrothermal reaction process and the macropores are derived from the aggregation of HAP crystallines in the crystal growth.However, without the templates, only the macropores existed in size of about 50 nm (Fig.3(b)).The specific surface area of HAP microspheres was determined to be 48.50 m2·g-1for L-Glu and 40.01 m2·g-1for the blank sample by Brunauer-Emmett-Teller (BET) model.And the high specific surface area endows the adsorbents with higher adsorption capacity and adsorption efficiency for heavy metal ions.

Table 1Parameters of HAP microspheres analyzed by N2 adsorption isotherms
As afore mentioned that, the direct factor for regulating the morphology and diameter of HAP microspheres might be ascribed to the crystallinity.Therefore, a series of XRD spectrum analysis were carried out and the results were shown in Fig.3(c).As the figure shows, all the location of diffraction peaks had no significant changes by comparison with the blank sample(HA).Among them,(002),(211)and(112)were the main diffraction peaks,which were confirmed by the appearance of additional peaks in the XRD pattern (JCPDS 09-0432).Meanwhile, the intensity of the diffraction peak at (002) crystal face for 2θ=25.9° was higher than other peaks, which further proved that the growth of HAP crystal was more favorable to along with theC-axis.Compared with the blank sample (HA), it was found that the characteristic peaks of XRD spectra with L-Glu as template were higher and sharper,indicating that the prepared microspheres have better crystallinity.This is because without the templates the mixture of calcium hydrogen phosphate(DCP)and HAP results into low crystallinity.In contrary,the L-Glu serves as a template to form a localized highly positive saturated region on products at the initial stage of the reaction,which is conducive to reduce the nucleation energy of the crystal and then increase the crystallinity.So, with, a single-phase HAP is obtained with L-Gluas template and shows better crystallinity.
Fourier transform infrared (FT-IR) spectrometry was applied to evaluate the chemical composition of the prepared HAP microspheres.As shown in Fig.3(d), the sharp peaks at 565 and 604 cm-1were ascribed to the υ4bending vibration of O-P-O, respectively.The weaker peak at 960 cm-1was ascribed to the υ1stretching vibration of O-P, and the peaks at 1033 and 1093 cm-1were ascribed to the υ3vibration of O-P bond.All these results indicated that the existence of PO43-[23].For the L-Glu as template,the broad peak at 3438 cm-1was ascribed to the stretching vibration of O-H,the split absorption peaks at 1420 and 1453 cm-1were ascribed to the asymmetric stretching vibration of CO32-[27].Traditionally,there are two kinds of substitution forms of CO32-when CO32-entered into HAP crystal-type A substitution (CO32-substituted OH-) or type B substitution (CO32-substituted PO43-).According to the location of peaks, it was classified to Type B substitution, that was, a certain amount of CO32-entered as a substitute for PO43-in the HAP crystal [28].The results showed that the fabricated HAP microspheres using L-Glu as template were partially carbonated hydroxyapatite and without other impurities.
As is shown in Fig.3(e),the HAP microspheres were analyzed by XPS to study the surface chemical composition.From this figure,there were three binding energy sites at 532.1, 347.3 and 133.1 eV, which indicated the elements of O, Ca and P elements in the samples, respectively.High resolution scanning XPS spectra of the O 1 s and P 2p lines were shown in the inserted images.Among them, the O 1s core energy peak at 532.1 eV was attributed to the O-H group on the surface of HAP,and the P 2p core energy peak at 133.1 eV was caused by the P-O bond in PO43-.And the calcium on the surface of the sample existed in two forms with binding energies of 347.3 and 350.7 eV.The XPS characterization expressly revealed the elementary composition of HAP microspheres, which further ensured the pure phase of hydroxyapatite.
