Applying nanotechnology to boost cancer immunotherapy by promoting immunogenic cell death
Lvqin Fu,Xianbin Ma,Yuantong Liu,Zhigang Xu,Zhijun Sun
a The State Key Laboratory Breeding Base of Basic Science of Stomatology(Hubei-MOST)&Key Laboratory of Oral Biomedicine Ministry of Education,School&Hospital of Stomatology,Wuhan University,Wuhan 430079,China
b Key Laboratory of Luminescence Analysis and Molecular Sensing,Ministry of Education,School of Materials and Energy &Chongqing Engineering Research Center for Micro-Nano Biomedical Materials and Devices,Southwest University,Chongqing 400715,China
1 These authors contributed equally to this work.
ABSTRACT Tumor immunotherapy,especially immune checkpoint blockade(ICB),has revolutionized the cancer field.However,the limited response of tumors to immunotherapy is a major obstacle.Tumor immunogenic cell death(ICD)is a death mode of tumor cells that can promote tumor immunity.ICD can induce strong antitumor immune responses through the ectopic exposure of calreticulin on the plasma membrane surface and the release of the non-histone nuclear protein high-mobility group box 1(HMGB1),ATP,and interferon(IFN),thus activating an adaptive immune response against dead cell-associated antigens and enhancing the therapeutic effect of tumor immunotherapy.Chemotherapy,radiotherapy,photothermal therapy,magneto-thermodynamics therapy,nanopulse stimulation,and oncolytic virus therapy can all induce a strong antitumor immune response by ICD.In addition,the application of nanotechnology can precisely target drug delivery and improve the efficacy of immunotherapy.Here we introduce the basic concepts and molecular mechanisms underlying the induction of ICD.Then,we summarize and discuss the progress in the application of nanotechnology in immunotherapy to promote ICD.Finally,we attempt to define the challenges and future directions in this area to extend the benefits of ICD to a broader patient population.
Keywords:Immunogenic cell death Nanotechnology Cancer immunotherapy Combination therapy Immune checkpoint blockade
1.Introduction
Research on tumor immunotherapy has created extended progress in recent years,suggesting that immunotherapy has the potential to attain general and adaptive cancer control[1,2].Current tumor immunotherapy includes adoptive T-cell transfer,immune checkpoint blockade(ICB),and injection of immune-related cytokines[3].However,the clearing of tumor cells by the immune system is smothered by various pathways[3–5].The abnormality of solid tumor blood vessels and,therefore,the high-pressure level of native tissues hinder the transport of immune cells[6].Growing tumor cells can even produce an associate degree immunosuppressive tumor microenvironment(TME)by secreting immunosuppressive molecules and attracting immunosuppressive cells,which has become a severe barrier to causing effective antitumor immunity and effective tumor immunotherapy[3,7].Although immune checkpoint blockade(ICB)has recently achieved clinical success,the therapeutic impact depends on programmed cell death ligand 1(PD-L1)expression in cancer cells and the immunogenicity of cancer[8,9].Tumors lacking T-cell infiltration are considered "cold tumors," exhibiting low immunogenicity and inadequate response to ICB[10–12].In addition,"cold tumors" are characterized by a low mutational load and the presence of various immunosuppressive cells[13].Tumors with a considerable T-cell infiltration are considered "hot tumors," exhibiting high immunogenicity and PDL1 expression[14,15].In contrast to "cold tumors","hot tumors"are less effective to ICB(Fig.1)[8,16].
Clinical results suggest that immunotherapy will doubtless accomplish systemic and adaptive cancer control,combined with additional treatment modalities necessary to maximize the advantages of immunotherapy and increase the proportion of a tumorspecific immune response[17].Many experiments have proven that tumor immunogenic cell death(ICD)can stimulate innate and adaptive immunity to control tumors effectively.When combined with checkpoint blockade,ICD can also control primary and distant tumors for extended periods[18].ICD can promote antigen presentation and T cell aggregation through a series of molecular mechanisms,which have specific auxiliary effects on tumor immunotherapy.ICD relies heavily on activating regulatory responses in dead cells,ultimately leading to the exposure or secretion of immune-stimulatory molecules.In an immunocompetent host,ICD will activate the adaptive immune response and regulate the immune response of dead cell-associated antigens[19–22].
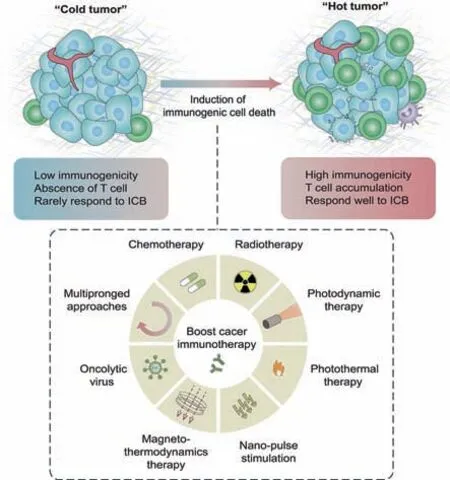
Fig.1.Transformation of low-immunogenic "cold tumors" into high-immunogenic"hot tumors" by inducing tumor immunogenic cell death.Inducing immunogenic cell death(ICD)through multiple strategies can effectively improve T cell infiltration in tumors and enhance immune checkpoint blockade(ICB)efficacy.
ICD can be induced by chemotherapy,radiotherapy,photodynamic therapy(PDT),photothermal therapy(PTT),magnetothermodynamics therapy(MTD),nano-pulse stimulation(NPS),and oncolytic virus(OVs),which can improve the immune microenvironment of the tumor.In this review,the application of nanotechnology in cancer therapy was reviewed to summarize the research progress in exploring enhancing the ICD of tumor cells in tumor therapy.In addition,we sought to identify challenges and future directions in this area to expand the benefits of ICD to a broader patient population.
