Process monitoring of the Au-S bond conversion in acetylene hydrochlorination
Lutai Song, Li Liu, Mingyuan Zhu,2,*, Bin Dai,2,*
1 School of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832000, China
2 Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi 832000, China
Keywords:Acetylene hydrochlorination Au-S bond Process monitoring Valence shift
ABSTRACT The continuous expansion of vinyl chloride production increases environmental pollution caused by mercury catalysts, which is an issue that urgently needs to be solved.Green and stable catalysts should be researched to alleviate this issue.In this research,Thiolactic acid acts as a ligand where sulfhydryl groups form a stable complex with Au on the surface of a spherical activated carbon(SAC).An Au-thiolactic acid/SAC catalyst was designed with a Au theoretical loading of 0.5%(mass)to overcome the disadvantages of traditional Au-based catalysts,such as a low conversion rate and poor life cycle.The ratio of Au to ligand was screened,and the activity was best when Au/S=1:8.The formation of the Au-S bond was proven by FT-IR and UV-vis.The longevity test of the Au1S8 /SAC catalyst was carried out at 1200 h-1 for 50 h.Samples with reaction times of 0 h, 5 h, 10 h, 20 h, and 50 h were taken to monitor the catalyst status.The XPS and TPR tests proved that the Au-S bond broke as the acetylene hydrochlorination reaction proceeded.The DFT calculation proved that the Au-S bond is the active site, and the sulfur atom promotes the adsorption of C2H2 by the catalyst.
1.Introduction
Currently, the production scale of vinyl chloride monomer(VCM) is expanding with the increase in polyvinyl chloride (PVC)production.Acetylene hydrochlorination has been widely used as the main process for producing vinyl chloride.In the past few decades, HgCl2has been regarded as the most important catalyst for acetylene hydrochlorination due to its high activity.However,because of its volatile, highly toxic, and other undesirable properties [1], mercury chloride does not meet the current green standards in the chemical industry, therefore the use of this catalyst was reduced and gradually abolished.Hence, it is necessary to develop a green catalyst with high stability and good activity.
Regarding metal-based non-mercury catalysts,such as Ru-,Cu-,or Pd-,stability is an important part that has to be considered.It is also true that Au-based catalysts are the most likely to replace mercury in the industrial production of vinyl chloride.The carbon-loaded AuCl3system is considered to be the best reaction system [2,3]due to its excellent performance.The survey mainly focuses on regulating the valence state of Au and how to maintain its high dispersion, since both of these are the core issues of the Au-based catalytic reaction of acetylene hydrochloride.There has been much work on extending the life of catalysts.The Au-SCN catalyst designed by Zhouet al.[4]achieved a catalyst lifetime of 3000 h under the conditions of 30 h-1and 180 °C and the activity was still maintained at 95%.Also,Zhanget al.[5]developed an Au-Co-Cu system that ran stably for 6513 h at 30 h-1and 180°C.Zhaoet al.[6]prepared Au-Cu-K/AC and carried out a small-scale pilot test., 89% acetylene conversion rate still existed after 1600 h reaction at 40 h-1.Excellent stability is maintained in different ways.Also,improving the dispersion of active species helps expose more reactive sites, and a key factor affecting dispersion is the Au particle size.The performance of catalysts on the nanoscale or even on the monoatomic scale is significantly better than that of large particles and the regulation of the valence state is even more important.The three valence states of Au (Au3+, Au+, Au0) [7]as different active phases are the most central part of the study of acetylene hydrochlorination.Most scholars believe that the inherent cations in the Au catalyst (Au3+, Au+) are the main active sites that determine the catalytic performance [8].It has also been reported that Au0will undergo a valence transition during the reaction, and Au nanoparticles will dynamically transform into a monodispersed state.In either case, the retention of Au in the cationic state is necessary for the hydrochlorination of acetylene.
Carrier modification has also been a subject of extensive research in the field of catalysis.For example, the N-modified carbon carriers can improve the chemical environment of Au, especially the electron cloud density [9,10].There has been less research regarding S and P modified carriers.Generally,S as a single substance for carrier doping is not advantageous.Numerous researchers have even proposed a negative effect on the reaction when using S and N for co-doping.Wanget al.[11]introduced S to nitrogen-doped AC.This result proved that the N-species originating in pyrrole was concentrated by introducing S,and therefore it showed that pyrrole nitrogen stabilized Au.When the S species was used as a functional group to functionalize the AC surface, S also became an excellent species for anchoring Au.Strong metalsupport interaction (SMSI) at the Au-carrier interface was most likely the cause for the stabilized Au.In the AuCl3/AC system, this was reflected in the interaction of Au and the oxygen-containing functional groups on the carbon surface.Based on this theoretical point, Duanet al.[12]studied the effect of different S-containing species (oxidized and reduced states) on the reaction stability of Au-based catalysts from the perspective of the carrier modification.They mainly compared the effects of—SO3H and-SH groups on the modified AC.According to the influence of Au-based catalyst, it was found that —SO3H maintained a high oxidation state of Au in the manner of Au-O-S-C,while the Au-S-C linkage of-SH was unfavorable to the reaction.Carrier modification is often based on heat treatment, which consumes a large amount of energy.From the perspective of environmental protection and industrial production,carrier modification may be limited.