To investigate the flow hydrodynamic property,the HAP microspheres were loaded in chromatographic column and measured by a HPLC system,by which the permeability of the HAP microspheres was calculated by Darcy’s equation and the corresponding results were listed in Table 2.For the mobile phase, water, THF and ethanol were separately induced to the flow process in an extraordinary experiment as shown in Fig.4.Apparently, the results showed that the back pressure had a good linear relationship along with the increase of flow rate with different mobile phases.By comparing the results of the bed permeability such as organicpolymerization monolithic column [13], porous cellulose microspheres [29]and agarose microspheres [30], the prepared HAP microspheres exhibited higher hydraulic fluidity.This is mainly due to the formation of interconnected macropores within HAP microspheres, which reduced the fluid resistance and increased the column permeability to a certain degree.It is expected that the high permeability would save the energy consumption in the separation process.In summary, the flow hydrodynamic results show the high permeability, good mechanical strength of HAP microspheres.Given the merits,the HAP microspheres are considered as an kind of promising candidate for high efficient adsorption.
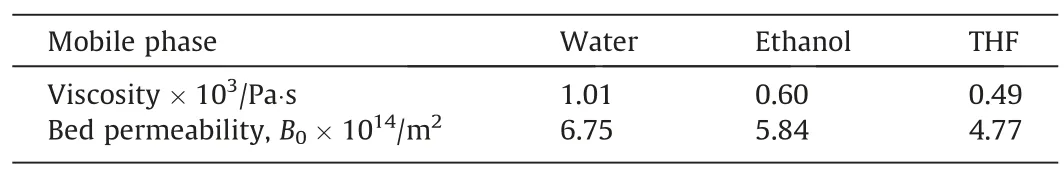
Table 2Column permeability of HAP microspheres with different mobile phases
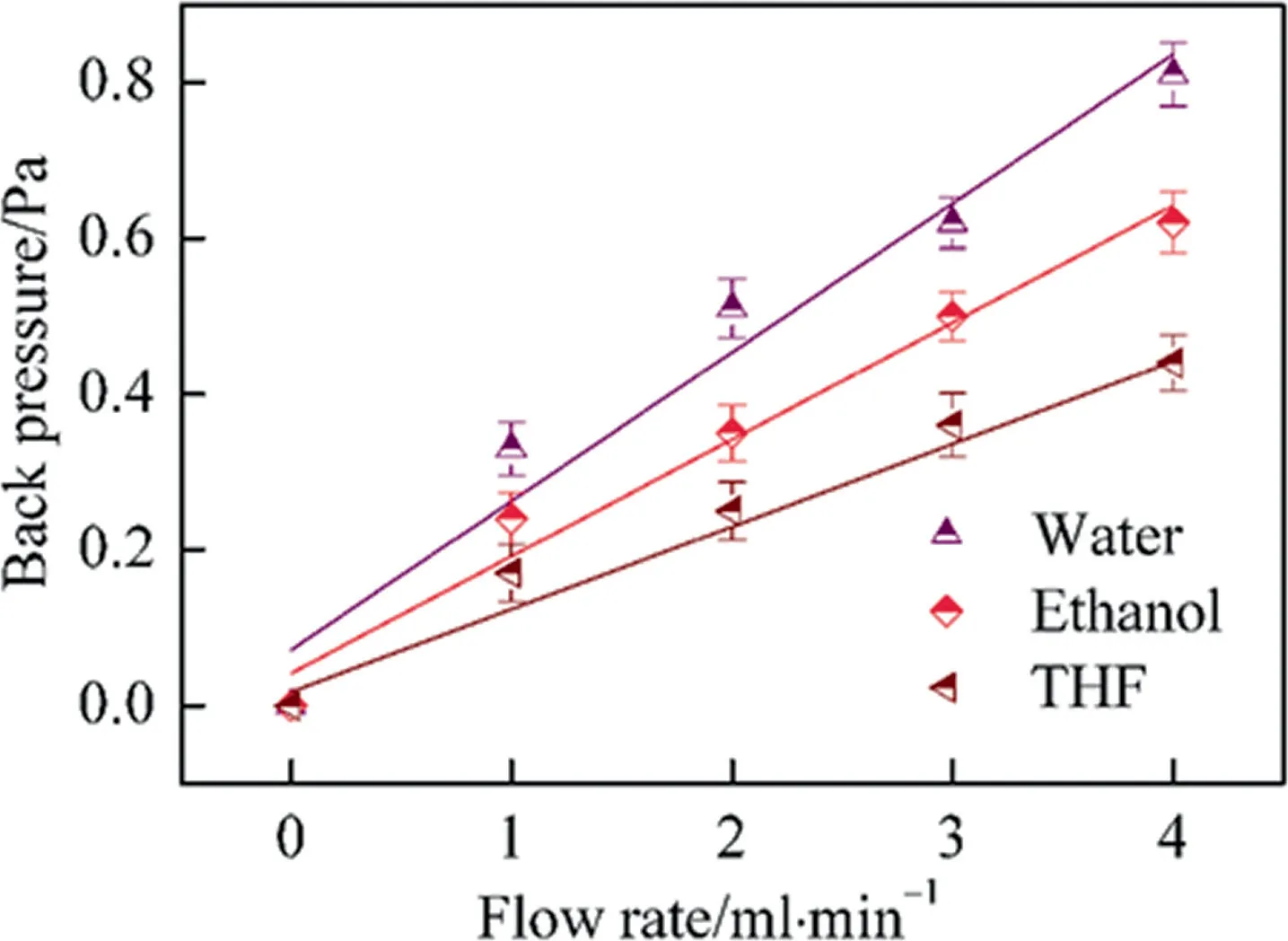
Fig.4. Relationship of back pressure and flow rates on HAP microspheres loaded monolith.
3.2.Adsorption of Ni2+ on hydroxyapatite microspheres