2.Immunogenic cell death
2.1.Salient features of ICD
ICD is a cell death type that may activate the adaptive immune response against the dead cell-associated antigen within the active immune host.ICD-activated immune cell recognition and antitumor immune response rely on damage-associated molecular patterns(DAMPs),including the exposure of calreticulin(CRT),the secretion of ATP,ANXA1,type I interferons(IFNs),and the passive release of the non-histone nuclear protein high-mobility group box 1(HMGB1)due to plasma membrane rupture(Fig.2)[5,23–27].
ATP and HMGB1 are secreted into the TME.ATP binds to P2RX7,promoting the accumulation of dendritic cells(DCs),and HMGB1 binds to toll-like receptors 4(TLR4)to promote DCs to support the optimal processing of tumor antigens and their presentation to cytotoxic T lymphocytes(CTLs)while secreting interleukin(IL)-1β.Type I IFNs can stimulate the activation of DCs,macrophages,and natural killer(NK)cells.The subsequent adaptive immune response was also involved in the production of IL-17-producingδT lymphocytes.γ δT cells andαβCTLs mediate the direct antitumor effectviathe secretion of IFN-γand granzyme-perforin[28].Eventually,the immune system is activated,and then the immune response targeting abnormal cancer cells is activated,and some CTLs obtain a memory phenotype,which lays the foundation for the establishment of long-term immune protection(Fig.3)[29].Other DAMPs,such as the chaperones of the heat shock protein(HSP)family and IFN-α,have been found and confirmed been shown to contribute to ICD[30].
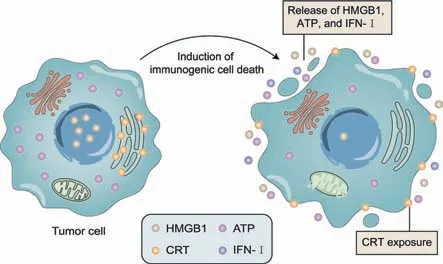
Fig.2.Schematic representation of immunogenic cell death(ICD).When ICD is induced in tumor cells by chemotherapy and radiotherapy,tumor cells rupture and express damage-associated molecular pattern(DAMP)hallmarks.These hallmarks include exposure of the endoplasmic reticulum protein calreticulin(CRT)and release of high-mobility group box 1(HMGB1),ATP,and type I interferons(IFNs)into the extracellular space.
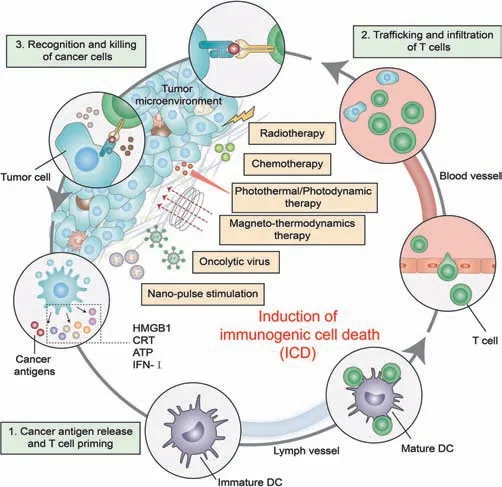
Fig.3.ICD activates the host’s adaptive immunity to dead cell-associated antigens.ATP promotes the accumulation of DC cells,calreticulin(CRT)exposure promotes the phagocytosis of DC cells to antigen,HMGB1 promotes the antigen presentation of DC cells and finally realizes tumor-specific immunity.
2.2.Common ICD inducers and their induction mechanisms
According to current research,ATP secretion relies on autophagy,and type I IFNs can be secreted in response to virus or bacterial infection[31].The endoplasmic reticulum(ER)stress response is related to the exposure of ER chaperones(such as CALR,PDIA3,and HSP70),converting to ICD-promoted signaling.The inhibition of protein glycosylation,accumulation of misfolded proteins,and relaxation of Ca2+and redox homeostasis regulation inhibit the homeostasis of the ER Golgi network,leading to activation of the ER stress response[32].At present,the molecular mecha-nism of HMGB1 and ANXA1 release from ICD cells remains elucidated[31].The current gold standard method for determining whether cell death is immunogenic depends on vaccination experiments injecting dead cells into syngeneic mice with immune activity[25].
3.Application of nanoparticles in enhancing tumor ICD
Current experiments have proven that the cancer treatment methods that can induce ICD include chemotherapy,radiotherapy,PDT,PTT,NPS,MTD,and oncolytic virotherapy.There are various ways of inducing ER stress,chemotherapy drugs(anthracycline,oxaliplatin),ultraviolet light,OVs,hydrostatic pressure,PDT,and so on[33,34].All of these can stimulate ER stress and produce ICD related DAMPs directly or indirectly.The role of nanotechnology in promoting ICD in tumor immunotherapy is enhancing the original ICD-promoting effects by improving the targeted tumor delivery and increasing drug accumulation in tumors(Table 1).The use of nanotechnology can also help achieve the combined application of multiple therapies and improve the TME and immune response by designing appropriate nanoparticles.Nanotechnology also helps accurately control drug dosage.For example,Zhanget al.designed micellar delivery to maintain a desired drug ratio ICD can be induced by quercetin and alantolactone at a molar ratio of 1:4[35].In addition,nanotechnology can be used in nonpharmacological cancer treatments to stimulate ICD,such as NPS.