The introduction of ligands is also considered to be a good way to achieve the maintenance of high oxidation state Au.This is because ligands increase steric hindrance, which will prevent the agglomeration of Au nanoparticles [13], thereby achieving an increase in catalyst life.The main reason for seeking different ligands is that ligands containing heteroatoms(N,S,P,etc.)change the electron density of the active center and introduce HCl adsorption sites.In recent years, there have been many reports on the study of Au-based ligands for the hydrochlorination of acetylene.Huanget al.[14]used Schiff bases to stabilize cationic gold and propose the Au-N interaction.When the GHSV=280 h-1,the acetylene conversion rate reached 95%.Zhouet al.[4]prepared a novel Au-based catalyst by complexing Au with thiocyanate(-SCN).The introduction of thiocyanate(-SCN)reduced the electrode potential of Au3+from 0.926 V to 0.662 V,thereby reducing the reduction of Au3+by acetylene.Even though the activity and stability of the gold catalyst were improved,their study did not reveal the role of N or S of the thiocyanate group on Au.Donget al.[15]synthesized a new type of complex AuPPh3Cl through a P-containing compound.After loading the AC, the activity and stability of the acetylene hydrochlorination reaction were significantly improved.Hutchingset al.[16]also mentioned that when using the(NH4)3Au(S2O3)2catalyst,the Au-S bond may be the key factor to maintain the stability of Au in the system.This is because the Au-S bond fixes and anchors the Au species, helps prevent the sintering or reduction of active materials, and improves the stability of Au cations.The 0.1% Au/C catalyst prepared by supporting Na3Au(S2O3)2is used in industrial applications and results in the production of 1000 kg of vinyl chloride with 1 kg of catalyst.
Sulfur-containing groups may stabilize the valence state of Au,therefore the weakly reducing-SH has also been a subject of many researchers.The Au-S bond and its stability are often mentioned in the photocatalysis and targeted drug therapy.Denget al.[17]developed an Au-SH-S-COF material,which relies on-SH to anchor Au,causing the Au.The light stability of the clusters was enhanced during the photocatalysis process, and the charge separation efficiency was improved.Liet al.[18]used a sulfhydryl-modified nucleic acid aptamer combined with an Au cluster as a probe to specifically identify certain diseased cells.This shows that the Au-S bond is not only stable, but also has good biocompatibility and low cytotoxicity.Therefore, it is speculated that sulfur with a specific chemical state should form a stable coordination with Au to maintain a high dispersion and high valence of Au.In this work,a sulfur-containing ligand(—SH)was chosen as the Au ligand,and a simple impregnation method was used to complete the preparation of the catalyst.The entire catalyst preparation process is green,acid-free,and uses a low Au loading[0.5%(mass)].The sulfhydryl group has weak reducibility and can reduce Au3+to Au+,forming a stable complex.An Au-SH complex was obtained and this catalyst is a good system for studying the role of the Au-S bond in the reaction.The catalyst activity was tested at a high space velocity, and samples were taken at 0 h, 5 h, 10 h, 20 h, and 50 h to characterize the catalyst over five time periods.The changes of the Au-S bond were characterized as the reaction progressed.
2.Experimental
2.1.Raw materials
Drugs used in this study were as follows:The SAC was produced by Shanghai, HAuCl4·4H2O was provided by Tianjin Yingda Rare Chemical Reagents Factory,the thiolactic acid was purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD, and NaOH was provided by Tianjin Guangfu Reagents.
2.2.Catalyst preparation
Gold-sulfur complex was synthesized using a simple procedure.HAuCl4·4H2O(1.0 g)was dissolved in 100 ml of deionized water to obtain the Au precursor solution.The sulfur-containing ligand solution (Na—SH) was obtained by the reaction of thiolactic acid and sodium hydroxide.3.14 ml of the HAuCl4·4H2O solution was added to a 50 ml beaker and was stirred.After stirring for 0.5 h,a certain amount of Na—SH was added to the solution.The mixture produced a white precipitate and cleared quickly.Then,a 1 mol·L-1NaOH solution was added dropwise to the mixture to adjust the pH to 11.The reaction mixture took 8 h of vigorous stirring at room temperature to react completely.3 g of SAC was added to the above mixture and stirring continued for 12 h, this was followed by vacuum filtration,washing,then drying at 60°C for 12 h.By adjusting the molar ratio of Au and S,different catalysts were obtained as follows: AuxSy/SAC (x= 1,y= 2/4/8/12/20).
As a control, Au/SAC catalyst was prepared.3.14 ml of HAuCl4·4H2O solution was poured into a beaker and stirred continuously for 0.5 h.Also, 5 ml of deionized water was added to the solution.Next, 3 g of SAC was added to the mixture, and stirring continued for 12 h.This was followed by vacuum filtration, washing,and drying at 60°C for 12 h.The prepared Au/SAC catalyst was then collected.
2.3.Catalyst evaluation
The catalytic performance was monitored in a fixed-bed microreactor (10 mm diameter).1 ml of the catalyst was put into a stainless-steel tube, placed on a fixed-bed reactor, and the tube was purged with N2(20 ml·min-1) for half an hour.The reaction tube was heated to 180 °C at a rate of 10 °C·min-1.After heating,the gas conditions were set to C2H2/HCl = 1:1.15 and GHSV = 1200 h-1.Also, an exhaust gas absorption device was added downstream of the reactor to absorb any unreacted HCl,which can reduce air pollution.And the products were also collected and sent to the gas chromatograph(GC-2014C)for analysis.
2.4.Catalyst characterization
To obtain the detailed microstructure information of the catalyst, standard characterization methods were used.X-ray diffraction (XRD) was used for phase analysis using a Bruker D8 advance X-ray diffractometer.Transmission electron microscopy(TEM,JEM-2010)was used to observe the morphology of the catalyst.The specific surface area and pore diameter were measured by a Micromeritics ASAP 2046 instrument using the Brunauer-Emm ett-Teller (BET) method.The carbon depositions of the catalysts were determined by thermogravimetric analysis (TGA) using air atmosphere (air-TG), and the decomposition of the Au-S bond was tested under a nitrogen atmosphere (N2-TG).Fourier transform infrared spectroscopy (FTIR) was used between 500 and 4000 cm-1on a Thermo Nicolet Avatar 360 to observe functional group changes.A Micromeritics Chemisorb 2720 (Micromeritics Instrument Corporation, USA) was employed for the TPR with 10% H2in Ar as the reducing gas.Au 4f and S 2p of XPS analyses were performed by Thermo ESCALAB 250XI(USA)with 16 kV voltage and a 14.9 mA current.The distribution of elements and chemical combinations were also obtained from XPS.Ultraviolet and visible spectrophotometry (UV-vis) in the 200-800 nm wavelength range was performed on a UV-3600 (Shimadzu, Japan) for the analysis of the precursor solutions.Inductively coupled plasma optical emission spectrometer (ICP-OES) was used to get Au loading on the Agilent 5110.