Varied parameters,like pH,initial adsorption concentration and adsorption time,are very vitalin evaluating the adsorption capacity of heavy metal ions.As for the adsorption equilibrium isotherms and the adsorption kinetics, they are important measurements in diagnosing the adsorption mechanism and evaluating the adsorption efficiency.In this experiment, a series of batch equilibrium experiments were carried out to identify the effects of pH (Fig.5(a)), initial adsorption concentration (Fig.5(b)) and adsorption time (Fig.5(c)) on the adsorption of Ni2+.In addition, fitting these adsorption data gave the relative parameters,which were listed in Table 3.For the adsorption mechanism of Ni2+,they were adsorbed on HAP microspheres, which mainly relied on the ion exchange through substituting Ca2+from the apatite lattice by a diffusion process according to Eq.(7):
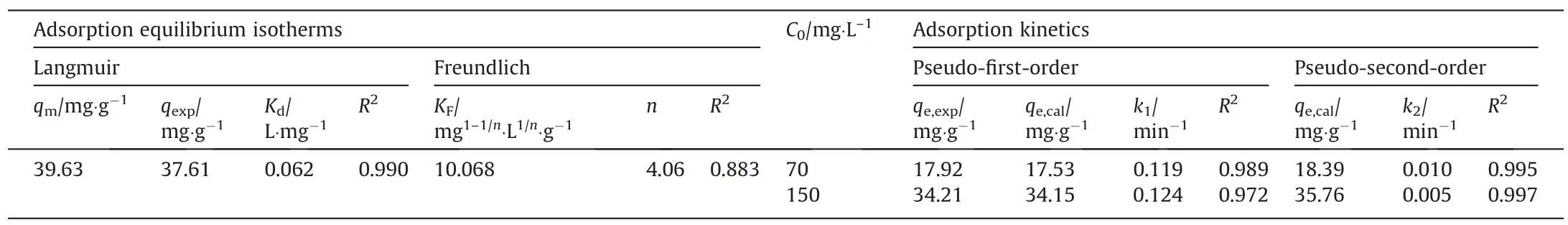
Table 3Constants of adsorption equilibrium isotherms and adsorption kinetics of Ni2+ adsorption on HAP microspheres

From Fig.5(a), it was seen that the adsorption capacity of Ni2+increased gradually with the increase of initial pH value and finally reached the maximum adsorption capacity of 35.54 mg·g-1at pH 6.5.This phenomenon might be assigned to the decreasing competition between positive H3O+and adsorption sites,which was beneficial to reduce the hindrance of repulsive force for Ni2+approaching the adsorption sites as a reduction of H3O+concentration.In addition,the formation of Ni2+complexes changed with the variation of the pH values.From Fig.5(a),it revealed that only pure Ni2+dominated when the solution pH was below 6.5, indicating that the ion exchange was carried out effectively.However, as the pH raised up, a variety of nickel complexes, such as NiOH+(pH >6.5), Ni(OH)2(pH >8.7 and 9.0) emerged and occupied the adsorption sites irreversibly.It was not conducive to perform the ion exchange process especially with the precipitation of Ni(OH)2when the pH value exceed 6.5.Therefore, the optimal pH value of Ni2+adsorption on HAP microspheres was determined to 6.5.