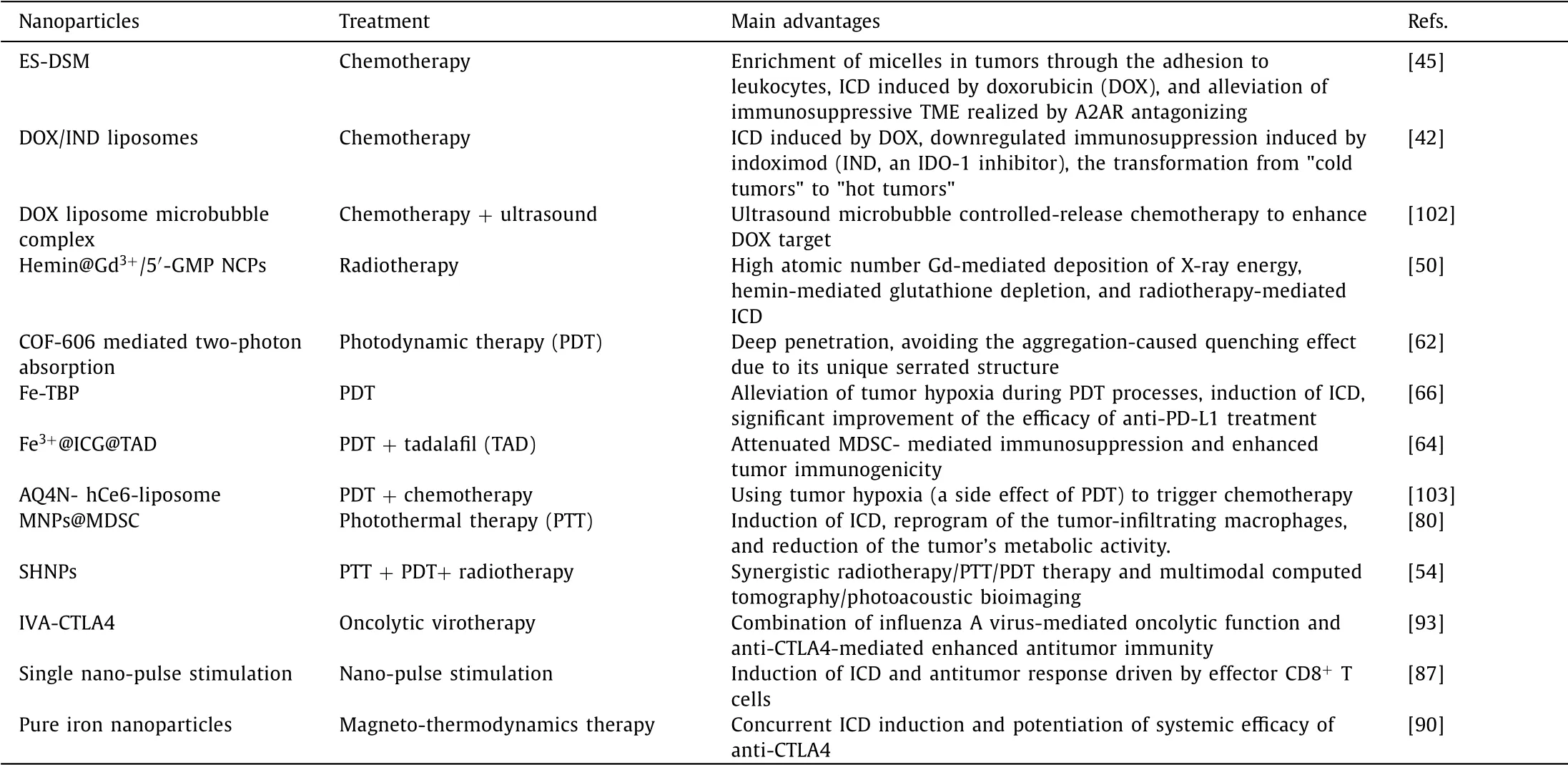
Table 1 Examples of nanoparticles in enhancing tumor immunogenic cell death(ICD).
3.1.ICD induction by chemotherapeutic drugs
Not all chemotherapeutic drugs can induce ICD.Currently,the chemotherapeutic agents including doxorubicin(DOX),epirubicin,idamycin,mitoxantrone(MIT),bleomycin,botezomib,cyclophosphamide,and oxaliplatin(OXA)and so on,which are currently capable of being identified,may be related to their pharmacological actions[36,37].The advantage of the delivery of chemotherapeutics with nanocarriers is that the ability to decrease the dose by improving the dynamic mechanical properties of drug delivery and increasing the concentration of drugs at the target tumor.The common nanoforms of chemotherapeutic drugs include polymer nanoparticles,micelles,dendrimers,and liposomes[38].
Royet al.prepared poly(lactic acid glycolic acid)(PLGA)-based paclitaxel(PTX)-and SP-LPS-containing nanoparticles(TLNPs)using the double emulsion method(w/o/w)[39].Compared with the commercial paclitaxel treatment group,the TLNP group had a higher PTX content in tumor tissue,antitumor activity,and activated immune cells.This result suggested that although nanoparticle encapsulation does not confer ICD-mediated antitumor ability to non-ICD inducers,nanoparticle-encapsulated ICD inducer therapy can significantly enhance ICD,thereby improving the antitumor effect.Furthermore,Jihoon Kim uses a new delivery system(pPTX/pCD-pSNO)formed by polymerized PTX(pPTX)and polymerizedβ-cyclodextrin(pCD-pSNO)multivalent host-guest incorporation of nitric oxide to solve the drawbacks of traditional therapy.Their experiments observed that pPTX/pCD-pSNO could induce ICD and a more robust immune response to cancer cells than the free preparations used alone or in combination,induce the activation of DCs and the expansion of T cells which had a more significant killing effect on tumor[40].Therefore,ICD induction combined with other therapies can effectively inhibit tumor growth and reconstruct and improve TME.
