Dynamic swelling performance of hydrophobic hydrogels
Hui Guo,Junxin Chen,Zing Wng,Hong Lei Guo,Wei Hong,Xiolin Wng
a School of Chemical Engineering and Technology,Sun Yat-sen University,Zhuhai 519082,China
b Department of Mechanics and Aerospace Engineering,Southern University of Science and Technology,Shenzhen 518055,China
c School of Pharmacy and State Key Laboratory of Quality Research in Chinese Medicine,Macau University of Science and Technology,Macao,China
1 These authors contributed equally to this work.
ABSTRACT Conventional gels manifest monotonous swelling or shrinking performance upon immersing in solvents until reaching an equilibrium state.Recently,we discovered that the“hydrophobic hydrogels”prepared from hydrophobic polymer networks demonstrated dynamic swelling performance without equilibrium states.Upon water immersion,the gels expanded tremendously at the first stage until reaching a swelling peak;subsequently,the gels shrunk at an extremely slow rate.While this phenomenon endows the material with an unusual feature,more efforts are highly demanding for the full understanding of this performance.Herein,we systematically investigate the hydrophobic hydrogels’swelling kinetics by screening the organic solvent dependence,polymer effect,and temperature impact.It is revealed that the chemical structure of gels greatly influences the swelling kinetics.The higher the networks’hydrophobicity,the slower the swelling kinetics.Meanwhile,organic solvents demonstrate a limited effect on the dynamic swelling performance.Moreover,higher temperature significantly accelerates the whole volume change process.Based on the swelling performance,we further develop hydrogel-based soft devices with timeprogrammable two-dimensional and three-dimensional shape-shifting performances.
Keywords:Hydrophobic hydrogels Swelling kinetics Shape-shifting device Time-dependence Thermodynamically unstable state
Gels are defined as three-dimensional crosslinked polymer networks infiltrated with solvents.While the networks maintain gels with elastic performance,the solvent fillers endow the materials with satisfactory biocompatibility,super wetness,and suitable softness[1].During the past few decades,gels have received intensive interest for versatile engineering and biological applications[2–7].All of the functions are highly dependent on the intrinsic features of gels.In particular,one favorable property of gels is their ability to change volume when immersing in a solvent[8].Based on this particularity,these soft materials can serve as potential candidates for soft actuators[9],valves[10]and rapid hemostasis[11].
Normally,a polymer hydrogel swells in a thermodynamically compatible solvent from the preparation stage.In contrast,the gel tends to shrink(deswell)in a noncompatible solvent driven by osmotic pressure.From a statistical macroscopic theory,the equilibrium state is achieved by a minimum of the Gibbs free energy[12].More precisely,the thermodynamic force of gels’volume change is determined by the change of free energy of mixing,change of elastic energy,and mixing of ions with solvent according to Flory and Rehner[13].Once a gel attains a thermodynamically stable state(swelling equilibrium state),no driving force exists to swell or shrink the gel as the osmotic pressure gap across the gel’s interface disappears.Consequently,the swelling/deswelling of gel manifests a monotonous performance under fixed environmental conditions.Starting from the swelling equilibrium state,a gel may undergo volume change to fulfill versatile functionalities only if the material is imposed with environmental triggers(e.g.,temperature[14–16],pH[17,18],light[19,20],magnetic field[21],salt[22],solvent[23])or undergo chemical change(e.g.,hydrolysis[24],crosslinking[25],isomerization[26]).
Recently,a kind of“hydrophobic hydrogels”composed of hydrophobic polymer networks while maintaining super high water content has been discovered[27,28].Besides this particular composition,these hydrophobic hydrogels have a unique fruitlike structure,selective water absorption capacity,and more interestingly,dynamic swelling processes.The gels were prepared by immersing hydrophobic organo-gels with omniphilic organic solvents in water.Upon such solvent exchange,instantaneously that the hydrophobic polymer chains phase separate to form condensed surface layers,which serve as a semipermeable membrane to maintain the organic solvent inside gels while staying open to water.Therefore,the organic solvent forms high osmotic pressure to absorb external water inside and swell the materials.Macroscopically,the gels expanded at the first stage and reached a swelling peak,thereafter the gels shrunk at an extremely slow rate(Fig.1).During the whole process,no swelling equilibrium state is observed as that for conventional gels,and the swelling ratio demonstrates obvious time dependence.