Fig.5. Effects of pH (a), initial adsorption concentration (b) and adsorption time (c) of Ni2+ adsorption on the HAP microspheres.
For the adsorption equilibrium isotherms,it revealed from Fig.5(b) that as the Ni2+concentration increased from 5 to 300 mg·L-1,the experimental adsorption capacity increased apparently and reached the equilibrium adsorption capacity of 37.61 mg·L-1,which was similar to the reported adsorbents [31,32].The initial increase of adsorption capacity was mainly ascribed to the enhancement of mass transfer as the metal ions concentration raised up, which was in favor of the intensity of the ion exchange for Ca2+to metal ions in HAP lattices [33-35].But the traditional ion exchange usually acts on the surface layer of adsorbents, so the adsorption sites for HAP microspheres were confined to a single surface layer and reached adsorption equilibrium at a critical concentration.To further demonstrate the mechanism, Langmuir and Freundlich models were applied to fit the experimental data and the results were shown in Table 3.In this table,the coefficientnof Ni2+was greater than 2, indicating that the adsorption on the HAP microspheres was more heterogeneous.Meanwhile, theR2of Langmuir model was closer to 1 than Freundlich one, which demonstrated that the adsorption matched better to Langmuir model and belonged to monolayer coverage.
For dynamic adsorption experiments as shown in Fig.5(c), it showed that the adsorption capacity of Ni2+increased sharply within the first period of about 60 min and gradually reached equilibrium at about 120 min.The equilibrium experimental data of Ni2+were fitted by pseudo-first-order and pseudo-second-order equations, by which the parameters were fitted and listed in Table 3.In all parameters, the adsorption kinetic parameters (k1andk2) are extremely important factors because they directly determined the adsorption efficiency of certain metal ions.The results showed that the HAP microspheres had faster adsorption rate than other reported adsorbents [30].This might be due to the prepared HAP microspheres in this experiment contained mesopores in size of about 4 nm,which could increase the specific surface area and further improve the adsorption rate.Once the surface adsorption sites were occupied,the remaining metal ions had a weak trend to enter the inner layer of HAP for ion exchange,which might reduce the adsorption efficiency in the subsequent stages [36,37].By comparing the fitting residuals of two models,the results showed that theR2of the pseudo-second-order equation was closer to 1 than the pseudo-first-order equation, which indicated that the adsorption of Ni2+on HAP microspheres matched better to chemical adsorption.
3.3.Proteins adsorption
Subsequently, Hb and BSA were applied in batch protein adsorption experiments by using HAP@Ni and HAP as adsorbents,respectively.It found that pH value had a positive influence on improving the adsorption efficiency for proteins because it might not only impact the stretching out of heme groups for Hb but also affect the surface charge of adsorbents.Thus,a range of batch equilibrium experiments were conducted to explore the optimum adsorption pH value on the adsorption of Hb and BSA, and the results were shown in Fig.6.