Studies have found that nanocarriers to achieve chemotherapy drugs and other antitumor immune-related drug coadministration can improve the effectiveness of antitumor treatment.The immunosuppression caused by indoleamine 2,3-dioxygenase(IDO)tends to prevent ICD from exerting antitumor effects.NLG919 can down-regulate IDO-1-mediated immunosuppression and inhibit regulatory T cells(Tregs).Fenget al.used a pH and glutathione(GSH)dual sensitive multifunctional NP system(BCPN)to encapsulate NLG919 and OXA,which can change from negative charge to positive charge in the acidic environment of the tumor,thus promoting the accumulation and penetration of drugs in the tumor site,and then OXA and NLG919 are activated under reducing conditions[41].OXA triggers ICD in cancer cells,which effectively inhibits breast cancer growth in combination with NLG919.Luet al.designed a chemical immunotherapeutic method to synthesize dual-channel DOX/IND liposomes[42].Indoximod(IND)is an IDO-1 inhibitor.Compared with pure DOX liposomes(DOX-NPs),double carriers can significantly enhance the antitumor immune response of primary and metastatic breast cancer sites.In addition,DOX/IND liposomes generate an immune replete "hot" breast cancer TME,which can effectively combine with anti-PD-1 to eliminate lung metastasis.
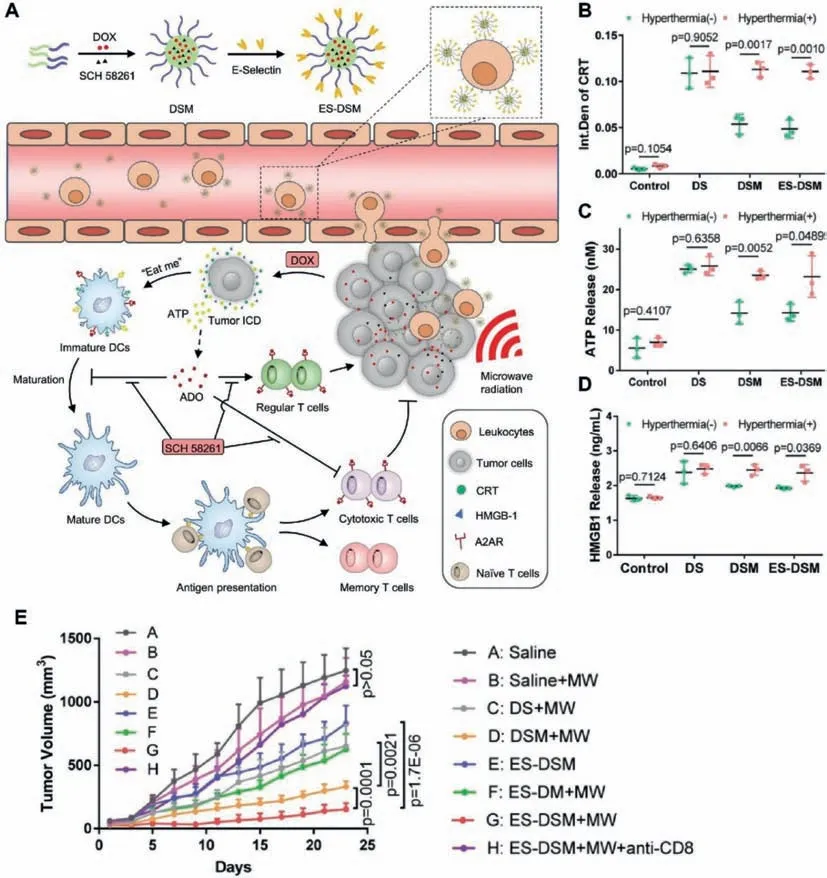
Fig.4.Schematic illustration of the E-selectin-modified thermal-sensitive micelles(ES-DSM).(A)Preparation process and the antitumor mechanism of ES-DSM.(B)Semiquantitative analysis of CRT exposure of 4T1 cells observed by confocal microscopy after different treatments.(C)ATP secretion was detected by luciferase conversion assay.(D)HMGB1 release was measured by ELISA kits.(E)Tumor growth curves of mice after various treatments.Reproduced with permission[45].Copyright 2021,Nature Publishing Group.
The occurrence of ICD in tumor cells is accompanied by the release of large amounts of ATP.Large amounts of ADO are generated when ATP is catalyzed by two ectonucleotidases,CD39 and CD73[43].ADO can bind to A2AR,which is widely present on the surface of various immune cells,and exacerbate the immunosuppressive TME[44].Therefore,the contradiction between ICDinduced antitumor immunity and ADO-mediated immunosuppression is a great challenge.Qiet al.designed a thermal-sensitive micelle co-loaded with DOX and the A2AR antagonist SCH 58261 and modified the surface with E-selectin(Fig.4)[45].The E-selectinmodified thermal-sensitive micelles(ES-DSM)adhered to the surface of leukocytes after intravenous injection and migrated to the tumor site with the leukocytes,achieving a high enrichment at the tumor site.Under local microwave stimulation,DOX is rapidly released and induces ICD in tumors,activating antitumor-specific immune responses.SCH 58261 alleviates the immunosuppressive TME by effectively antagonizing A2AR,ultimately realizing the synergistic antitumor effects of chemotherapy and immunotherapy.
3.2.ICD induction by radiotherapy
Radiotherapy involves irradiating cancerous tissue with radiation,such as X-ray,γ,and high-energy electron beams.The energy carried by radiation causes DNA double-strand breaks,which are not limited by tissue penetration,to counter the cancer cells that rapidly grow and divide.Surface exposure to CRT and the release of HSP70 and HMGB1 suggest that radiotherapy induces ICDin situ,stimulates DC maturation,and induces IFN-γ-producing T cellsin vitroandin vivo[46].