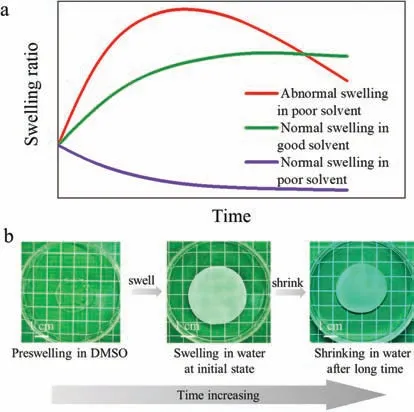
Fig.1.(a)Scheme of swelling kinetics of hydrophobic hydrogels and conventional hydrogels upon solvent exchange.(b)Photos of PMEA gels swelling performances at different stages.
Benefitting from this unusual swelling process,it is feasible to program the volume change of hydrogels with time.In this work,we report the systematical investigation of factors that influence the hydrophobic hydrogels’swelling kinetics,including organic solvent dependence,polymer effect,and temperature impact.Both gels’ chemical structure and environmental temperature greatly influence the swelling kinetics and ratio.Based on the swelling performance,we further develop time-programmable soft shape-shifting materials,where the two-dimensional(2D)and three-dimensional(3D)shape-shifting performances can be finely tuned by controlling immersing time.
Upon the solvent exchange by immersing organo-gels in water,only three components are on the scene:organic solvent,polymer network,and water.Water may enter the gels with osmotic pressure as a driven force,while organic solvents infiltrated the organo-gels may come out to mix with water.The two processes compete with each other and lead to abnormal swelling or normal shrinking of gels.According to our previous study[27],only the organo-gels with omniphilic organic solvents have a higher affinity for water than for polymer network displayed such type of abnormal volume expansion.Taking our previous system of polymer poly(methyl acrylate)(PMA)gels for example,DMSO(dimethyl sulfoxide),DMF(N,Ndimethylformamide),NMP(N-methyl-2-pyrrolidone),DMAc(N,Ndimethylacetamide),and NMF(N-methylformamide)are such kind of solvent(termed as“swelling solvent”)to swell PMA gels,while other solvents such as acetone,THF(tetrahydrofuran)and MeCN(acetonitrile)are account for the normal shrinkage of PMA gels.
In the first place,we investigated the influence of organic solvent on the gels’swelling performance with PMA gels.The fabrication of PMA organo-gels was simply realized with UV-cure polymerization with monomer and crosslink molecules in(DMSO).Subsequently,the PMA discoid gels were immersed omniphilic organic solvent to reach an equilibrium state,followed by the solvent exchange in a large amount of water.As depicted in Fig.2a,PMA organo-gels pre-equilibrated in the five swelling organic solvents all manifested obvious swelling performance upon immersion in water.Just after a few hours’solvent exchange process,the gels’volume boosted 5-8 times.In contrast,the organo-gels pre-swelled in acetone,THF,and MeCN led to fast shrinkage(Fig.S1 in Supporting information).To characterize the swelling kinetics,we first eliminated the effect induced by gels’size effect.According to Fick’s Laws of diffusion[29],the diffusion time of given species across a fixed layer thickness is proportional to the square of the layer’s thickness.For better comparison,the swelling time is normalized by the square of the initial sample thickness(time/t2).From our previous work,this normalization approach well fits samples with different initial thicknesses.In addition,two parameters are defined to simplify our following discussion:Qmax,i.e.,the maximum swelling ratio;tmax,i.e.,the normalized time to reachQmax.From the time profiles of the swelling ratio depicted in Figs.2b and c,no evident difference occurs among 5 groups of samples concerning theQmax,as all the maximum swelling ratios are around 6-8.Similarly,theTmaxdemonstrates weak dependence on organic solvents,as most of the values are around 20 h/mm2.The only exception is DMF,which may be attributed to the large gap in sampling.Moreover,the diffusion coefficient of water during the solvent exchange was also carried out to assess the organic solvent impact with a typical gravimetric method[30].Similar to the other parameters,all the samples from different organic solvents demonstrate comparable diffusion rates.From all these data,it is clearly demonstrated that organic solvent has a rather limited effect on the kinetics of such abnormal swelling.