2.5.Calculation details
In this work,all the density functional theory(DFT)calculations were performed by using the Guassian09 software package.In the process of geometrical optimizations,the hybrid density functional method M062X[19]with the def2-TZVP[20]basis was set for C,O,H, S, Cl, Au atoms and pseudo-potential def2-TZVP for Au atoms.All the energies involved in the calculation are taken as the zeropoint corrected electronic energies.No symmetry constraints were imposed on the geometry optimizations.For the Hessian matrix calculation,all of the stationary points were characterized as minima (no imaginary frequency).We also calculated adsorption energy (Eads) during the study, which were defined as follows:Eads=Eads-state- (EC2H2/H2O+Ecatalyst).
The Multiwfn 3.7 program [21]was used to calculate Hirshfeld charges.All reactions were carried out in the gas phase.
3.Results and Discussion
To begin, performance tests were completed for catalysts with different theoretical Au and S ratios.As shown in Fig.1, it was found that the Au1S8/SAC had the highest activity within 10 h and reached the highest conversion rate of 71.89% after 10 h.As the S content increased, the acetylene conversion rate first increased and then decreased.Therefore,Au:S =1:8 was the optimal ratio.This implied that the chemical environment of Au under the ratio was in a better state, so the activity was different from other ratios.However, regardless of the ratio of Au to S, the Au-S system catalyst had better stability.Meanwhile, the selectivity of almost all the catalysts to vinyl chloride exceeded 99.5%.Therefore,to fully verify the activity of the Au-S catalyst, extreme conditions were used to quickly deactivate the catalyst [5].The long-term activity change was then tested (Fig.2).After 50 h, the acetylene conversion rate was reduced by 13.2%.The catalysts were sampled at 0 h, 5 h, 10 h, 20 h, and 50 h, and the corresponding characterization was performed to explore the effect of the Au-S bond on the hydrochlorination of acetylene.

Fig.1. (a)Acetylene conversion and(b)selectivity of VCM of the Au-based catalysts under the reaction conditions of T = 180 °C, GHSV(C2H2) = 1200 h-1.
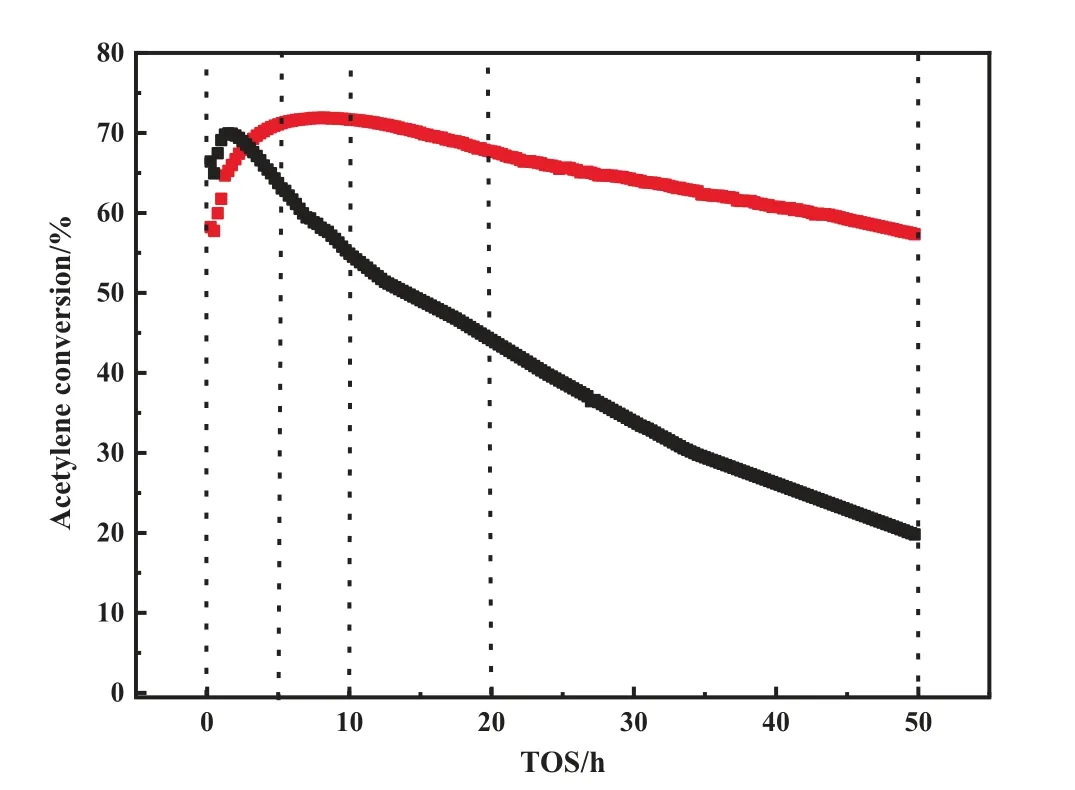
Fig.2. Long-term activity test of Au/SAC (black) and Au1S8/SAC (red) under the reaction conditions of T = 180 °C, GHSV(C2H2) = 1200 h-1.
