Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
Yilin Song,Huaqing Jing,Long Binh Vong,Jinping Wang,Nan Li
a Tianjin Key Laboratory of Drug Delivery &High-Efficiency,School of Pharmaceutical Science and Technology,Tianjin University,Tianjin 300072,China
b Key Laboratory of Molecular Biophysics of Hebei Province,Institute of Biophysics,School of Sciences,Hebei University of Technology,Tianjin 300401,China
c School of Biomedical Engineering,International University,Ho Chi Minh 700000,Vietnam
d Vietnam National University Ho Chi Minh City(VNU-HCMC),Ho Chi Minh 700000,Vietnam
ABSTRACT Atherosclerosis(AS),mainly caused by the changed immune system functions and inflammation,is the central pathogenesis of cardiovascular disease,which is a leading cause of death in the world.In modern medicine,the development of carriers precisely delivering the therapeutic agents to the target sites is the primary goal,which could minimize the potential adverse effects and be more effective in treating lesions.Due to the precise location,real-time monitoring,AS microenvironment response,and low toxicity,stimuli-responsive nano-based drug delivery systems(NDDSs)have been a promising approach in AS treatments.Herein,we will systematically summarize the recent advances in stimuli-responsive NDDSs for AS treatment,including internal stimuli(reactive oxygen species,enzyme,shear stress,and pH)and external stimuli(light,ultrasound,and magnetism)responsive NDDSs.Besides,we will also summarize in detail the classification of stimuli-responsive NDDSs for AS,such as organic NDDSs(e.g.,lipid-based and polymer-based nanomaterials),inorganic NDDSs(e.g.,metal-based nanoparticles and nonmetallic nanomaterials),and composite multifunctional NDDSs.Finally,the critical challenges and prospects of this field will also be proposed and discussed.
Keywords:Atherosclerosis stimuli-responsive Nano-based drug delivery systems Theranostics Target therapy
1.Introduction
In recent years,cardiovascular disease(CVD)remains the leading cause of death and disability in the world,the etiology of which often involves atherosclerosis(AS)[1–4].As the common pathological basis of many diseases such as myocardial infarction(MI),stenocardia and coronary heart disease,AS is a destructive disease affecting many individuals[5–8].Generally,AS mainly occurs in the human body’s large or medium-sized elastic blood vessels,showing the main characteristic of inflammatory responses and lipid metabolism disorders.During the development of AS,the thickening and hardening of arteries are attributable to inflammatory reaction and lipid accumulation,which further causes narrowing of the arterial lumen and difficulty for blood to pass through,resulting in various ischemic cardiovascular and cerebrovascular diseases[9–11].The pathology of AS makes it difficult for drugs to reach the lesion site,so treating it is a medical problem that humans urgently need to overcome.
Despite the less thorough understanding of its pathogenesis,many primary and clinical studies show that AS is a chronic inflammatory disease of the arterial wall.The central cells involved in AS include endothelial cells,smooth muscle cells,macrophages,T-lymphocytes,neutrophils,foam cells[12–14].From the perspective of pathogenesis,the pathological process of AS can be divided into the following four stages.Firstly,the damage and dysfunction of endothelial cells are the initial stages of AS,which may be caused by many factors,such as increased levels of low-density lipoprotein(LDL),high blood pressure,diabetes,genetic diseases,and other pathogens.This pathological stage increases the adhesion of endothelial cells to leukocytes,neutrophils and platelets,leading to imbalances in the vascular endothelial environment and forming oxidized low-density lipoprotein(Ox-LDL).AS lesions have developed in this process,but there is no visible vascular obstruction in the arterial vessels[15].Secondly,if this inflammatory process is not effectively inhibited,smooth muscle cells will proliferate due to inflammatory stimuli and then cause endothelial lesions.In addition,inflammatory cells such as macrophages,T-lymphocytes,and neutrophils will accumulate in the vascular le-sions to further aggravate endothelial damage[16,17].Besides,excessive reactive oxygen species(ROS)are released from damaged endothelial cells and macrophages,which then promote oxidative stress and Ox-LDL formation[18–20].As a result,the arterial vessel wall gradually thickens and expands.Thirdly,after the vascular endothelium is damaged,the monocytes in the blood will enter the vascular intima and transform into macrophages.Under the action of scavenger receptors,macrophages will engulf Ox-LDL to form foam cells.Excessive foam cells accumulate to form lipid streaks and even AS plaques,further leading to tissue and organ ischemia necrosis.After necrosis,the tissue can heal by fibrosis,but in most cases,the AS plaque will pass directly through the necrotic phase and enter the fibrotic phase due to prolonged ischemia.For example,prolonged ischemia and fibrosis in the kidney can lead to renal failure[21–25].Finally,the foam cells accumulated in the blood vessel wall will induce cytokines and chemokines and further promote the recruitment of monocytes in the blood circulation,leading to severe inflammation.More importantly,the rupture of AS plaque will also cause thrombosis,which can block the blood flow in the arteries,leading to fatal clinical events such as MI,heart failure,and ischemic stroke in the internal cerebral artery[26–28].In clinical practice,surgery and drug treatment are the main methods for AS therapy.Surgical treatment refers to dredging and reconstruction of narrowed arteries or performing vascular stent surgery to improve blood supply,which has the advantage of high efficiency and minimally invasiveness.Still,the problems of postoperative restenosis and thrombosis cannot be ignored[29,30].In addition,due to the significant risk and common ischemic emergencies such as acute symptomatic stroke,surgery is mainly used for the last stage treatment of AS[31].On the other hand,drug treatment plays an essential role in this disease’s early and middle stages.There are some widely used drugs,such as lipid-lowering drugs,antiplatelet drugs,vasodilator drugs(Table 1)[32–35].
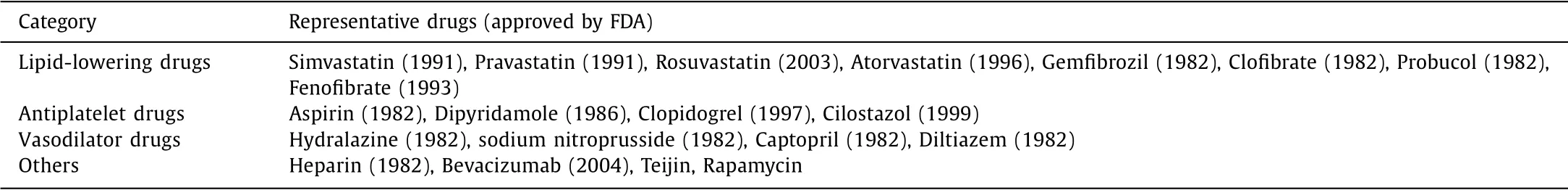
Table 1 Current therapeutic agents of AS.
However,traditional drug delivery methods have many shortcomings,such as low efficiency,non-specific distribution,and difficulty to reach the target site.Thus,long-term use of the above drugs will bring many unnecessary side effects,which will affect their further clinical applications.Fortunately,the emergence of nanotechnology in recent years has provided better options for drug delivery systems(DDSs)[35].Since the size of nanoparticles is usually around 100 nanometers with a high specific surface area,the nano-scale effect allows them to selectively and effi-ciently load various therapeutic drugs and avoid rapid elimination from the body[36].Compared with traditional DDSs,the stimuliresponsive nano-drug delivery systems(NDDSs)have better pharmacokinetic parameters and tissue specificity,higher drug loading and encapsulation efficiency improving the solubilization,half-life and biodistribution of drugs,and simultaneously reduce their systemic toxicity and adverse effects[37].Based on the local pathological characteristics of AS,many researchers have constructed microenvironment-responsive NDDSs,which can achieve targeted and responsive drug release,resulting in highly effective outcomes[38].Therefore,the development of stimuli-responsive NDDSs for the treatment of AS has essential application prospects and potential clinical value.
This review will focus on the recent advances in stimuliresponsive NDDSs for AS therapy,including internal stimuli(ROS,enzyme,shear stress,pH)and external stimuli(light,ultrasound,magnetic field)responsive NDDSs,which will be introduced in detail.Then,we will systematically summarize the classification of NDDSs for AS therapy as shown in Fig.1,such as organic NDDSs(lipid-based nanomaterials,polymer-based nanomaterials),inorganic NDDSs(metal-based nanomaterials,nonmetallic nanomaterials)and composite multifunctional NDDSs(Table 2).In the end,the opportunities and further deliberations on the critical challenges of stimuli-responsive NDDSs in AS therapy will be discussed for further direction.