From our previous preliminary work,it has been verified that the organic solvent residues played a critical role in the type of abnormal swelling performance[27].The more organic remained,the higher osmotic pressure was generated,and the larger swelling degree was achieved.Indeed,unlike the conventional hydrogels where miscible solvent escapes from the soft materials rapidly during the solvent exchange,still a large amount of organic solvents was left inside the gel after swelling in water for 16 h(Fig.S2 in Supporting information).Between different swelling solvent groups,a minor difference exists,which brings about a slight deviation in osmotic pressure between different gels.Consequently,no significant difference in swelling performance presents.
Next,the significance of the polymer effect has been fully screened.Whereas the organic solvent infiltrated inside the network displays a slight influence on the swelling ratio and kinetics,the role played by the polymer network is extremely prominent.As shown in Fig.3a,the swelling kinetics of 9 polymers organogels demonstrated a striking difference.Almost all the samples exhibited a dynamic swelling performance with no swelling equilibrium state,except for poly(phenyl acrylate)(PPA)sample that exhibited an equilibrium-like swelling process with a relatively lower maximum swelling ratio.Compared to other samples with polymer glass transition temperature(Tg)lower than observation temperature(25 °C),the PA polymer has a rather highTg(63 °C).Therefore,the swelling performance for PPA gels can be account for the plasticity of the frozen phase separation structure during swelling,which increases the swelling resistance but diminishes the contractile elasticity.From the supplementary rheological test,the rubbery like PPA-DMSO organo-gel turned into a fragile plastic PPA-hydrogel after just swelling in water for 2 min(Fig.S3 in Supporting information).As a result,the gel maintained a constant volume after long-term immersion even without enough osmotic pressure induced by residue solvents.In contrast,immersed at a temperature higher than Tg,this gel presented again a dynamic volume change without equilibrium state,while the maximum swelling degree was remarkably enhanced compared to that at low temperature(Fig.S4 in Supporting information).At the same time,the other 8 types of organo-gels exhibited different swelling ratios and kinetics in water(Fig.3b).On one hand,the polymer gels which has only poly(ethylene glycol)(PEG)side chains,such as PMEA,PCBA,tended to swell rapidly.This is especially the case for PMEA organo-gels,which took only around 1 h/mm2to reach thetmax.On the other hand,the polymers that have bulk aryl hydrophobic groups,such as PBnA,PPHEA,manifested a very long swelling process prior to the maximum swelling degree.Thetmaxachieved by PBnA gel(73.1 h/mm2)is nearly two decades higher than that of PMEA.Meanwhile,the longer swelling period of PBnA and PPHEA led to the highest swelling ratio.Between the two critical situations,other polymer gels are located with mild swelling kinetics and maximum swelling ratio.The difference induced by polymer is more prominent and comparative by comparing the diffusion coefficient(D).Whereas PBnA gels showed a D value of 5.9 × 10-13m2/s,PMEA demonstrated a more than 100 higher value of 8.0 × 10-11m2/s.
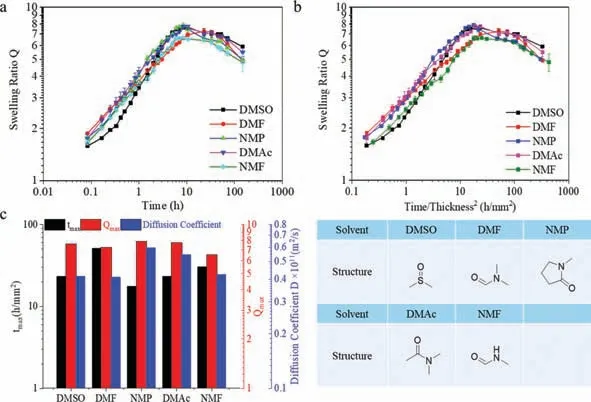
Fig.2.(a)Time profiles of swelling ratio(Q)of PMA organo-gels from the different organic solvent after being immersed in water at 25 °C.(b)Time/thickness2 profiles of Q of PMA organo-gel from different organic solvent.(c) tmax, Qmax and water diffusion coefficient(D)of the different PMA organo-gels.The initial size of the disc shape samples was 33 mm in diameter and around ~0.67 mm in thickness.The chemical structures of the solvent are illustrated below the figure.