The result of ICP-OES indicated the 0.41% (mass) Au loading in Au/SAC and the 0.40% (mass) Au loading in Au1S8/SAC, which showed that the difference in catalyst activity was independent of Au loading.To explore the Au-S bond and find direct evidence of the interaction between Au and the ligand, FT-IR (Fig.3) and UVvis (Fig.4) were performed.The Au-Au interaction peak at 320 nm was observed for HAuCl4using the UV-vis.While Au was in a complex, this peak was not found.The inherent meaning may be that the addition of sulfur-containing ligands weakened the ‘‘Aurophilic interactions [22], which also proved the high dispersion of the Au species on the surface of the carrier.In the FTIR spectrum, the characteristic thiolactic acid band at 2526 cm-1was due to S—H stretching[23].The loss of the—SH peak was considered as an indication of the Au-S bond formation.
The most important factor for complex catalysts, especially those used in thermal catalytic systems,is thermal stability.Therefore,a N2-TG test(Fig.5)was conducted and Au1S8/SAC had a severe mass loss at 293 °C, which was determined to be the decomposition of the Au-S complex.Since the boiling point of Thiolactic acid is 117°C and the reaction temperature was 180°C,the decomposition temperature of the compound was appropriate.In addition to a N2-TG test, an air-TG was also completed (Fig.6).The purpose of testing the mass loss of the catalyst was to measure the carbon deposition level of the catalyst.The results followed what was expected and the carbon deposition of the Au/SAC reached 3% within 10 h, where the carbon deposition of Au1S8/SAC within 50 h was only 0.6% (Table 1).It is worth mentioning that the activity of Au1S8/SAC was reduced by 13.8%in 50 h,while the activity of Au/SAC was reduced by 14.2%in 10 h.The decrease of carbon deposition was due to the high dispersion of Au species,and the self-polymerization of acetylene was obviously inhibited.When the reduction in the activity level was similar, the amount of carbon deposition was different.It was speculated that the reduction of Au1S8/SAC activity did not come from the carbon deposits.
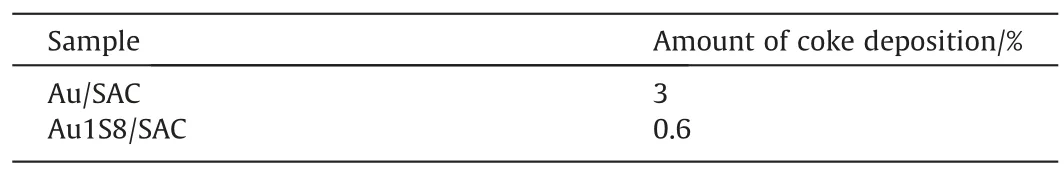
Table 1The amount of coke deposition on the used catalysts
Due to the small number of carbon deposits,the cause of deactivation needed further exploration.Therefore, XRD analysis was performed on the catalysts during different periods of the reaction(Fig.7).Two characteristic diffraction peaks at 24.4° and 43.7°appeared for all prepared catalysts, which corresponded to the(0 0 2) and (1 0 1) facets of the activated carbon [9,24].The Au/SAC samples had Au(1 1 1)crystal planes at 38.5°before and after the reaction, while there was no observation of Au peaks for the Au1S8/SAC in the first 20 h.This indicated that the introduction of ligands had a regulatory effect on the chemical state of Au.The dispersion of active components in the carbon carrier was also improved, and most importantly, the anti-sintering performance was enhanced.At 50 h, the Au (1 1 1) peak was observed, hinting that the Au0species appeared and the crystal face was exposed after the reaction.In addition, compared with used Au/SAC, the 38.5° peak intensity of the Au0in Au1S8/SAC after the 50-h reaction was significantly weaker.
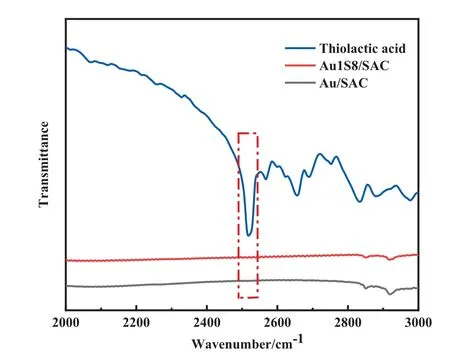
Fig.3. FT-IR curves of catalysts.