2.Stimuli-responsive mechanism of AS treatment
To further improve the safety and delivery efficiency of the nano-delivery system for AS,it is necessary to integrate nanoagents and drugs into different stimuli-responsive NDDSs and thus release the drugs in specific sites.The type of stimulation is divided into internal and external stimuli.Internal stimuli-responsive systems can achieve drug positioning release through the unique physiological environment stimulation in the dis-ease site,generally including ROS,shear stress,pH,enzymes,etc.(Table 3).While the external stimuli-responsive NDDSs usually control the drug releaseviaextra physical methods such as ultrasound(US),magnetism,and light.According to different drug release requirements,these stimuli-responsive NDDSs could enhance the safety of AS therapy and the target delivery effect of these active agents,which provided a promising approach for personalized medicine[39].Here we will introduce the detailed internal and external stimuliresponsive NDDSs using for the treatment of AS.
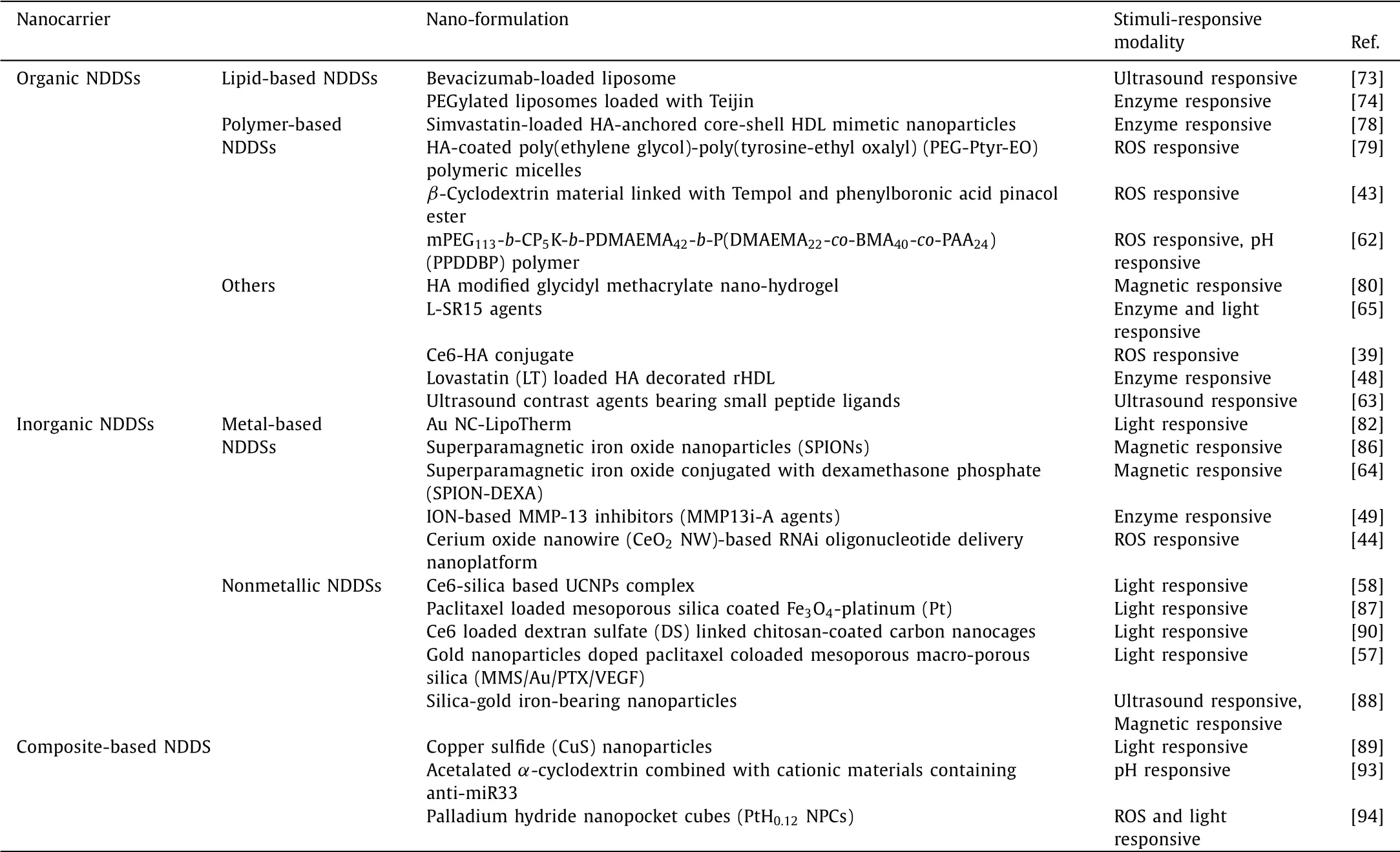
Table 2 Summary of stimuli-responsive targeting NDDSs for AS treatment in recent years.
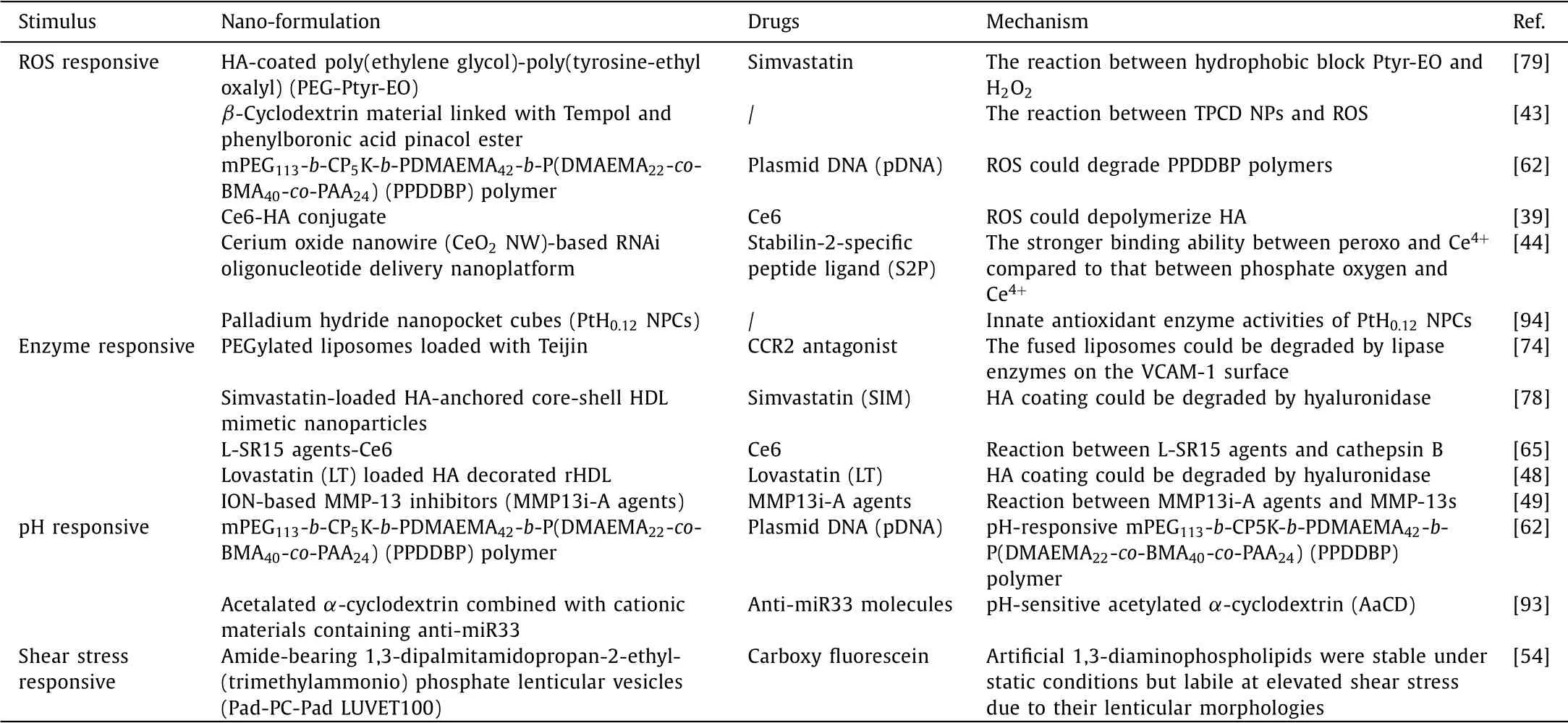
Table 3 Recent advances in internal stimuli-responsive NDDSs for atherosclerosis treatment.