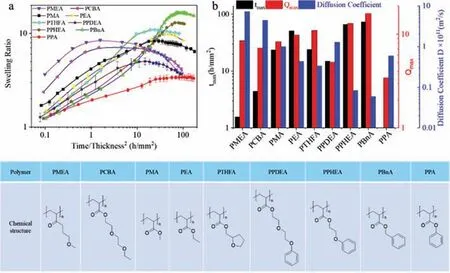
Fig.3.(a)Time profiles of swelling ratio(Q)of different polymer organo-gels from DMSO after being immersed in water at 25 °C.(b) tmax, Qmax and water diffusion coefficient(D)of different organo-gels.The initial size of the disc shape samples was 33 mm in diameter and around ~2.0 mm in thickness.The chemical structures of the linear polymers are illustrated below the figure.Note that all the linear polymers bear glass transition temperature(Tg)lower than room temperature,except PPA whose Tg is 63 °C[32].
This distinct performance can be elucidated with the aid of the semi-permeable membrane hypothesis developed in our previous study[27].Upon solvent exchanging from good solvent to poor solvent,the phase separation of polymer network restrains a large amount of organic solvent and generates high osmotic pressure to swell the gel at the first stage.Over time,organic solvent slowly leaking from the phase-separated membrane decreases the osmotic pressure,therefore gives rise to the further deswelling of the materials.The more hydrophobic the membrane,the less susceptible that organic solvent and water diffuse across the system,the higher the osmotic pressure generated.Consequently,the materials achieve the higher maximum swelling ratio.These are typically the stories for PBnA and PPHEA gels,where the hydrophobic benzyl side chains form solvent-proof membranes and the release of organic solvent becomes rather time-consuming.Consequently,slow swelling kinetics are revealed.In contrast,if the polymers are somehow hydrophilic with PEG side chains,the structure of the membrane is not finely fixed,thus facilitates the flux of both organic solvent and water and lead to fast swelling kinetics.
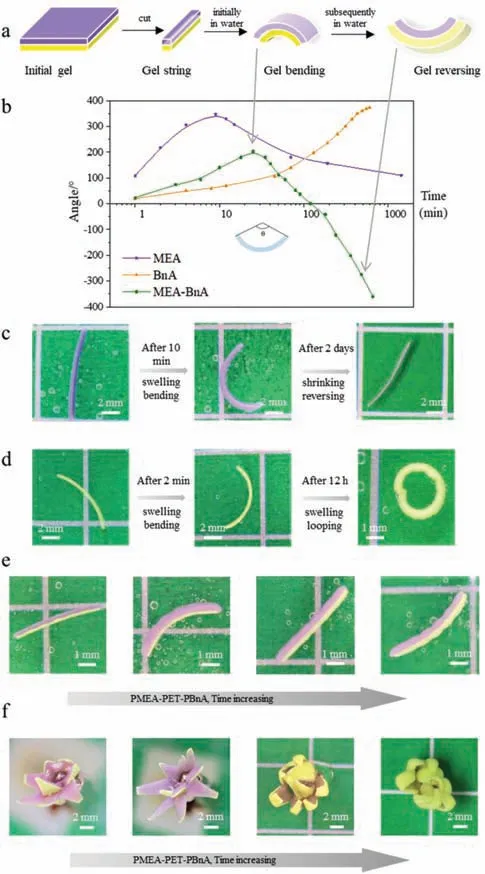
Fig.4.Shape-shifting behaviors of hydrophobic hydrogels.(a)Scheme of the sandwich-like soft device upon solvent exchange process.(b)Time profiles of bending angle of three soft shape-shift materials(PMEA-PET film,PBnA-PET film,PMEAPET film-PBnA),the inset shows the definition of the bending angle(θ).(c-e)Twodimensional(2D)shape-shifting performances of three types of materials(PMEAPET,PBnA-PET,PMEA-PET-PBnA).(f)Three-dimensional(3D)shape-shifting performance of PMEA-PET-PBnA hydrogel.The initial size of the organo-gel layer was 0.2 mm in thickness.