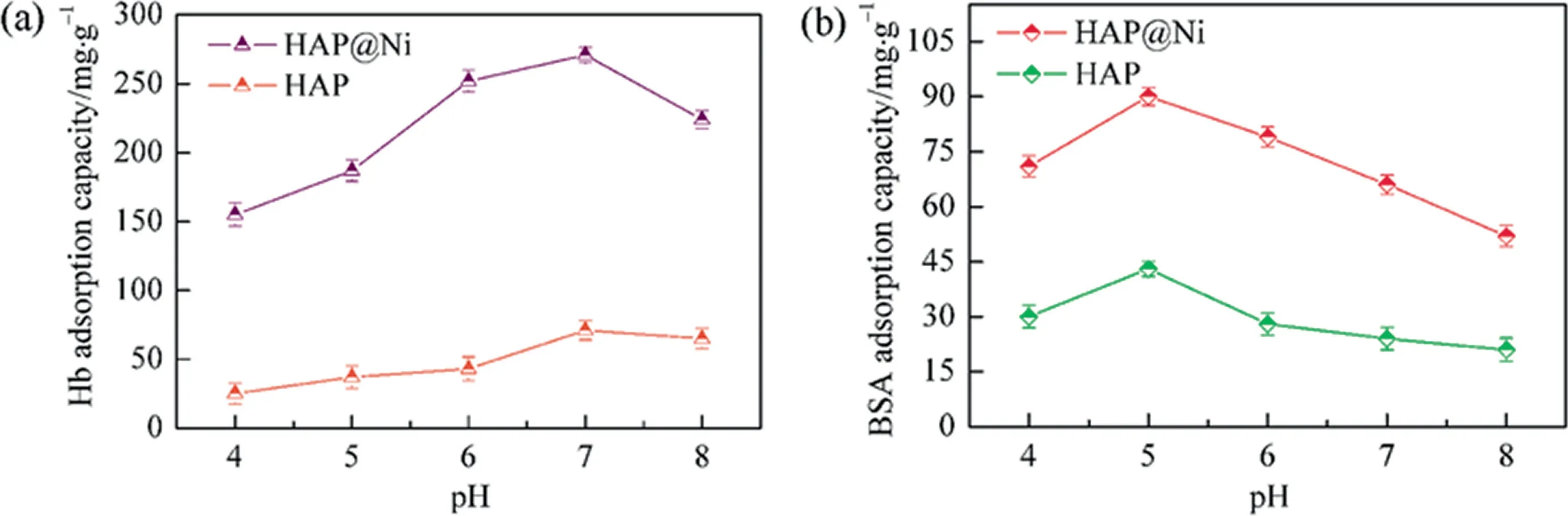
Fig.6. Effect of initial pH values on the adsorption capacities of HAP@Ni and HAP adsorbents for Hb (a) and BSA (b).
From Fig.6(a),it revealed that as the initial pH increased to 7.0(pIHb=6.8), the Hb adsorption capacity of HAP and HAP@Ni gradually increased to 69.2 and 268.7 mg·g-1, respectively.It was almost 4 times in adsorption capacity enhancement for HAP@Ni to HAP microspheres.However, as the pH value greater than 7.0,the adsorption capacity gradually decreased.The results might be interpret in two factors, on one hand, when the pH value reached 7.0, the heme group stretched out completely and its four pyrrole subunits were able to chelate Ni2+by coordination bonds,thus,the association affinity effectively enhanced by doping Ni2+on HAP microshperes, but as for HAP, the adsorption mainly contributed to mesopores and intermolecular hydrogen bonds, which was a relatively weaker force.In addition, Fig.6(b) presented the effect of pH values for BSA adsorption, likewise the maximal adsorption capacities of HAP and HAP@Ni gradually increased to 44.1 and 89.2 mg·g-1when the solution pH approached 5.0, which was closed to the pIBSA(4.8), but the maximal adsorption capacity of HAP@Ni decreased dramatically compared with Hb.From the molecular structure as we know, the adsorption for BSA on HAP@Ni mainly contributed to the chelation of Ni2+by sulfydryl,which also enhanced the association affinity to some extent compared to HAP adsorbents.Therefore, the experiments demonstrated that the adsorption capacity for Hb and BSA were efficiently enhanced by doping Ni2+onto HAP microspheres when the pH approached the pI value of proteins.