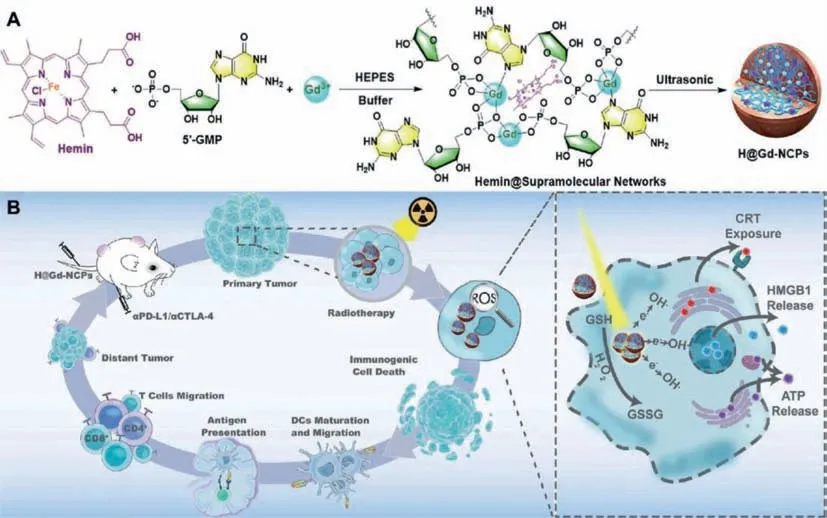
Fig.5.Mechanism of antitumor immune responses induced by H@Gd-NCPs.(A)Preparation process of gadolinium-hemin based nanoscale coordination polymers(NCPs)Hemin@Gd3+/5′-GMP NCPs(H@Gd-NCPs).(B)Schematic illustration of the antitumor mechanism of H@Gd-NCPs.Reproduced with permission[50].Copyright 2021,Nature Publishing Group.
However,tumor cells have a poor absorption capacity for Xrays,and there are also many hypoxic regions within solid tumors,especially overexpressed H2O2and abnormally regulated GSH[47–49].These factors considerably reduce the efficacy of radiotherapy,resulting in insufficient radiotherapy-induced ICD and making it difficult for radiotherapy to elicit a systemic antitumor immune response.Huanget al.constructed gadolinium-hemin based nanoscale coordination polymers(NCPs)Hemin@Gd3+/5′-GMP NCPs(H@Gd-NCPs)by supramolecular self-assembly technique(Fig.5)[50].As a nanoscale radiotherapy sensitizer,H@Gd-NCPs effectively realize the integration of diagnosis and treatment.H@Gd-NCPs can effectively deposit X-ray energy at relatively low radiotherapy doses using high atomic number Gd to increase the local radiotherapy effect.In addition,H@Gd-NCPs can utilize the overexpressed H2O2and GSH in the TME through hemin with peroxidase-mimic catalytic activity to disrupt the abnormally regulated redox balance in tumor cells.Ultimately,H@Gd-NCPs induce more potent tumor ICD in concert with radiotherapy and improve the response rate of ICB.
Chenet al.[51]encapsulated water-soluble catalase(Cat)in the nucleus and loaded imiquimod(R837),which is a TLR-7 agonist,into the PLGA shell to prepare polylactic acid-co-ethanol-based shell nanoparticles of PGA(PLGA)PLGA-R837@Cat.Cat can effectively alleviate tumor hypoxia and relieve the limitation of tumor hypoxia-related radiation resistance.In the study,R837 can induce ICD in the tumor,and then tumor-associated antigen can induce a robust antitumor immune response.Combined with checkpoint blocking therapy of cytotoxic T lymphocyte-associated antigen-4(CTLA-4),R837 can eliminate the tumor and effectively inhibit tumor metastasis.In addition,Chaoet al.also intratumorally injected131I Cat sodium alginate preparation in the presence of endogenous Ca2+[52].Soluble polysaccharides quickly transformed into hydrogels to fix131I-Cat at the tumor site.Simultaneously,preparations supplemented with immunostimulatory cytosine-phosphateguanine(CpG)oligonucleotides were treated with local radiotherapy at low doses and combined with systemic checkpoint blockade therapy using anti-CTLA-4 antibodies,which led to suppression of metastasis and prevention of tumor recurrence.This finding offers new options for patients with advanced cancer that cannot be cured by surgery or radiation.After the combined treatment,the survival rate reached 80% after the 50thday,and no metastasis was observed after 90 days.
Radiotherapy can be combined with other immunotherapies to demonstrate a more potent induction of ICD and stimulate a more robust antitumor immune response.Hadiet al.combined radiotherapy with radiofrequency(RF)hyperthermia by gold-coated iron oxide nanoparticles(Au@IONPs),acting as the thermoradiosensitizer.The results showed that the combination of radiotherapy and RF had a synergistic effect on improving the survival rate of normal tissue cells,the level of apoptosis induction of tumor cells,and tumor treatment.In contrast,radiotherapy and RF alone had no significant effect compared with the combination[53].Guoet al.designed BiOI@Bi2S3@BSA(bovine serum albumin)semiconductor heterojunction nanoparticles(SHNPs)for triple-combination radiotherapy/PDT/PTT cancer therapy and multimodal computed tomography/photoacoustic(CT/PA)bioimaging[54].SHNPs have a strong X-ray attenuation ability due to their highZcontent,which is expected to be a potential radiosensitizer.SHNPs can provide reactive oxygen species through PDT and have strong infrared light absorption capacity,which can efficiently convert light energy into heat energy and provide PTT.Studies have shown that any combination of radiotherapy,PDT,and PTT can achieve a more significant therapeutic effect than any single therapy.