2.1.Internal stimuli-response
2.1.1.ROS stimuli-response
As a chronic inflammatory disease,the essential and ultimate common mechanism of AS is oxidative stress[40].While ROS is a group of small reactive molecules that can regulate pluripotent cellular function,including hypochlorous acid(HOCl),peroxynitrite(ONOO-),hydrogen peroxide(H2O2),superoxide(O2·–),and hydroxyl(HO·)radicals,etc.Generally,the ROS concentration is crucial to vessel homeostasis.However,excessive ROS production could cause blood vessel damage and lead to endothelial dysfunction,thus promoting OX-LDL generation,DNA damage,WBC migration and differentiation,vascular smooth muscle cell proliferation and collagen degradation,worsening the AS condition.Based on this finding,many researchers designed the ROS-responsive nanomaterials to realize the target drug delivery to treat AS disease[40–42].
Kimet al.have designed and prepared a macrophage-targeted AS diagnostic and therapeutic nano-delivery system(Mac TNP)based on chlorin e6(Ce6)-hyaluronic acid(HA)conjugate.When macrophages internalize the Mac TNP,the overexpressed ROS(mainly1O2)inside the AS environment could degrade the nanomaterials by cutting the hyaluronic acids chemical bonds and releasing the Ce6 from the Mac TNP,which further leads to singlet oxygen generation under the light irradiation to kill the Raw 264.7 cells.Due to the high target-to-background ratio of the Mac TNP,such ROS responsive NDDS could provide great potential in anti-AS photodynamic therapy(PDT)with minimum side effects[39].
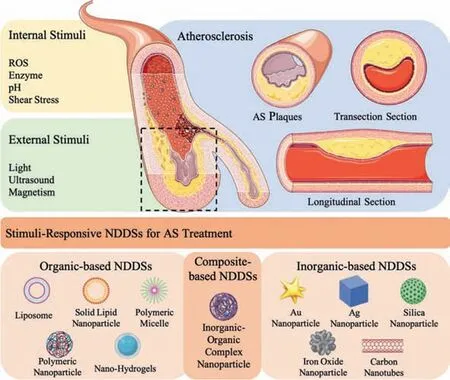
Fig.1.Targeted NDDSs based on different stimuli-responsive mechanisms and nanomaterial classification for AS therapy.
In another interesting work,Wanget al.synthesized a broadspectrum ROS-eliminating nanomaterial through conjugating covalently,a hydro-gen-peroxide-eliminating compound of phenylboronic acid pinacol ester and Tempol(a superoxide dismutase mimetic agent)onto a cyclic polysaccharideβ-cyclodextrin(abbreviated as TPCD)and processed into nanoparticles(TPCD NP)as shown in Fig.2.Such TPCD NPs could remarkably attenuate cell apoptosis in macrophages and ROS-induced inflammation by clear-ing the excess ROS in the cells by the enzyme reaction with Tempol.Besides,the TPCD NPs could thus effectively inhibit foam cells’formation in macrophages and VSMCsviadecreasing oxidized lowdensity lipoprotein internalization.Bothin vitroandin vivoexperiments demonstrated that such TPCD NPs have high biocompatibility and bioactivity and great potential for ani-atherosclerotic therapy.Moreover,the combined therapeutic approaches could be achieved through loading such nanomaterials with diverse chemo-drugs for the management of atherosclerotic diseases[43].
Gaoet al.designed and synthesized an engineered cerium oxide nanowire(CeO2NW)-based RNAi oligonucleotide NDDS,which could effectively suppress mTOR expression and the progression of AS.The CeO2NW was modified with a plaque-penetrating peptide(stabilin-2-specific peptide ligand,S2P)and PEG and further wrapped with two diverse mTOR ASOsviametal coordination(Fig.3).After internalized into the plaque cells,the CeO2core could pierce the endosome membrane and facilitate the loaded ASOs escape from the endosome lysosomal degradation.With the existence of hydrogen peroxide(H2O2),which was overexpressed in plaque cells,the loaded ASOs were realized from the surface of CeO2owing to the stronger binding capacity between Ce4+and peroxo(in comparison with that between Ce4+and phosphate oxygen).With the existence of H2O2,such NDDS could escape from endosomes effectively and release ASOs into the cytoplasm,leading to significant suppression of the mTOR expression and atherosclerotic plaque formation[44].
2.1.2.Enzyme stimuli-response
Several enzymes are actively involved in the development of AS,including matrix metalloproteinases(MMP),hyaluronidases,cathepsin and so on[39].MMP is a group of proteolytic enzymes that could cause extracellular matrix(ECM)proteins degradation,like gelatin(by MMP-2s and MMP-9s),collagen(by MMP-1s,MMP-8s and MMP-13s),fibrin(by MMP-3 and MMP-10)and elastin(by MMP-12s)[45],while hyaluronidase is a group of enzymes that could degrade HA primarily and specific to HA,playing an essential role in tissue response to injury[46].What is more,cathepsin is a type of proteolytic enzyme which is mainly present in lysosomes.Post being released from macrophages,cathepsin could inactivate the relative enzymes,degrade the proteins,and promote the inflammatory activity of AS after micro-environment imbalance[47].In recent years,these enzymes have become attractive stimulants and AS imaging and drug delivery targets.
In the year of 2014,Liuet al.designed and synthesized an NDDS based on HA-reconstituted high-density lipoprotein(rHDL)loaded with Lovastatin(LT),which was a classical anti-hyperlipidemic drug(HA-LT-rHDL)(Fig.4).The LT-rHDL with positive charge was synthesized firstly through sodium cholate mediation post-thinfilm dispersion and then served as the cationic core for HA coating by electrostatic adsorption.After being coated with HA,HA-LTrHDL could prolong the circulation time of NDDSs in the bloodstream and increase the accumulation of NDDSs in AS lesionsviaactively targeting HARE and CD44 receptors.Post degradation of HA coating by the specific enzyme(hyaluronidases),naked r-HDL would be internalized by macrophage-derived foam cellsviaSRBI receptor-mediated endocytosis.Bothin vitroandin vivostudies demonstrated the enzyme responsive HA-LT-rHDL provided a new approach for AS treatment[48].
Apart from HA as the targeted enzyme,MMPs also serve as important targets for drug delivery to AS plaques as they can specifically target macrophages.Quillardet al.[49]constructed the IONbased MMP-13 inhibitors(MMP13i-A agents)for enzyme responsive AS treatment.The fluorescence of such NDDSs kept quenched while delivering in the bloodstream.After reaching the plaque lesions,the NDDSs could be cleaved by the over-expressed MMP-13s and activated for AS theranostics.The NDDSs attenuated MMP-13 activities by ~53% and ~76% inactivated human-and murinemacrophages,respectively.Such inhibition caused thicker fibrous caps owing to elevated collagen contents in the AS plaques.
2.1.3.Other internal stimuli-response
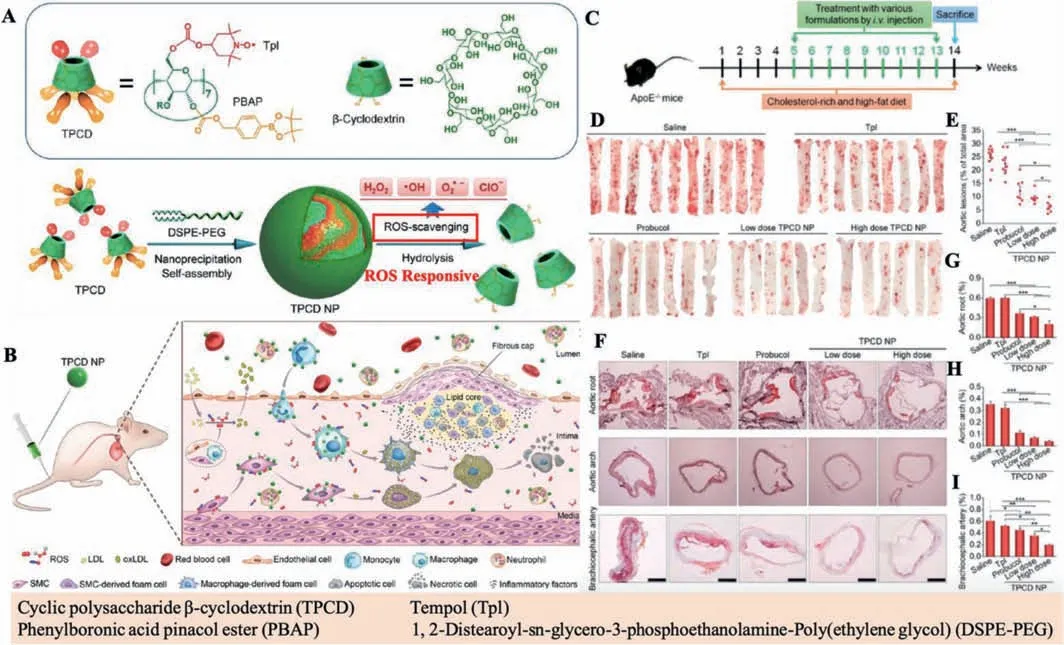
Fig.2.(A)Synthesis procedure of TPCD nanoparticle(TPCD NP).(B)Illustration of targeted treatment of AS by eliminating ROS.(C)Illustration of the treatment protocols.(D)Representative photographs of enface ORO-stained aortas from mice after diverse treatments.(E)Quantitative analysis of the aortas lesion area.(F)ORO-stained cryosections.(G-I)Quantitative analysis of the relative plaque area.Reproduced with permission[43].Copyright 2018,American Chemical Society.