Besides intrinsic property,environmental factors such as temperature also significantly influence the swelling kinetic.As shown in Fig.S5(Supporting information),the kinetics is greatly enhanced upon raising the temperature.For PMEA gels,thetmaxat 60 °C is more than 20 times shorter than that at 5 °C.Consequently,the diffusion rate experiences a significant enhancement upon heating.At the same time,the maximum swelling ratioQmaxremains less unaffected at lower temperatures but presents descending trend at higher temperatures.
Benefitting from this dynamic swelling process of such hydrophobic hydrogels,it is feasible to program the volume change of materials.For this purpose,sandwich-like materials were fabricated by two hydrophobic hydrogels with divergent swelling kinetics combining with non-swellable backing.As a representative illustration,PMEA and PBnA were prepared with polyethylene terephthalate(PET)film in the middle as a dimensional stable bonding medium(Fig.4a).In the first few minutes after soaking the sandwich-like PMEA-PET-PBnA sample in water,the violet PMEA layer swelled much more promptly and greatly than the opposite yellow layer of PBnA hydrogel,driving the bilayer hydrogel strip to bend in the direction of the yellow side(Figs.4b-e).Later,after the swelling equilibrium state of PMEA and with the continuous expansion of PBnA part,the swelling volume difference between the two layers decreases,resulting in the full recovery of binding performance.Then the bilayer hydrogel strip started to shift the bending direction to the violet side since the swelling volume of PBnA hydrogel is more important than that of the PMEA hydrogel.Besides the sandwich-like samples,hydrogel-PET singlelayer materials also demonstrate similar dynamic shape-shifting performance.Both of the single-layer hydrogel strips could finally bend to full or nearly complete circles.Moreover,heating can significantly accelerate the shape-shifting performance and shorten the response time.
In addition to the 2D variation,3D self-actuation like a flower has been designed as well.As shown in Fig.4f,a piece of PMEAPET-PBnA organo-gel was cut into the shape of a long-sawtooth and then was rolled one or two circles along the long side to form the shape of a flower bud.When it was immersed in water,the blossom opened to the maximum at the first few minutes and then began to close up like an Epiphyllum.Accordingly,by rationally adjusting the composition of the two layers,as well as the temperature of the permeant solvent,the asymmetricity in the hydrophobicity of the hydrogel can be changed and the amplitude along with the speed or the duration of self-actuation behavior can be regulated.Furthermore,by designing the shape of the bilayer gel or the patterned distributions[31]of the different gels,the hydrogel could deform into 2D or 3D complex structures spontaneously,indicating considerable potential as time-programmable soft shape-shifting materials.
In this work,we systematically investigate the hydrophobic hydrogels’dynamic swelling process upon solvent exchange.The organic solvent dependence,polymer effect,and temperature impact are systematically studied by extracting three parameters,namely:the maximum swelling ratio(Qmax),time to reach maximum swelling ratio(tmax),and the diffusion coefficient of water(D).It is verified that the chemical structure of gels greatly influences the swelling kinetics.The higher the polymer networks’hydrophobicity,the larger thetmaxand the lower the D.Meanwhile,organic solvents that can swell the gels during the solvent exchange process demonstrates a limited effect on the dynamic swelling performance.Moreover,higher environmental temperature significantly accelerates the whole volume change process.Based on the swelling performance,we further develop timeprogrammable soft shape-shifting materials with distinct polymer gels,which demonstrate rapid 2D and 3D shape-shifting materials upon solvent exchange.
With this study,we hope to inspire readers to take a new look at gels’swelling process.Other dynamic swelling processes are also likely by finely tuning the swelling driven force.In addition,we anticipate that the dynamic swelling performance of the hydrophobic hydrogels may endow them with other promising applications in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China(NSFC,Nos.51903253,51903257),Natural Science Foundation of Guangdong Province of China(Nos.2019A1515011150,2019A1515011258),Macau University of Science and Technology Foundation(No.FRG-19-003-SP),and the Science and Technology Development Fund of Macao(Nos.FDCT 0009/2019/A,0083/2019/A2,0007/2019/AKP,0009/2020/AMJ).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.015.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