The adsorption equilibrium isotherms were applied to determine the adsorption capacity and diagnose the adsorption nature of HAP and HAP@Ni adsorbents.From Fig.7, it was seen that the static adsorption capacities of HAP and HAP@Ni adsorbents increased gradually and reached the maximal adsorption capacities of about 275 and 51 mg·g-1for Hb adsorption and of about 98 and 39 mg·g-1for BSA adsorption, when the concentration of Hb increased from 0.5 to 4.0 mg·L-1and of BSA increased from 0.2 to 1.6 mg·L-1, respectively.The results demonstrated that the initial proteins concentration provided a large driving force to conquer the mass transfer resistance of proteins between the aqueous phase and solid phase.By fitting the experimental results with Eq.(2) and Eq.(3), the results demonstrated that the fitting curves gave a better fit to Langmuir over the entire range of protein concentrations than Freundlichfor HAP@Ni adsorbents,indicating that the protein adsorption conformed to a single monolayer adsorption, but for HAP adsorbents, theR2of Langmuir and Freundlich were both closed to 1.0, this might be due to the stack of proteins.The according adsorption parameters were presented in the Table 4.
In addition,the adsorption kinetics was applied to evaluate the dynamic adsorption efficiency and mechanism of proteins.Fig.8 showed the kinetic adsorption curves with adsorption time ranging from 20 to 160 min and the experimental results were fitted by Eq.(4)and Eq.(5).From Fig.8,it found that the proteins adsorption on HAP and HAP@Ni were both rapid and up to maximum uptake within 40 min and reached adsorption equilibrium at 60 min, the final equilibrium adsorption capacities for Hb and BSA of 1000 mg·L-1on HAP@Ni reached 180 and 47 mg·g-1and HAP reached 46 and 33 mg·g-1,respectively.By fitting the experimental data with pseudo-first-order and pseudo-second-order equations,the fitting curves revealed that the adsorption behavior on the HAP@Ni matched better with pseudo-second-order equation,indicating that the adsorption behavior conformed better to chemical adsorption but on the HAP adsorbents, both fitting curves were close to 1.0, indicating that chemical and physical adsorption existed synchronously on the adsorption process.Comparing the kinetic coefficients(k1andk2,Table 5)of HAP@Ni and HAP for proteins,it appeared apparently that the values ofk2were bigger thank1on average,which suggested that mass transfer was dominated by chemical adsorption.Initially, the adsorption sites were easily accessible for proteins and a higher adsorption rate could be seen at the beginning, but once the outer adsorption sites consumed,the residual proteins had to adsorb in the inner mesopores, which then compromised the adsorption rate.