It is well known that extracellular fluid at inflammatory sites is acidic.The hypoxic state of atherosclerotic lesions increases the concentration of lactic acid due to the accumulation of activated macrophages.Activated macrophages actively engulf ox-LDL with extremely high energy demand,forcing macrophages to produce ATP through anaerobic glycolysis,and uncontrolled secretion of lactate resulting in a decrease in the pH value of tissue fluid of atherosclerotic lesions[39,50].Naghaviet al.used two pH-sensitive fluorescent dyes to assess the pH of atherosclerotic plaquesin vivoand showed that the pH of atherosclerotic lesions in humans and rabbits was around 6.5–8.5 and 5.5–7.5,respectively.More importantly,the lysosomes were acidic,indicating that the pH range of macrophages in atherosclerotic lesions was around 4.7–4.8.Thus,in atherosclerotic lesions(pH about 6.0–6.8),drug release can be controlled under acidic conditions of macrophages and lysosomes(pH below 5.0)[51].
The development of AS causes vascular narrowing,which increases hemodynamic shear stress and vascular wall shear stress(WSS)in the atherosclerotic sites.The range of WSS in plaque was predicted to be 31.90–136.09 dyne/cm2,while WSS in normal vessels was 1–10 dyne/cm2.The shear stress of the fluid can be locally increased by one or two orders of magnitude,from 70 dyne/cm2in normal blood vessels to more than 1000 dyne/cm2[39,52].This abnormal shear stress can be used as a stimulus to trigger drug release and deliver the drug locally to the AS.In AS and vascular injury,platelet plays a key role.Due to the narrow and thrombosis blood vessels in the disease site,normal circulating platelets could be activated by the high shear stress in these areas and quickly adhesion in narrow veins,which is the main factor of the unstable atherosclerotic plaque formation[52].In this case,most micro and nano-scale materials for shear-mediated drug delivery mimic the action of platelet profile.Nowadays,with advanced researches,the materials of shear responsive NDDSs are not limited to platelet simulators,some polymer aggregates,hydrogels and emulsions are also studied[53].
Such high shear stress in AS provided us a novel method to develop shear-stress-responsive nanoplatform for AS treatments.Holmeet al.[54]constructed the shear-stress-responsive lenticular vesicles to target vulnerable atherosclerotic plaques for AS therapy.These obtained Pad-PC-Pad LUVET100 with the average size of 114 nm,were based on artificial 1,3-diaminophospholipids that were stable under static conditions but labile at elevated shear stress due to their lenticular morphologies,so the drugs could easily be released in the locations with high shear stress.More than 95% of carboxy fluorescein fluorophores could be successfully released from Pad-PC-Pad NPs while passing through a constricted artery with shear stress at ~50 dyne/cm2.
2.2.External stimuli-response
2.2.1.Light stimuli-response
In recent years,photo-responsive nanomaterials have been widely developed for imaging purposes and therapeutic applications.Compared with the visible region(400–700 nm),the light in the near-infrared(NIR)region(700–1700 nm)usually has deeper tissue penetration.More importantly,NIR photothermal agent could convert NIR light into heat energy,applied in temperatureresponsive drug release.Besides,such NIR agents could be stimulated by corresponding light to produce singlet oxygen as well.Therefore,NIR responsive agents are of great interest in AS studies[55,56].
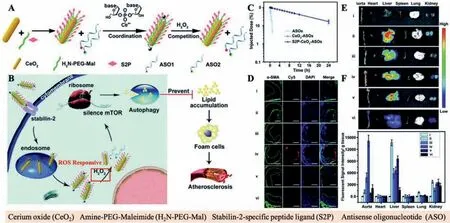
Fig.3.(A)Synthesis procedure of the H2O2-responsive and plaque-penetrating nanoplatform,S2P-CeO2-ASOs.(B)Illustration of targeted mTOR gene silencing to attenuate AS.(C)Pharmaco-kinetics study of diverse treated groups.(D)Fluorescence images of diverse treated groups 24 h post-injection.(E)Immunofluorescence micrographs of different treated groups.Reproduced with permission[44].Copyright 2018,Royal Society of Chemistry.
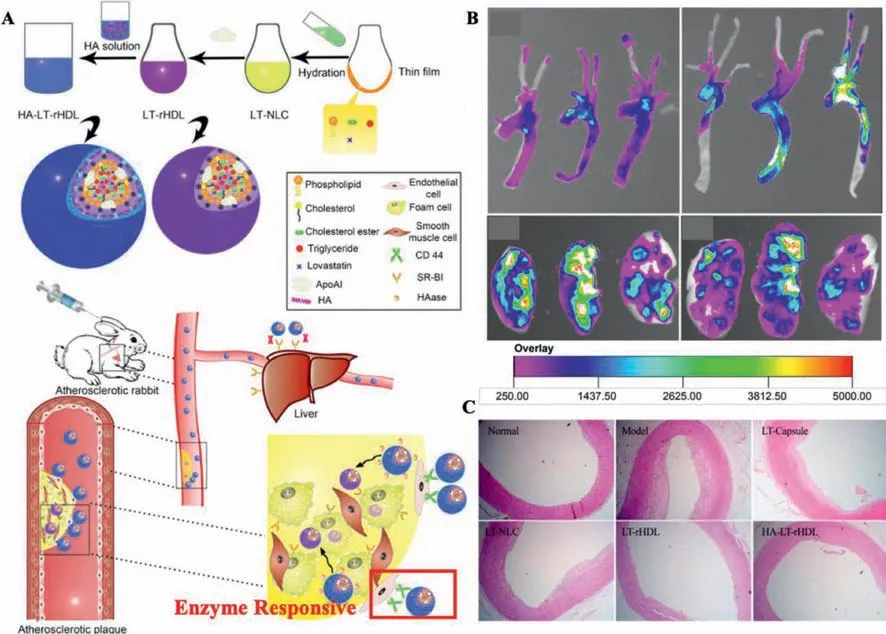
Fig.4.(A)Synthesis procedure of Lovastatin loaded HA-reconstituted high-density lipoprotein(HA-LT-rHDL).(B) Ex vivo imaging of livers and aortic trees in treated groups and control group.(C)H&E stained sections of all groups.Reproduced with permission[48].Copyright 2014,Elsevier Ltd.
Liet al.[57]have constructed the gold nanoparticles(Au NPs)doped paclitaxel(PTX)and vascular endothelial growth factor(VEGF)coloaded mesoporous macro-porous silica(MMS/Au/PTX/VEGF).Such nanoplatforms could slowly release PTX to prevent long-term tissue proliferation and rapidly release VEGF to repair blood vessels with NIR stimulation in AS treatments(Figs.5A and B).After the NIR stimulation,Au NPs could generate heat,and drugs can be released by suppressing the hydrophobic interaction and increasing diffusion due to the increased temperature.Such“on-demand”sequential release was beneficial to decrease the smooth muscle cells proliferation post endothelial repair.Moreover,the PTX release profile was closer to the first-order model under NIR stimulation,demonstrating that the PTX was more likely released from the multichannel.Due to VEGF existed in the macro-porous tubular structure,the release profile of VEGF confirmed to the Peppas model.