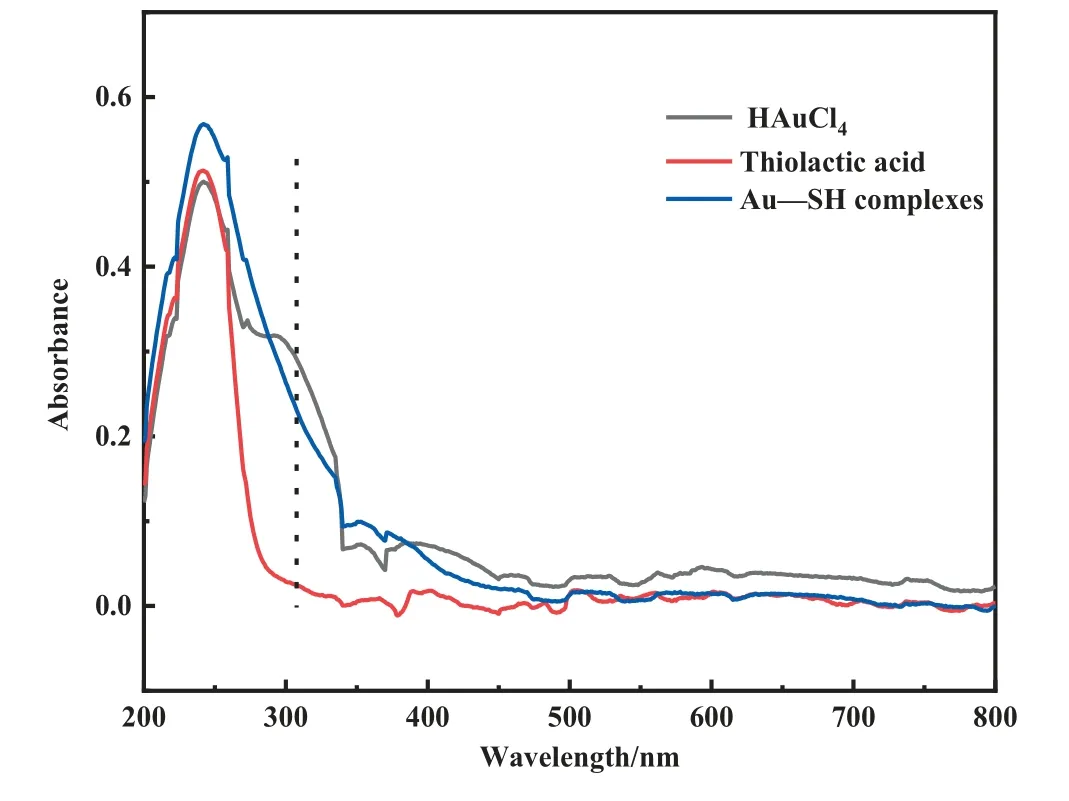
Fig.4. UV-vis absorption spectroscopy of HAuCl4, Thiolactic acid, and Au—SH complexes solution.
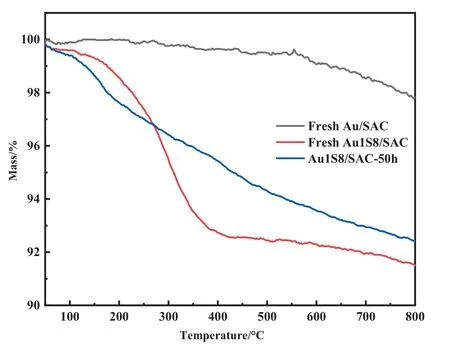
Fig.5. N2-TG pattern of catalysts.
Subsequently, the TEM analysis was completed.In the Au/SAC image (Fig.8), gold nanoparticles can be observed.The size of the gold particles in the used Au/SAC was significantly increased.Something unusual was also observed,the surface of the gold particles was completely covered by carbon deposits,which was consistent with the previous air-TG results.The formation of carbon deposits came from the tandem reaction of C2H2and HCl.The self-polymerization of acetylene on the surface of the Au species and its inability to leave the Au after the formation of vinyl chloride led to the deactivation of the catalyst [25].The unexpected carbon deposits were not found in Figs.8 and 9.Also, no shaped particles were observed in the unreacted or reacted Au1S8/SAC catalysts(Figs.8 and 9).However, the Au species should have existed in a highly dispersed manner on the surface of the catalyst.Comparing the fresh and used Au1S8/SAC catalyst mapping images, there was a different dispersion of Au and S atoms.The Au and S atoms in the reacted catalyst increased significantly on the same scale.This also confirmed the results of the XRD data since the Au species did appear to agglomerate after the 50-h reaction ( Fig.10).

Fig.6. Air-TG curves of catalysts.
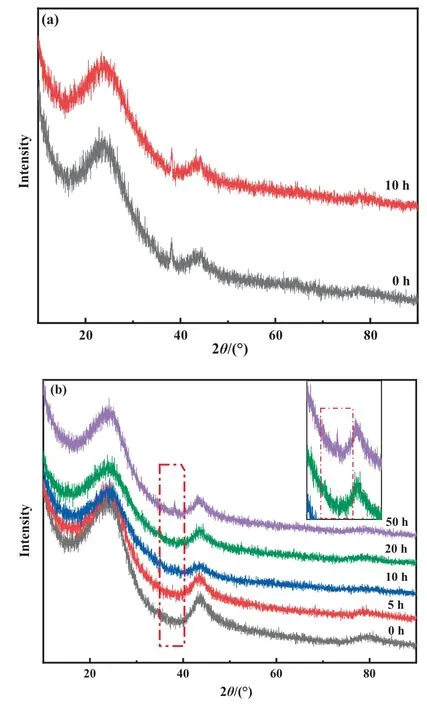
Fig.7. XRD profiles of (a) Au/SAC, (b) Au1S8/SAC.
The catalyst carrier was a spherical activated carbon which had the advantages of a high specific surface area and uniform pore size distribution.The BET test of the catalyst(Table 2)showed that the specific surface area of the Au/SAC decreased by 18.7%within 10 h.The specific surface area of the Au1S8/SAC decreased by 0.9%within 10 h and by 7% within 50 h.This result proved that the amount of carbon deposition on the Au1S8/SAC during the reaction was far less than that of the Au/SAC, and pore blockages did not appear.This showed that the presence of the ligand was beneficial to the catalytic reaction and prevented the self-polymerization of acetylene.