3.3.ICD induction by PDT
PDT produces reactive oxygen species(ROS,such as1O2)by combining nontoxic photosensitizers,light,and tissue oxygen to achieve local tumor control/rejection[38].Photosensitizer produces highly cytotoxic reactive oxygen species in the presence of oxygen,which can cause ER stress to promote ICD,leading to oxidative stress-based cell death and destruction of the tumor vasculature[55].In general,PSs are hydrophobic and aggregate in aqueous media,resulting in reduced solubility and decreased reactive oxygen species.Nanoparticles are ideal for overcoming tumor conditions that limit PDT and are designed to increase tumortargeting transportation to reduce side effects in normal tissues[56,57].The results showed that PDT mediated by nanoparticles could induce ICD,activate the innate immune system and adaptive immune system of TME,and effectively improve the activity of the host immune system.When combined with checkpoint blocking immunotherapy,PDT could enhance the efficacy and improve the systemic antitumor response[58,59].
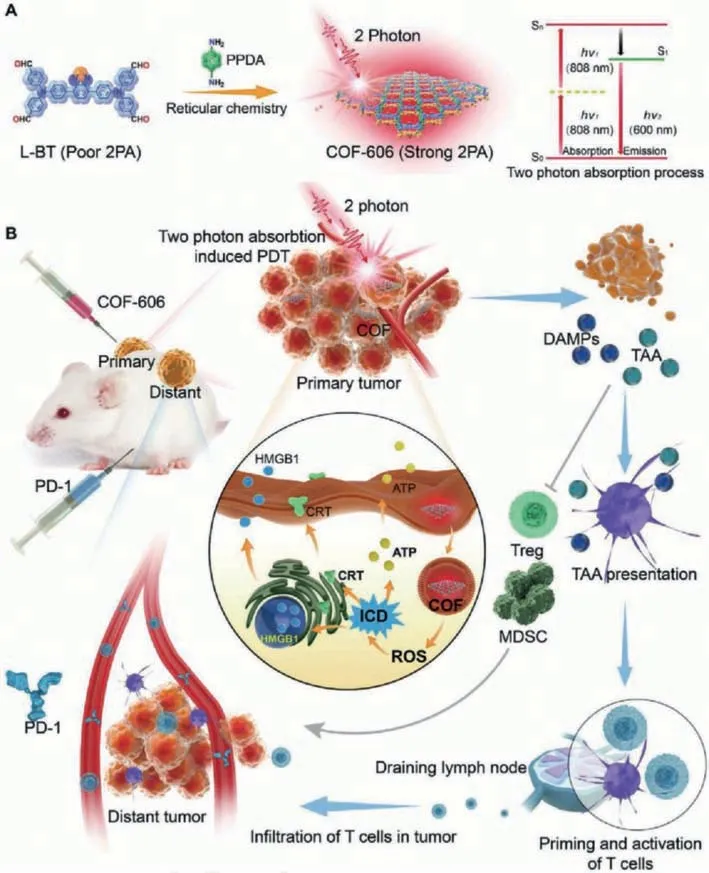
Fig.6.Schematic of COF-606 mediated two-photon absorption(2PA)induced PDT to enhance cancer immunotherapy by combining ICD and anti-PD-1.(A)Fabrication of COF-606 with strong 2PA.(B)The antitumor mechanism of COF-606 mediated 2PA induced PDT by combining ICD and anti-PD-1.Copied with permission[62].Copyright 2021,Wiley-VCH.
The limited penetration of light limits to some extent the synergistic application of PDT with immunotherapy.Two-photon absorption(2PA)can penetrate deep tumor tissues and stimulate robust antitumor responses[60].However,conventional 2PA photosensitizer nanoparticles undergo strong aggregation-caused quenching(ACQ),limiting ROS production and the therapeutic effect of PDT[61].Covalent organic framework(COF)-606 avoids the ACQ effect due to its unique serrated structure and has good 2PA performance and photostability.Yanget al.used COF-606 as a 2PA photosensitizer to induce PDT(Fig.6)[62].2PA-induced PDT mediated by COF-606 effectively induced ICD in breast cancer and significantly reduced Tregs and MDSCs in TME.In addition,COF-606-mediated PDT increased T-cell infiltration at tumor sites and further enhanced anti-PD-1 therapy.
Myeloid-derived suppressor cell(MDSC)is a population of immature myeloid cells that can suppress antitumor immunity by producing high levels of arginase and PD-L1[63].The combination of PDT and targeting MDSC has significant antitumor effects.Zhanget al.used Fe3+as a mediator to assemble tadalafil(TAD)and ICG into a supramolecular system(Fe3+@ICG@TAD,FIT)(Fig.7)[64].The higher affinity of Fe3+to the S oligopeptides of GSH in the TME caused the FIT NPs to dissociate,releasing TAD and ICG in the tumor site.TAD effectively suppresses the function of MDSC in the TME,while ICG-mediated PDT enhances the immunogenicity of tumor cells by inducing ICD to produce anin situ"vaccine." The nanoparticles effectively improve the defects of ICG instabilityin vivoand the side effects caused by TAD and can also be used for near-infrared fluorescence and photothermal imaging.Overall,FIT NPs attenuates MDSC-mediated immunosuppression and enhances tumor immunogenicity,effectively promoting DC maturation and T cell infiltration.