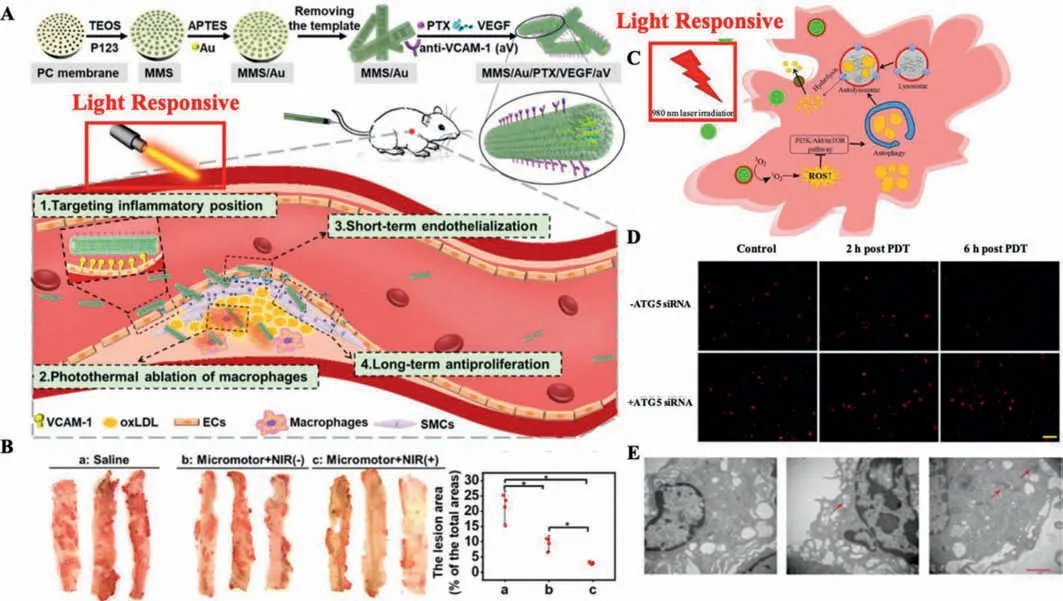
Fig.5.(A)Synthesis procedure of MMS/Au/PTX/VEGF/aV and mechanism illustration of AS therapy.(B)ORO staining and quantitative analysis of lesion area.Copied with permission[57].Copyright 2021,American Chemical Society.(C)Mechanism illustration of UCNPs-Ce6-mediated PDT induced autophagy.(D)PDT effect on the intracellular lipid burden.(E)Transmission electron microscopy of treated groups and control group.Reproduced with permission[58].Copyright 2017,Springer Nature Limited.
Hanet al.have built the Ce6 loaded up-conversion fluorescent nanoparticles(UCNPs)to achieve THP-1 macrophage-derived foam cells cholesterol efflux and explored the possible mechanism of NIR induced anti-AS PDT as shown in Figs.5C-E.They noticed that PDT could remarkably increase the autophagy induction and cholesterol efflux in peritoneal and THP-1 macrophage-derived foam cells.The ATG5 siRNA and 3-methyladenine(autophagy inhibitor)significantly attenuated NIR-induced autophagy and further inhibited the ABCA1-mediated cholesterol efflux.These findings verified that NIR induces cholesterol efflux through autophagy,and the ROS/PI3K/Akt/mTOR signaling pathway in peritoneal and THP-1 macrophage-derived foam cells could also mediate the autophagy[58].
2.2.2.Ultrasound stimuli-response
Ultrasound(US)has many advantages,such as low cost,noninvasive,real-time,high tissue contrast and security(no radiation)[59].Since the first generation of microbubble AlbunexR received FDA approval,the stability and biocompatibility of US contrast agents have been developed.And at the same time,the US contrast nanoagents have also been modified with ligands or antibodies that specifically and actively target certain diseases.With the help of the US forces,the drug can also be released from the ultrasound responsive NDDSs[60,61].
Wanget al.have constructed a vulnerable vascular plaque targeted material based on lipid nanoscale microbubbles(NBs)that promised faster drug release upon high acoustic power ultrasound stimulation.To release the entrapped drugs or gas in the NBs,an ultrasound stimulus was applied through a cavitation process known as sonoporation to affect the integrity of cellular membranes,thereby enhancing the drug delivery system,both endocytosis and transcytosis.Thein vivoresults verified that the targeted NBs could serve as the US imaging contrast agents with good bioactivity and biocompatibility,which demonstrated the potential application of the NBs to offer advantages to identify atherosclerotic plaques[62].Besides,Moccetti and coworkers have also tested the endothelial-targeted microbubble(MB)US contrast agents loaded small peptide ligands of the human-ready approach for molecular imaging of markers of high-risk atherosclerotic plaque[63].
2.2.3.Other external stimuli-response
Except for the above external stimuli-responsive NDDSs,magnetic nanoparticle(MNP)is another effective system to carry the drugs to the target siteviathe extra magnetic force.For example,Matuszak and co-workers[64]developed a magnetic drug targeting system based on superparamagnetic iron oxide nanoparticles(SPIONs)conjugating dexamethasone phosphate(SPION-DEXA)for accumulating drugs in specified vasculature regions.The SPION formulation used in this study could serve as the magnetic responsive drug carrier.It could be actively targeted to the lesion site using the external alternating-current magnetic stimulation,spin backward and forward at high velocity and eventually produce heat,thus released the DEXA for further AS treatments.However,MNPs in AS treatments are mainly focused on atherosclerotic plaques detection and drug intervention monitoring.The development of MNPs for AS theranostics still needs to be further explored.
Besides,as the reactivity to over one stimulus could increase the nanoagents performance,many studies have been focused on constructing multi-stimuli responsive NDDSs.Kimet al.[39]produced a dual-modal responsive nanoplatform(Mac TNPs)which could be activated under ROS and light stimulation.Mac TNPs will keep inactive(non-photo toxic and nonfluorescent)only under light exposure.Moreover,after internalized by macrophages and exposed to ROS,especially peroxynitrites,HA coating of the nanoplatforms will be degraded,which further causes photosensitizer release.As a consequence,the nanoplatform could emit fluorescence and produce singlet oxygen which causes apoptosis of macrophages.Similarly,Shon and co-workers[65]have constructed a dual-responsive nanoplatform(L-SR15 agents)which were active in response to the enzyme(cathepsin B)and light stimulation for AS treatments.Without the existence of specific enzyme-cathepsin Bs,the L-SR15 nanoplatforms were still inactive.Such L-SR15 nano-platform could realize NIRF imaging-guided deplete macrophages selectively by PDT,thus leading to cathepsin B activity reduction.
3.Classification of stimuli-responsive NDDSs for AS therapy
Over the past few decades,stimuli-responsive nanomaterials have been extensively investigated to treat various diseases ranging from cancer to inflammation,owing to their superiorities in precise,controllable,and targeted drug release[66–69].Generally,nano-materials are composed of organic,inorganic,and hybrid organic and inorganic nanomaterials.In this part,the progress of different stimuli-responsive NDDSs in the prevention and treatment of AS is summarized,and the development trend of nanomedicine will also be discussed.
3.1.Organic stimuli-responsive NDDSs
Apart from the common superior properties of organic NDDS,such as high compatibility,low toxicity,and natural targeting ability,some unique nature of both lipid-based and polymer-based nanomaterials belong to the category of organic nanomaterials,which can be prepared into liposomes,polymer micelles,polymer hydrogels,polymer conjugates and other drug nanocarriers for the mitigation of AS[70].These organic nanoparticles can control the active agent’s release by various stimuli-responsive chemical bonds or ligands.Here,we will detail introduce the different organic stimuli-responsive nanomaterials.
3.1.1.Lipid-based NDDSs
Generally,most phospholipids show the phase change at a constant temperature,thus some stimuli-responsive liposomes fabricated by these lipids can realize the temperature-responsive drug release.In addition,these liposomes could also be modified by stimuli-responsive chemical bonds,ligands or antibodies to achieve targeted and controlled drug delivery.In the context of treating AS,liposomes can preferentially accumulate in pathological sites such as the endothelial cells or macrophages,which are widely used because they meet most of the ideal conditions for NDDSs[71,72].
For example,Klegermanet al.[73]developed echogenic liposomes(ELIP)as an ultra-sound-enhanced controlled-release nanocarrier for AS therapy.Then,they loaded the therapeutic monoclonal antibody bevacizumab(BEV)to ELIP,which enables the appreciable ultrasound-triggered release of BEV,establishing a controlled-release drug delivery construct.The liposome structure can explain the possible ultrasound-responsive mechanisms.In general,the rarefaction phase of the sound wave must cause expansion of the air pocket and stretch the bilayer.Then,if the stress is sufficient to stretch the liposome membrane beyond its elastic limit,the liposome contents will be released.These studies initially established the application of external-responsive drug released liposomes for the treatment of AS.Still,there are relatively few relevant studies so far,which deserve further in-depth exploration for researchers.