Table 4Adsorption equilibrium isotherm parameters of Hb and BSA on HAP@Ni and HAP adsorbents
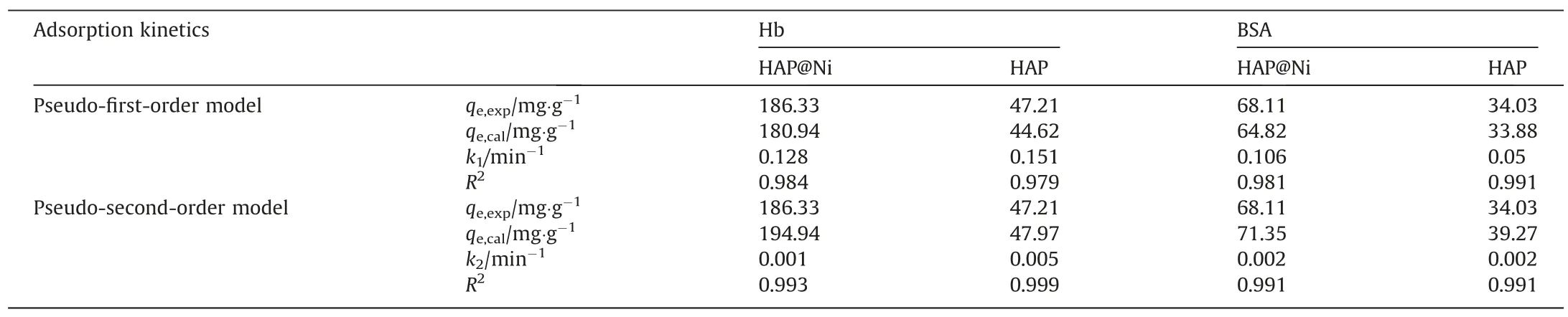
Table 5Adsorption kinetic parameters of Hb and BSA on HAP@Ni and HAP adsorbents
3.4.Desorption and repeatability
Batch desorption and repeatability experiments were carried out by adding NaCl solutions with different pH values as eluents to evaluate the regenerability of the adsorbent, the proteins were eluted from the adsorption sites of the adsorbent mainly through salting-in effect and competitive adsorption of hydrogen ions and the results were shown in Figs.9 and 10.From Fig.9, it revealed that the optimum desorption pH value was close to pI as the previous researches reported.And with the increase of the salt concentration reached 1.0 mol·L-1, the maximum desorption efficiency approached 93% for Hb and 63% for BSA, respectively.Through the repeatability experiments,it was obvious that the eluents had an advantage for Hb than BSA because the binding energy and salting-in effect of Hb were relatively lower than BSA.The prepared HAP microspheres still have high adsorption capacity after 7 times of reuse,indicating that the microspheres have good performance stability.The morphology of HAP microspheres after 7 cycles was shown in the Fig.S1,it can also be seen that HAP microspheres still maintain a good spherical shape.
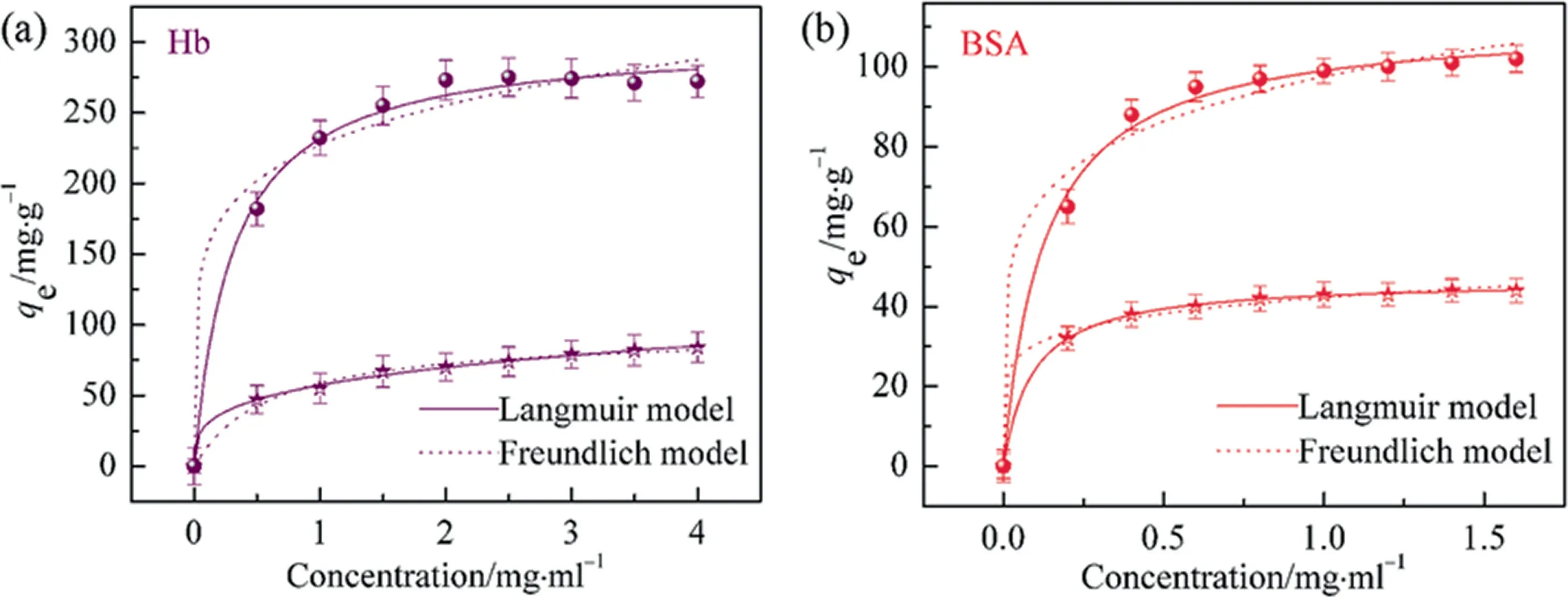
Fig.7. Adsorption equilibrium isotherms of Hb (a) and BSA (b) on HAP@Ni and HAP adsorbents (●: HAP@Ni, ★: HAP).
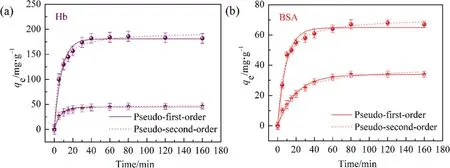
Fig.8. Adsorption kinetics of Hb (a) and BSA (b) on HAP@Ni and HAP adsorbents (●: HAP@Ni, ★: HAP).

Fig.9. Batch desorption experiments of Hb (a) and BSA (b) adsorption on HAP@Ni and HAP adsorbents in different solution pH values and NaCl concentrations.