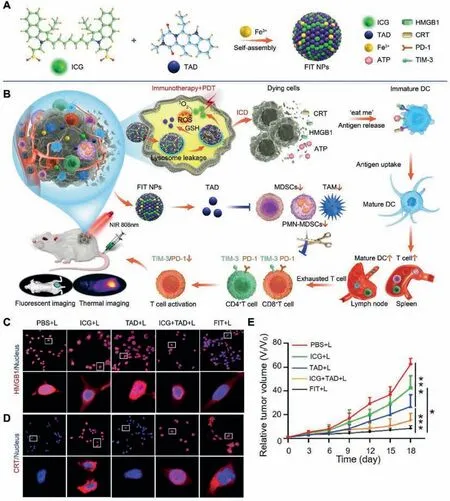
Fig.7.Schematic of FIT for cancer immunotherapy.(A)Schematic illustrating the fabrication process of Fe3+@ICG@TAD(FIT).(B)FIT-mediated antitumor immunity by inhibiting MDSC and enhancing tumor immunogenicity.(C)HMGB1 and(D)CRT staining in CT26 cells after different treatments.(E)The CT26 tumor growth curves.Reproduced with permission[64].Copyright 2021,Wiley-VCH.
Hypoxia,a common feature of most malignancies,has become a significant cause of limiting the efficacy of PDT[34,65].Lanet al.reported a kind of nano metal-organic framework,Fe-TBP,composed of iron clusters and porphyrin ligands[66].It can alleviate tumor hypoxia under normal temperature and may be used as a new nano photosensitizer to overcome tumor hypoxia,improve the treatment rate of PDT,and initiate tumor immunotherapy for noninflammatory tumors.In the study,PDT mediated by Fe-TBP could induce ICD and significantly improve the efficacy of anti-PD-L1 treatment.Liuet al.designed GSH-responsive active photosynthetic bacteria(Synechococcus 7942,Syne)to deliver HSA/ICG nanoparticles crosslinked by intermolecular disulfide bonds to resolve tumor hypoxia during photodynamic processes.S/HSA/ICG generates oxygen through photosynthesis under laser irradiation,and photosensitizers produce cytotoxic reactive oxygen to eliminate primary tumors,reverse the tumor’s immunosuppressive TME,and powerfully evoke a systemic antitumor immune response.They have an excellent effect on metastatic triple-negative breast cancer[67].
The ICD induced by PDT depends on ROS,hindered by a short half-life and limited diffusion ability.PDT combined with the strategy of enhancing the role of ROS has achieved good results[65].Denget al.designed a GSH-responsive drug delivery platform(Ds-sP/TCPP-TERNPs)loaded with the ER-targeted photosensitizer TCPP-TER to improve the half-life of ROS produced by photodynamics[68].TCPP can selectively accumulate in the ER and generate activity under laser irradiation.Oxygen induces ER stress,amplifies ICD,and activates immune cells.Drugs targeting the ER enhance subsequent ER stress by increasing the concentration of ROS in the ER.Maiet al.used platelet membranes(PM)as carriers of metformin(Met)and IR780(PM-IR780-Met-NPs),which have a longer circulation life and tumor cell adhesion ability.IR780,as a PDT agent,produces ROS.At the same time,Met,as a mitochondrial inhibitor,reduces oxygen consumption and simultaneously hinders the pathway by which MDSCs regulate immunosuppression due to the local hypoxic environment.Finally,many T cells are recruited and activated,effectively clearing the tumorin situand inhibiting metastasis[69].
Encapsulation of drugs with the cell membranes is a strategy to enhance drug targeting.Denget al.used NK cells membrane to wrap the photosensitizer TCPP to make the drug target tumor NK,to enhance the antitumor immunity by inducing or enhancing the polarization of pro-inflammatory M1 macrophages[70].Liuet al.used the cytomembranes(FMs)of hybrid cells acquired from the fusion of cancer and DCs to encapsulate nanophotosensitizers[71].Due to the expression of intact cancer antigens and immune costimulatory molecules on FM,not only can tumor-targeting be achieved,but the immune system can also be activated,not only inducing primary tumor ablation but also leading to the regression of radiation-free distant tumors.Zhanget al.designed a nanoparticle(PCPK-SR)modified with targeting peptide on the cell membrane for loading photosensitizer(PpIX)[72].Under laser irradiation,active oxygen was generated to inactivate membrane-related proteins,trigger lipid peroxidation,and destroy PM,further inducing rapid DAMPs(HMGB1 and ATP)release,producing an antitumor immune response.When combined with PD-L1 therapy,this strategy can effectively prevent tumor recurrence.
3.4.ICD induction by PTT
PTT is a process in which a photothermal agent transforms the radiation energy into heat energy under laser irradiation.The temperature of tumor cells is raised to above 42 °C to clear cancer cells.The laser can irradiate the tumor area locally with high therapeutic effects and fewer side effects.The common near-infrared photoreactive inorganic nanoparticles include gold nanoparticles,carbon nanotubes,and copper sulfide nanoparticles[73–76].Elevated temperature can induce unfolded protein response,activate ER stress,promote ICD and subsequent antitumor immune response.Nanotechnology is helpful to precisely control the heating effect of PTT,control the temperature in a proper range(39–45 °C),increase its targeting,and maximize the therapeutic effect while controlling the side effects[65,77,78].Experiments have shown that PTT therapy can increase the response of tumors to ICB therapy,suggesting that cell death caused by PTT can activate autoimmunity[79].
MDSC can migrate naturally to tumor sites[63].Yuet al.developed biocompatible bone MDSC membrane-coated ferric oxide magnetic nanoparticles(MNPs@MDSCs)(Fig.8)[80].This work observed the distribution of MNPs@MDSCs in mice by photothermal imaging,PET imaging,and magnetic resonance imaging.MNPs@MDSCs can aggregate at tumor sites and enhance the onset of immunogenic cell death of tumor cells.In addition,this strategy can reprogram tumor-infiltrating macrophages,and reduce tumor metabolic activity,thereby enhancing the antitumor response[80].