In 2015,Calinet al.reported VCAM-1 directed PEGylated targeted-sensitive liposomes loading Teijin.In this formulation,the nanomaterials were prepared by DOPE,DOPA and a functionalized phospholipid anchor(Mal-PEG-DSPE),which covalently couple the peptide with an affinity for VCAM-1 to the liposome surface.Thus,most of the fused liposomes will be degraded by lipase enzymes on the VCAM-1 surface and collapse immediately,thereby releasing the entrapped Teijin.This study achieved efficiently AS therapy by the targeted enzyme-sensitive drug release and inhibiting the adhesion and transmigration of monocytes to activated endothelial cells[74].
3.1.2.Polymer-based NDDSs
In recent years,stimuli-responsive polymeric nanomaterials have been widely used in the therapy of AS and have become a hot research topic due to their excellent biocompatibility and drug delivery ability.Many types of polymer-based nanocarriers are synthesized by stimuli-responsive chemical monomer to achieve drug delivery and controlled release,such as polymer nanoparticles,polymer-drug conjugates,polymer nano-assembly,and polymer nanogel polyelectrolyte nanocomplex[75–77].
In 2015,Zhanget al.designed simvastatin(SIM)-loaded HAanchored core-shell HDL mimetic nanoparticles for AS therapy.The enzyme-responsive polymer nanomaterials contained a biodegradable PLGA core within a lipid bilayer,and apolipoprotein apoA-I absorbing on the lipid bilayer was covalently decorated with HA.Due to the hyaluronidase is abundant in AS plaque,the nanoparticles realized the SIM releaseviaapoA-I after HA coating degraded by hyaluronidase and also achieve targeting of endothelial macro-phages.Thus,as an efficient endothelial macrophagestargeted enzyme-responsive NDDS,hyaluronidase-responsive HDL mimetic nanoparticles are promising nanocarriers for AS therapy[78].
In 2020,Muet al.established SIM-loaded biodegradable polymeric micelles coated with HA for AS treatment,which assembled from an amphiphilic copolymer poly(ethylene glycol)-poly(tyrosine-ethyl oxalyl)(PEG-Ptyr-EO)(Fig.6).Due to the ROSresponsive nature of PEG-Ptyr-EO,the micelles can react with H2O2,and this process only occurs at the pathologic sites where the H2O2concentration is far above normal tissues.Therefore,the accumulation of pro-inflammatory macrophages and oxidative stress is alleviated.Importantly,SIM-loaded HA-coated micelles showed excellent AS plaque-targeted property,which exerts a synergistic effect for the effective treatment of AS[79].
3.1.3.Others NDDSs
Intelligent nano-hydrogels with a size of 1-1000 nm can also respond to external or internal stimuli with potential application in AS therapy.For example,in 2020,Yaoet al.developed composite hydrogel NDDSs with mechanical-sensitive drug release and targeting macrophages at thrombus.In this formulation,the HA modified with glycidyl methacrylate(GMA)is a freely cross-linkable macroscopic hydrogel precursor(HAGMA).An amphiphilic block copolymer micelle(CBC)was synthesized with tertbutyl acrylate(tBA),hydroxyethyl methacrylate(HEMA)and 2-ethylhexyl acrylate(EHA).Cross-linking HAGMA with CBC further improves the mechanical properties of the composite hydrogel(HACBC).Especially,the mechanical properties of hydrogels are enhanced by adding a block polymer micelle that can be freely cross-linked,thereby preparing a shear-responsive smart hydrogel.The HACBC is sensitive to mechanical stress and may realize the gradually stabilized to release the drug effectively.Thus,the conception and preparation of the hydrogel NDDSs propose a new feasible solution for AS treatment[80].
3.2.Inorganic stimuli-responsive NDDSs
By harnessing the unique magnetic,optical,and/or physicochemical properties of metallic inorganic nanomaterials,the selective release of therapeutic cargoes for AS therapy from the drugdelivery systems in response to a variety of remotely applied and local stimuli such as light,magnetic fields,US,and pH can be achieved.Generally,therapeutic drugs can be physically loaded inside the hollow or mesoporous nanostructures or chemically bonded to the surface of the nanomaterials to achieve targeted delivery of the drugs.Here we will introduce the metal-based and nonmetallic nanomaterials for AS drug de-livery and“on-demand”release.
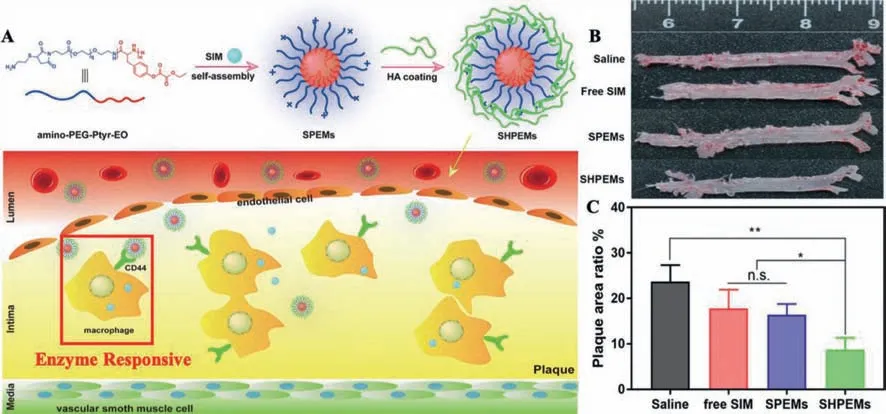
Fig.6.(A)Schematic illustration of preparation procedure and targeted delivery of SIM from the SHPEMs.(B)Representative photographs of ORO stained aortas.(C)Plaque area ratio of diverse treated groups.Reproduced with permission[79].Copyright 2020,Springer Nature Limited.
3.2.1.Metal-based NDDSs
Due to the unique properties,metal-based nanomaterials could exert diverse effects in AS theranostics.Herein,due to the extensive biomedical evaluation in animal andin vitrostudies,we address the importance of gold and iron oxide nanomaterials in AS detecting,specific responsive drugs and stem cells delivery.
Au NPs were initially fostered for cancer management and employed as drug delivery systems,contrast media and radioenhancers.The most common Au NPs were Au nanoshells,Au nanocages,Au nanorods and Au nanospheres.Among these Au nanomaterials,Au spheres and Au nanorods have been investigated as AS theranostic platforms.In the interesting work reported by Qinet al.,Au nanorods were constructed as theranostic agents for CT and NIR guided inflammatory macrophages photothermal ablating under light stimulation[81].
Recently,Bejaranoet al.developed gold nanoclusters(Au NC)complex nanoplatforms for angiotensin-(1–9)[Ang-(1–9)]NIRresponsive release[82].Au NC was grown on the thermal-sensitive liposome and displayed high energy absorption in the NIR wavelength range.Under NIR irradiation,Au NC could convert light energy to heat and cause a high temperature in the diseased sites.After reaching the phase transition temperature of the liposome(43 °C),Ang-(1–9)could re-lease from the Au NC-based nanoplatform and further elicit improved left developed ventricular pressure(LVDP).
Iron nanomaterials are clinically approved as biocompatible systems for drug delivery and MRI contrast agents in cancer therapy.For AS management,iron nanomaterials are primarily designated for atherosclerotic plaque tailoring and stem cell applications[83,84].In 2020,Liuet al.reported the development of Fe3S4NPs,which was expected to show photothermal and magnetothermal properties and be used to eliminate inflammatory macrophages in AS treatment.As expected,Fe3S4NPs could respond to light and magnetic,thus converted into heat energy and killed inflammatory macrophages,thereby inhibiting the progression of AS in ApoE-/-mice[85].In addition,these NPs also showed T2 MRI intensity(52.8 mm-1)culturedin vitro,which was the first study to combine photothermal and magnetic hyperthermia for NPs design.