Fig.10. Recycling experiments of Hb (a) and BSA (b) adsorption on HAP@Ni microspheres.
4.Conclusions
In summary, hierarchical porous HAP microspheres were successfully synthesized by ‘‘one-pot” hydrothermal method with good spherical shape, high specific surface area of 48.5 m2·g-1,and hierarchical porous structure.The hierarchical porous structure endows the HAP microspheres with excellent permeability.In the adsorption experiments, the obtained Ni-doped HAP microspheres (HAP@Ni) show high adsorption capacities of 275.11 and 97.55 mg·g-1for Hb and BSA, respectively, indicating the great potential in protein separation.
CRediT Authorship Contribution Statement
Yaling Li:Formal analysis, Data curation, Writing - original draft.Hao Ai:Investigation.Liangzhi Qiao:Writing - review &editing.Yinghong Wang:Data curation, Resources.Kaifeng Du:Conceptualization, Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by the National Natural Science Foundation of China (21676170).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.020.
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Notes for Contributors
- Solubility determination and thermodynamic modeling of bis-2-hydroxyethyl terephthalate (BHET) in different solvents
- Insights into the cross-amyloid aggregation of Aβ40 and its N-terminal truncated peptide Aβ11-40 affected by epigallocatechin gallate
- Numerical study on hydrodynamic characteristics of spherical bubble contaminated by surfactants under higher Reynolds numbers
- Spray-drying assisted layer-structured H2TiO3 ion sieve synthesis and lithium adsorption performance
- Innovative hydrophobic/hydrophilic perfluoropolyether (PFPE)/polyvinylidene fluoride (PVDF) composite membrane for vacuum membrane distillation