The deeper exploration of the Au-S bond was reflected in the XPS results.The Au 4f spectrum that was recorded for each catalyst involved three species of Au0(84.1 eV, 87.8 eV), Au+(85.0 eV,88.7 eV),and Au3+(86.2 eV,89.6 eV)[8,26].The S 2p spectrum also encompassed two species,S2-(164.1 eV)and S4+(167.6 eV)[12].It was found that the unreacted Au1S8/SAC had significantly more high-valence Au than fresh Au/SAC,which has been proven pivotal for acetylene hydrochlorination.Interestingly, comparing the XPS data in the reaction process, some patterns were detected.The amount of Au3+in the Au/SAC was very small.This conclusion was consistent with previous studies.Cation Au was the active center and directly determined the catalytic performance, which was also the main reason for the low catalytic performance of Au/SAC.The valence shifts apparently appeared for Au1S8/SAC,as the reaction progressed, the value of Au3+/Au+first increased and then decreased (Table 3).In addition, it was also mapped to the activity diagram(Fig.2).The analysis of the S 2p XPS spectrum was also completed(Fig.11).After the TPR test(Fig.12),additional evidence of the Au-S bond fracture process was obtained.Based on the production of Au+,this was believed to be obtained by the reaction of S2-in sulfydryl with Au3+.We believed that the rising of S valence is another evidence for the reduction of Au3+, which also reflects the role of Au-S mediately.As the reaction progressed,the S4+content gradually decreased,which was likely from broken Au-S bonds, and the S2-is the most direct explanation for reexposure(Table 4).Correspondingly,the breaking of the Au-S bond promoted the Au species to further oxidize from Au+to Au3+,which was seen in the Au 4f XPS spectrum Fig.13.In the Au-S system,Au was regarded as Au+, therefore the Au-S bond fracture released Au3+,and after 10 h,the Au3+decreased.This was due to the attack speed of acetylene on the active Au being greater than the release of Au3+.The TPR temperature of Au increased at first and then decreased and reached the highest value at 10 h,which also corresponded to the release of Au3+.
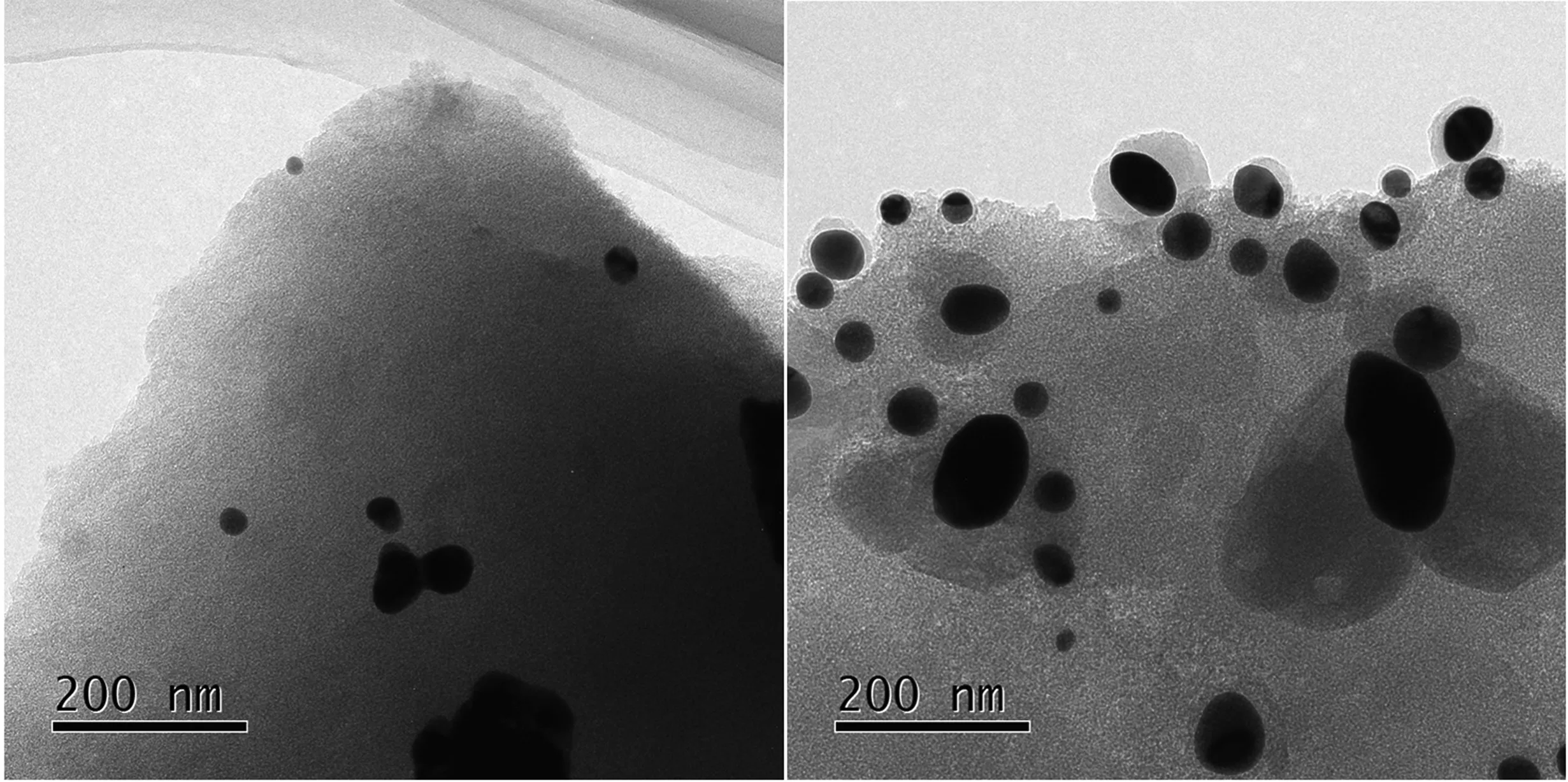
Fig.8. High resolution TEM images: left fresh Au/SAC and right used Au/SAC.
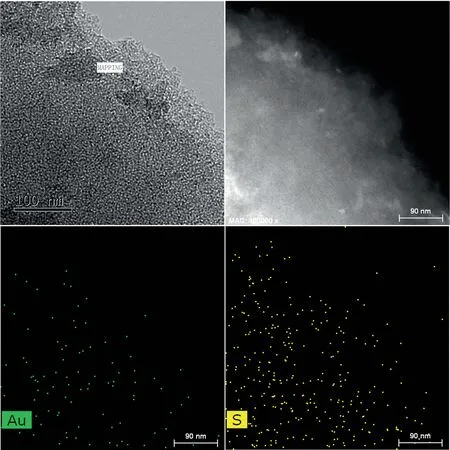
Fig.9. Elemental mapping images: Au and S of Au1S8/SAC-0 h.