Interesting work was demonstrated by Chandramouliet al.[86],who designed the superparamagnetic iron oxide nanoparticles(SPIONs)for plaque abrasion through magnetic-responsive hyperthermia.Such SPIONs could be actively targeted to the lesion site using the external alternating-current magnetic stimulation,spin backward and forward at high velocity,and eventually produce heat,reducing plaque’s hardness owing to transient thermal expansion.Such magnetic-responsive SPIONs could be applied in destroying the plaque by either interchange or rotation of MNPs until the plaque disappears from the blood vessel wall through abrasion.It is worth mentioning that,in cancer treatment,a high-frequency magnetic field could also be the driving force for drug release.However,magnetic hyperthermia applications have yet to be thoroughly studied for the controlled release of drugs in atherosclerosis management.
3.2.2.Nonmetallic NDDSs
Silica and carbon-based nanomaterials have received more attention in recent years for targeted drug delivery and treatment of diseases due to their porous structure and large surface area.Notably,the combination of metallic and nonmetallic inorganic materials has become a trend in nanotherapeutics,which will play a broader and more functional role in AS therapy.
Huanget al.[87]constructed paclitaxel-loaded platelet membrane bionic Janus mesoporous silica porous nanomotors(JAMS)that were obtained by asymmetric modification of platinum(Pt)nanoparticles.After the NIR stimulation,drugs could be released by suppressing the hydrophobic interaction and increasing diffusion due to the increased temperature.Meanwhile,the nanomotors can penetrate the plaque and enhance the drug retention efficiency,thus realize short-term photothermal elimination of inflammatory macrophages and the long-term anti-proliferation effect of the drug.The introduction of NIR-responsive nanomotor,platelet membrane coating and the combination of photothermal and drug therapy provided a new avenue for treating AS with high efficiency.Moreover,Kharlamovet al.[88]demonstrated multi-stimuli-responsive silica-gold iron-bearing nanomaterials for AS treatment.Such nanomaterials could be responsive to the US to improve targeting and imaging capacities and be delivered to the atherosclerotic lesions through a magnetic navigation system,which demonstrated the promising application for AS.
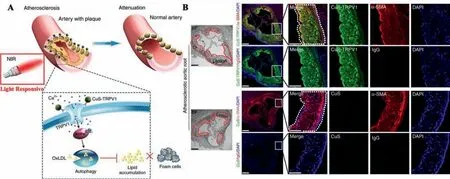
Fig.7.(A)Illustration of photothermal activation of TRPV1 signaling based on CuS-TRPV1 to attenuate AS.(B)Representative immunofluorescent micrographs of aortic roots sections.Reproduced with permission[90].Copyright 2018,Springer Nature Limited.
Recently,Liuet al.[89]firstly adopted Ce6 loaded,dextran sulfate(DS)linked chitosan-coated carbon nanocages(CS-CNCs)as a multifunctional theranostic nanoplatform,CS-CNCs@Ce6/DS.The high photothermal conversion efficiency of CS-CNCs ensured effective drug delivery during photothermal therapy(PTT).Moreover,a light-stimuli drug release profile was observed when the nanoplatform was under the 808 nm(NIR)laser irradiation,thus increasing the concentration of Ce6 in the atherosclerotic plaque area and the efficiency of photodynamic therapy(PDT).Sequential photodynamic/photothermal ablation of the activated macrophages decreased the pro-inflammatory cytokine secretion.Such nanoplatform could inhibit the proliferation and migration of smooth muscle cells,promising for the prevention of the AS-related disease.
3.3.Composite-based NDDS
Recently,the preparation of composite nanomaterials by combining organic and inorganic materials with different properties has been the focus of many researchers.For example,introducing inorganic nanomaterials into lipid or polymer nanomaterials for preparing multifunctional NDDSs containing contrast agents and therapeutic drugs,or modifying inorganic nanomaterials by using organic materials to improve their chemical and physical properties.Therefore,the composite of organic and inorganic materials to prepare composite multifunctional NDDSs with unique structures and diversified functions is a potential trend for NDDSs,which will have great significance for the treatment of AS.
Gaoet al.[90]designed and synthesized antibodies coupled with copper sulfide nanoparticles(CuS-TRPV1)for NIR-responsive AS treatments(Fig.7).Such nanoparticles could target transient receptor potential cation channel subfamily V member 1(TRPV1)and serves as a photothermal agent in VSMCs under NIR irradiation.The NIR-responsive temperature lift in the VSMCs could activate TRPV1 channels and lead to the Ca2+influx.Such enhanced intracellular concentration of Ca2+could reactivate the autophagic system and further inhibit the formation of foam cellsviathe AMPactivated protein kinase(AMPK)signaling pathway.
Notably,biomimetic nanoparticles have been employed in AS therapy,and different types of membranes have been used to fabricate biomimetic nanoparticles,such as macrophage membranes,red blood cell(RBC)membranes,and platelets.These membranecoated nanoparticles have merits of high biocompatibility,longcirculating half-life as well as disease-specific targeting.As a result,cell membrane-coated biomimetic nanoparticles have been employed in AS therapy.For example,in 2019,Wang’s group designed and constructed biomimetic NDDSs,consisting of rapamycin(RAP)-loaded PLGA nanoparticles as the core and RBC membranes as a cloak for effective treatment of AS[91].In the nanocomplexes,the biomimetic nature of the RBC interface resulted in less macrophage-mediated phagocytosis in the blood and enhanced accumulation of nanoparticles in the AS plaques,thereby achieving targeted drug-release.
In 2021,their group also designed and constructed macrophage membrane(MM)-coated biomimetic nanoparticles with RAPloaded PLGA copolymers(RAPNPs)[92].In this study,MM-coated RAPNPs(MM/RAPNPs)showed favorable sustained drug release and inhibited the phagocytosis by macrophagesin vitro.Additionally,in vivotherapy results showed that the MM/RAPNPs were effective for the targeted therapy of AS.More importantly,owing to the natural immune escape and specific recognition of inflammatory endothelial cells by macrophages,the nanomaterial also displayed desirable safety profile after long-term administration.Therefore,these membranes-functionalized biomimetic nanoparticles hold considerable promise as effective targeted NDDSs to treat AS.
In 2020,Li’s group developed site-specific microRNA-33 antagonism by pH-responsive monotherapies for the treatment of AS,as shown in Fig.8.In their study,the nanocarrier’s core comprises acetylatedα-cyclodextrin(AaCD),a pH-sensitive and biodegradable material that could be selectively hydrolyzed in subcellular endolysosomes mildly acidic conditions(pH 4.5–6.5).Additionally,the shell of the nanocarrier consists of PEG chains,can be further modified with targeted moieties.Thus,the nanocarrier can release the loaded anti-miR33 molecules subsequently in acidic endolysosomes when being endocytosed by target cells.From thein vitroandin vivostudies,anti-miR33 monotherapies could prevent AS progression efficiently,mainly through regulating vascular immune responses and promoting reverse cholesterol transport.Such nanobased therapy method is promising for targeted stimuli-responsive AS therapy[93].
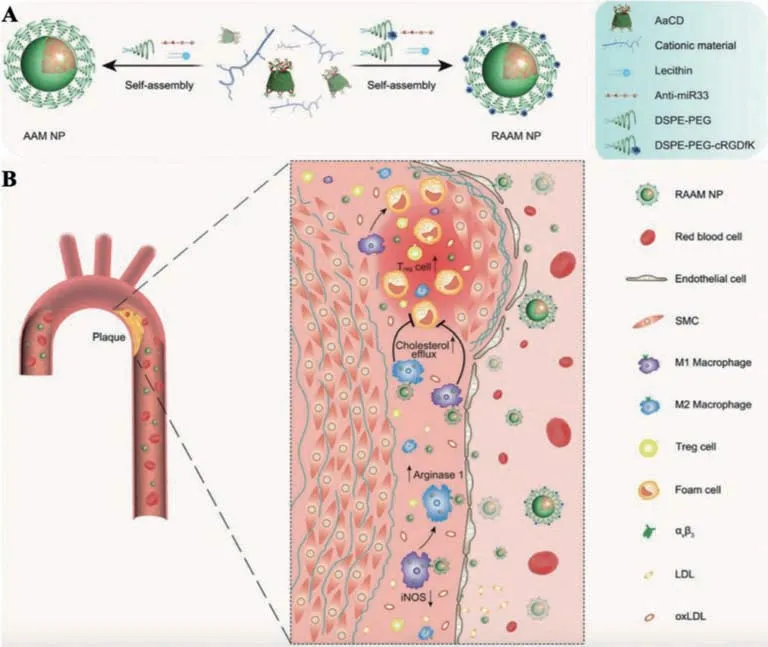
Fig.8.(A)Synthesis procedure of pH-responsive anti-miR33 nanoplatform.(B)Mechanism il-lustration of targeted AS treatment.Copied with permission[93].Copyright 2020,WILEY-VCH Verlag GmbH &Co.KGaA,Wein.