Fig.10. Elemental mapping images: Au and S of Au1S8/SAC-50 h.
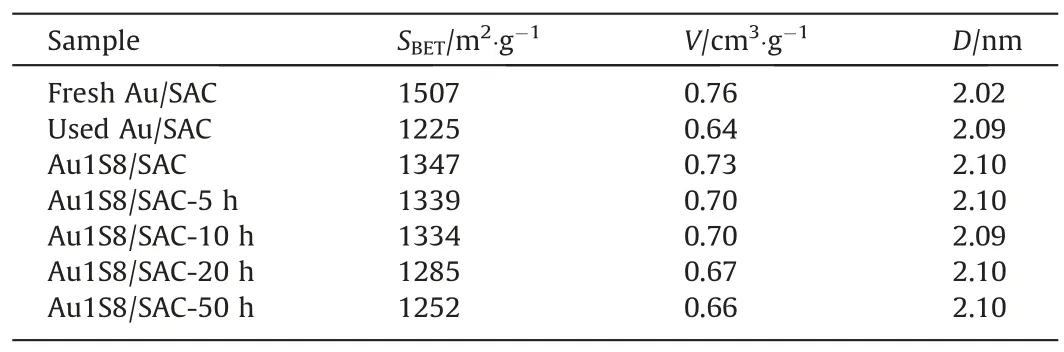
Table 2Texture parameter of catalysts, tested by low-temperature N2 adsorption/desorption experiments

Table 3The relative content of Au species from the Au 4f spectra of Au-based catalysts
Based on the AuCl3monomer adsorption model,we studied the chemical adsorption strength of Au-S complexes with different configurations to C2H2and HCl.As shown in Fig.14,the adsorption energy of Au-Cl3 for C2H2was greater than the adsorption energy for HCl, which belonged to the acetylene preferential adsorption mechanism.As shown in Fig.15,two complex configurations were shown: Au-S-Cl2 coordinated by a single sulfur ligand and Au-S2-Cl coordinated by a disulfide ligand.Similar to the mechanism of AuCl3/SAC, Au1S8/SAC also belonged to the acetylene preferential adsorption mechanism.For Au-S2-Cl, the adsorption energy of Au-S2 as C2H2adsorption sites was greater than the adsorption energy of Au-S as adsorption sites.And for Au-S-Cl2,the adsorption energy of Au-S was greater than Au-Cl.We have obtained the active sites of Au-S2 and Au-S respectively, which further illustrated the significance of the Au-S interaction.Table 5 showed the amount of charge in the Au center of the three configurations.The order of magnitude is Au-S2-Cl <Au-S-Cl2 <Au-Cl3.Based on the principle of ‘‘the more positive the charge, the more electrons are lost”, as the actual number of ligands increased, the electron cloud density of Au species increased.This result was consistent with the conclusion of XPS.Compared with AuCl3,the Au+content increased after the introduction of ligand.
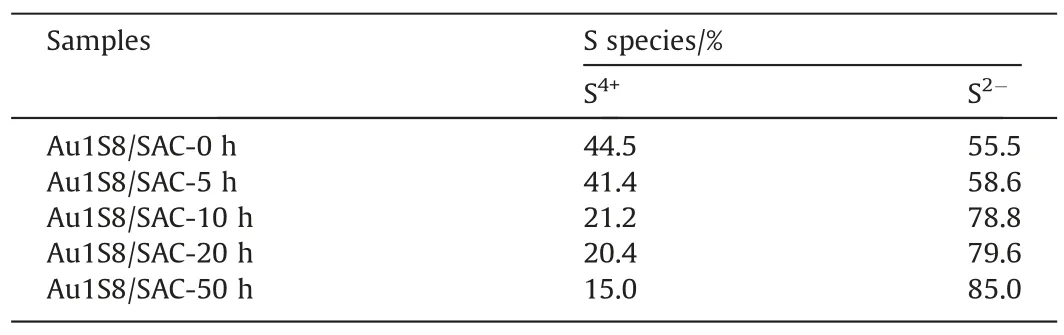
Table 4The relative content of S species from the S 2p spectra

Fig.11. S 2p XPS profiles of Au1S8/SAC-0 h and -50 h.
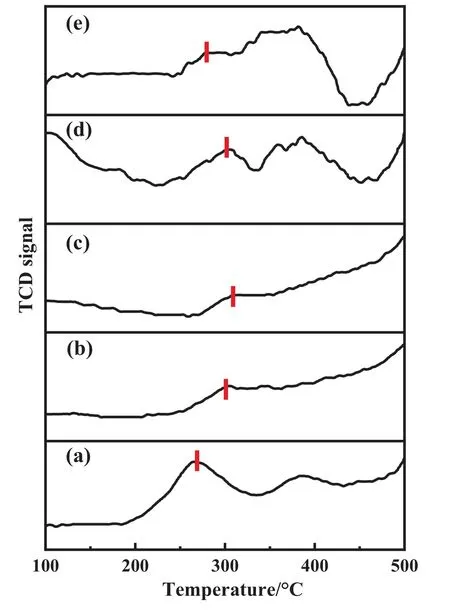
Fig.12. TPR pattern of Au1S8/SAC (a) 0 h (b) 5 h (c) 10 h (d) 20 h (e) 50 h.