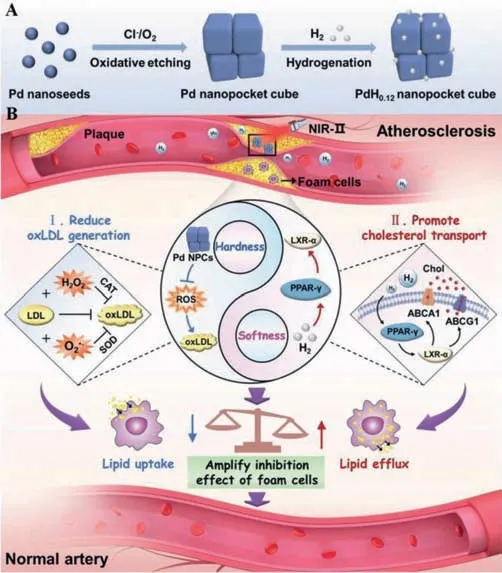
Fig.9.A“coupling hardness and softness”strategy of foam cell amplified-inhibition based on PtH0.12 NPCs for atherosclerosis attenuation.Schematic illustration of(A)the composition of the PdH0.12 NPCs and(B)the therapeutic mechanism for atherosclerosis.Copied with permission[94].Copyright 2021,WILEY-VCH Verlag GmbH &Co.KGaA,Weinheim.
In our very recent work[94],a“coupling hardness with softness”strategy was demonstrated through applying palladium hydride nano-pocket cubes(PtH0.12NPCs)to influence lipid uptake and efflux(Fig.9).The PtH0.12NPCs played the hardness role in response to ROS through the innate antioxidant enzyme activities of PtH0.12NPCs.Meanwhile,the hydrogen release profile could be controlled by light(near-infrared-II)and thus upregulate peroxisome proliferator-activated receptor gamma-mediated cholesterol transport.Consequently,ROS scavenging reduced lipid uptake and promoted cholesterol transport in-creases lipid efflux,resulting in the amplified-inhibition of foam cells.In vitroandin vivotests showed that PtH0.12NPCs significantly reduce lipid storage and plaque formation in Western diet-fed apolipoprotein E-deficient mice without obvious long-term toxicity.The dual ROS and enzyme responsive“coupling hardness with softness”strategy illustrated promising to be further developed as high efficacy and low toxicity method to treat atherosclerosis.
4.Conclusion and perspective
In this review,we systematically reviewed the recent advances in stimuli-responsive NDDSs for AS therapy in detail,including internal stimuli(reactive oxygen species,enzyme,shear stress,pH)and external stimuli(light,ultrasound,magnetic)responsive NDDSs.Besides,the classification of nanomaterials-based drug systems for AS therapy was summarized,such as organic NDDSs(lipid-based nanomaterials,polymer-based nanomaterials),inorganic NDDSs(metal-based nanomaterials,nonmetallic nanomaterials),and composite multifunctional NDDSs.Compared with traditional drug delivery methods,NDDSs could achieve better pharmacokinetic parameters and tissue specificity,higher drug loading and encapsulation efficiency,higher targeted and responsive drug release,which can improve the solubilization,half-life,biodistribution of drugs,and reduce the systemic toxicity and adverse effects[85,86].
Among these NDDSs,organic-based NDDS performed some superior properties,such as high compatibility,low toxicity,and natural targeting ability.Besides,some unique nature of both lipidbased and polymer-based nanomaterials belonged to the category of organic nanomaterials,which can be prepared into liposomes,polymer micelles,polymer hydrogels,polymer conjugates and other drug nanocarriers for the mitigation of AS[95],while the inorganic-based NDDSs possessed a series of excellent properties,such as strong near-infrared light absorption,high photothermal conversion efficiency,multi-model imaging capacity and easy preparation[96].However,poor biocompatibility and difficult biodegradation largely limited the development of inorganic-based NDDSs.Composite-based NDDSs could synergistically combine the advantages of organic and inorganic NDDSs,thus achieving targeting,controlled drug release,enhanced imaging,AS therapy,and low toxicity in the same time.The combination of organic and inorganic NDDSs signified the remarkable progress in the development of functional hybrids,which triggered the switching nature of the nanohybrid and displayed on-off behavior in response to various stimuli.Stimuli-responsive composite-based NDDSs have been applied in various fields,such as catalysis,drug delivery,gene delivery,sensing and imaging.Undoubtedly,considerable development has already been made with these NDDSs in AS treatments.
Although tremendous interesting studies reported in this field in the last few years,there are still some potential challenges against these stimuli-responsive NDDSs with structure design,biostability,targeting efficiency,toxicity and clinical transformation.The discovery of stimuli-sensitive NDDSs with good bioactivity and bio-compatibility is preferred in AS treatment.Nanogels or hydrogels with the capacity of encapsulating diverse biologically active molecules(genes,proteins,drugs,etc.)and programmed releasing the cargo under various stimuli(redox potential,enzymes,temperatures)hold great potential for stimuli-sensitive NDDSs[85].Moreover,to enhance drug delivery efficiency and prolong the blood circulation time,the multi-potential applications of inorganic nanoplatforms could be optimized by combined with organic nanomaterials.The application of NDDSs with imaging contrast capacity is an exciting option.The imaging modalities could detect through magnetic-,ultrasound-,or light-activation and enable realtime imaging-guided therapy,thus improving targeting efficiency[87].
Current nanomaterials-based approaches for AS treatments have mainly focused on the anti-inflammatory effectsviatargeting foam/macrophages cell developments and the monocytes recruitment in the AS plaques.However,monoclonal antibodies,gene therapy,and other therapeutic approaches could also be promising if combined with the potentially beneficial applications of nanomaterials[88].Especially,owing to the specificity and efficiency of the silencing effect on target genes,small interfering RNA(siRNA)technology has received widespread attention in the long-term treatment of AS.For instance,in 2018,Zhao and coworkers constructed advanced PLGA NPs to silence lectin-like ox-LDL receptor-1(LOX-1)on macrophages using siRNA for the AS treatment[97].In a recent study,Liuet al.fabricated graphene quantum dots(GODs)-miR-223 complexes,which shown advantages of organic and inorganic nanomaterials as well as biologic components.The nanocomplexes could deliver miR-223 for regulating the inflammation in the plaques,thereby demonstrated desirable therapeutic effects for AS[98].Inspiringly,the expression pattern and biological functions of siRNA have been partially elucidated in recent years.Therefore,it is believed that the role of siRNA in the treatment of AS will become increasingly prominent in the future[99].
Finally,in recent years,extrapolating the great advancements in preclinical studies,only some nanomaterials,albeit slowly,have been studied in clinical trials for AS.Typically,lipid-based and inorganic NPs are the main NDDSs that have been used in clinical studies for the treatment of AS.For example,lipid-based NPs,such as liposomal NPs encapsulating prednisolone phosphate(LN-PLPs,NCT01039103,NCT01647685,NCT01601106)and paclitaxel-loaded lipidic emulsion(PTX-LDEs,NCT04148833),acted as NDDSs to treat AS by targeting AS plaque macrophages in patients.Unfortunately,LN-PLP did not show satisfactory anti-AS effects in patients as they didin vitroandin vivostudies[100].PTX-LDE demonstrated inconclusive results regarding its efficacy,likely due to the small size study group[101].In addition,inorganic NPs,such as silica-gold NPs(NANOs,NCT01270139)have been used along with plasmonic PTT to reduce total atheroma volume[102].However,the potential cytotoxicity of metal NPs was a major concern for the use of inorganic NPs in large clinical trials.Due to the higher cost and longer time thanin vitroorin vivomodels,it has been a big challenge for the effective translation of NDDSs into clinical studies.Thus,in future studies,more attention may be devoted to evaluating the efficacy and safety of these NDDSs in AS therapy,thereby moving forward to future clinical translation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the financial support from the Young Elite Scientists Sponsorship Program by Tianjin(No.0701320001),Major Special Project of Tianjin(No.0402080005),Program for Excellent Innovative Talents in Universities of Hebei Province(No.BJ2021019)and Vietnam National University,Ho Chi Minh City(VNU-HCM,NCM2020-28-01).
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Applying nanotechnology to boost cancer immunotherapy by promoting immunogenic cell death