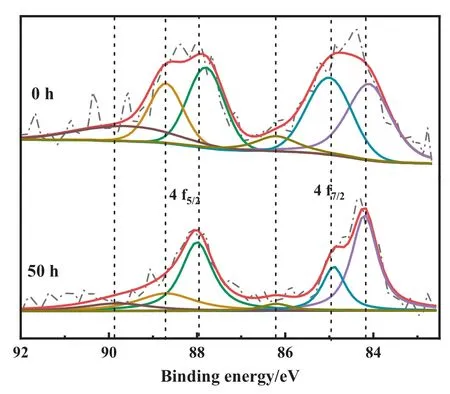
Fig.13. Au 4f XPS profiles of Au1S8/SAC-0 h and -50 h.
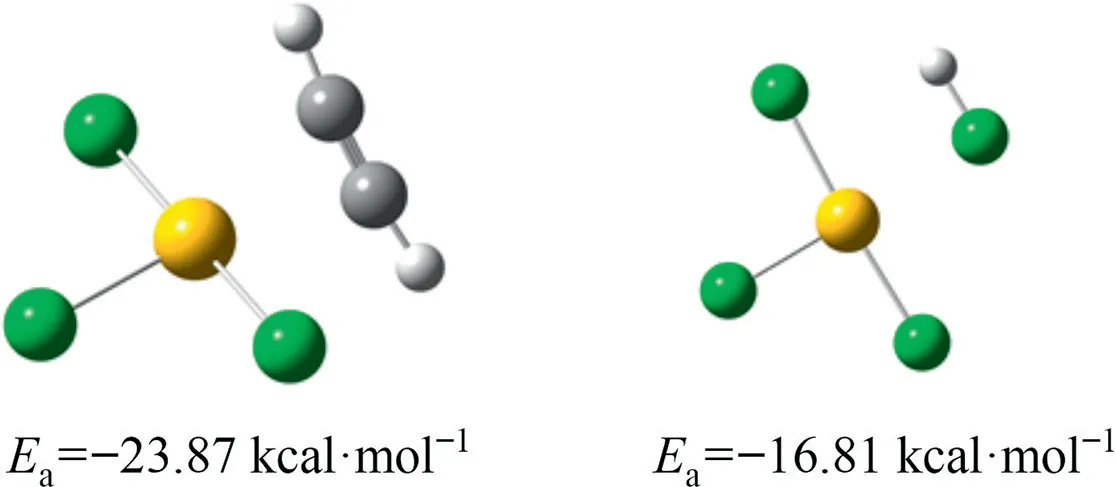
Fig.14. Adsorption model and energy of Au-Cl3 monomer on C2H2 (left) and HCl(right).(1 kcal=4.186 kJ)
4.Conclusions
Au1S8/SAC was prepared by a wet impregnation method.The life of the catalyst was tested over 1200 h-1at 180 °C.After 50 h reaction,the catalyst activity decreased by 13.2%.Based on a deactivation phenomenon, the properties of the catalyst at different periods in the acetylene hydrochlorination process were characterized.First, the introduction of sulfur-containing ligands significantly increased the electron density around the Au species.The sulfhydryl reducibility keeps Au+amount, and Au-S complexation protected Au.By analyzing the characterization results of the catalyst from XPS, XRD, TPR, BET, TEM, FT-IR, UV-vis and DFT, it was found that the tenth hour was the time point when the Au species started to change differently.Au3+started to change from increasing to decreasing and the Au-TPR also switched from increasing to decreasing.And the decreasing S4+species indicated that the Au-S system was always in the process of breaking.Carbon deposition rarely occurred on the reacted catalyst, and the reason for the decrease in activity may be attributed to the rupture of the Au-S bond.Although the complex decomposition temperature was higher than the reaction temperature of 180 °C, some slow decomposition still occurred at 180 °C.So, the fact that the Au-S bond broke was not difficult to be explained.Therefore,the process of Au-S bond-breaking included the release of Au3+, which also played a positive role.DFT calculations at the atomic level revealed that the introduction of S made Au1S8/SAC adsorb more acetylene,and the Au-S interaction was proven to be a true active site to promote acetylene hydrochlorination.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
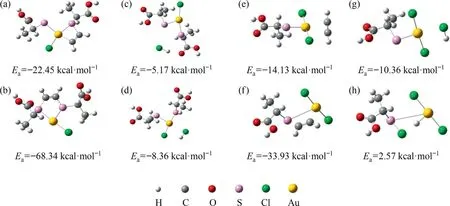
Fig.15. (a,b)Adsorption model and energy of C2H2 for Au-S2-Cl;(c,d)Adsorption model and adsorption energy of HCl for Au-S2-Cl;(e,f)Adsorption model and adsorption energy of C2H2 for Au-S-Cl2; (g, h) Adsorption model and energy of HCl for Au-S-Cl2.(1 kcal = 4.186 kJ)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21666033), the State Key Research and Development Project of China (2016YFB0301603), the International Corporation of S&T Project in Xinjiang Bingtuan (2018BC003),and the International Corporation of S&T Project in Shihezi University (GJHZ201701).
 Chinese Journal of Chemical Engineering2022年5期
Chinese Journal of Chemical Engineering2022年5期
- Chinese Journal of Chemical Engineering的其它文章
- Notes for Contributors
- Solubility determination and thermodynamic modeling of bis-2-hydroxyethyl terephthalate (BHET) in different solvents
- Insights into the cross-amyloid aggregation of Aβ40 and its N-terminal truncated peptide Aβ11-40 affected by epigallocatechin gallate
- Numerical study on hydrodynamic characteristics of spherical bubble contaminated by surfactants under higher Reynolds numbers
- Spray-drying assisted layer-structured H2TiO3 ion sieve synthesis and lithium adsorption performance
- Innovative hydrophobic/hydrophilic perfluoropolyether (PFPE)/polyvinylidene fluoride (PVDF) composite membrane for vacuum membrane distillation