PTT exhibits more potent immune stimuli when combined with other immunotherapies or when co-loading immunostimulatory factors[81].Yuet al.used a surface protection etching method to prepare rattle-type gold nanorods@void@porousSiO2(GVPS)with hollow internal structures for drug delivery[82].GVPS was further functionalized by polyethylene glycol(PEG)and RGD peptides to improve its biocompatibility and selectivity to targeted cancer cells.GVPSPR-DOX/TD nanocomposites showed good performance in the combination of photothermal chemotherapy.Compared with chemotherapy alone or PTT,combined therapy showed higher efficiency in killing tumor cells.Liet al.designed a double ER-targeting strategy to combine PTT and PDT.This nanosystem includes ICG-conjugated hollow gold nanospheres(FAL-ICG-HAuNS)and oxygen transfusion hemoglobin(Hb)liposome(FAL-Hb lipo).These nanosystems induced intense ER stress and CRT exposure on the cell surface under NIR light exposure,caused CD8+T cell proliferation and cytotoxic cytokine secretion,resulting in an immuneactivated tandem,effectively inhibiting tumor growth[83].
ICG,DiR,and other photothermal agents can also be used as imaging agents to observe the distribution of drugs in tumors.Bionic nanoplatforms are designed to improve drug retention in the body.The stimulant DiR and the IFN gene agonist Vadimethan and PD-1 blocker were encapsulated in synthetic high density lipoproteins(sHDLs)for imaging-guided combination therapy[84].sHDLs trigger immunogenic death,promote DCs maturation,sensitize tumors to PD-1 blockade,and provide scalable nanoplatforms for the combination of photothermal ablation and immunotherapy of liver cancer[84].Maet al.found that the light-induced thermal effect of NIR(II)light can be more uniform than that of NIR(I)light and that red light triggers the death of immunogenic cancer cells more deeply and is more effective than oxaliplatin[18].The 2D polypyrrole nanometers wrapped by the red cell membrane film used as a NIR(II)PTT transducer combined with checkpoint blockade therapy can eliminate 5/8 of primary tumors and effectively suppress distant tumors.
3.5.ICD induction by NPS
NPS is a non-thermal,drug-free treatment for cancer.To deliver shorter but larger amplitude pulses than those commonly used for electroporation to tumor cells,which can penetrate all the cells and organelles in the tumor,resulting in the rearrangement of intracellular ions and the formation of nanopores on the membrane[85].In this short period,calcium ions flow into the cytoplasm and extracellular space,causing ER stress and inducing ICD.At present,NPS has been used to treat non-viral tumors,inducing target cells to undergo ICD.The release of critical DAMPs of ICD triggered by NPS is equivalent to that of DOX and MIT,inducing an antitumor immune response of the innate immune system and then leading to tumor necrosis,which slowly subsides in a few weeks[86].
Guoet al.applied NPS therapy to human papillomavirus 16(HPV16)transformed the C3.43 mouse tumor cell model.In the experiment,NPS can effectively eliminate primary tumor cells by inducing ICD and increasing tumor-infiltrating lymphocytes.Tumorbearing mice treated with NPS showed an initial decrease in tumor volume and a significant increase in overall survival.At the same time,it was also observed that NPs therapy could be used to resect primary tumors and induce antitumor response driven by effector CD8+T cells and protect individuals from disease recurrence[87].
3.6.ICD induction by MTD
MTD treatment,as a new and effective systemic cancer treatment method,has recently received widespread attention.In a study,ferrimagnetic vortex-domain iron oxide nanoring and graphene oxide(FVIOs-GO)nanoplatform has high heat conversion efficiency,which can produce more reactive oxygen species under alternating magnetic field(AMF),and produce ER stress,exposing a large number of CRT on the surface of tumor cells[88].The dual effects of magnetocaloric effect and ROS-related immune effect can significantly increase tumor-infiltrating T lymphocytes and improve the traditional magnetic therapy[88,89].
Chaoet al.prepared pure iron nanoparticles(FeNPs)by chemical reduction and functionalized them with PEG/ dopamine(DA).The FeNPs modified by this biocompatible polymer have good stability in an aqueous solution and are an efficient MTD reagent,generating enough heat under a low-power atomic force microscope[90].Combined with anti-CTLA4,FeNPs mediated MTD can produce a systemic therapeutic response to inhibit tumor metastasis and prevent recurrence.
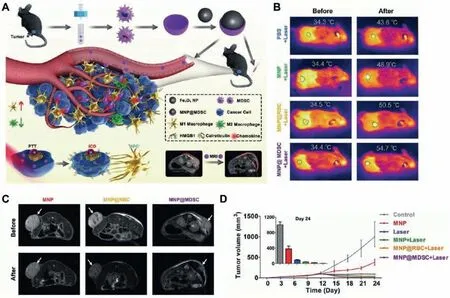
Fig.8.Schematic illustration of the fabrication process of MDSC membrane-coated ferric oxide magnetic nanoparticles(MNPs@MDSCs)and its application in cancer theranostics.(A)Synthesis of MNP@MDSC,and the mechanisms of MNP@MDSC in tumor diagnosis and treatment.(B)Infrared thermal images and tumor temperature measurements of B16-F10-tumor-bearing mice after different treatments.The circles indicate tumor sites.(C)T2-weighted magnetic resonance imaging of mice.(D)The tumor growth curves after different treatments.Reproduced with permission[80].Copyright 2018,Wiley-VCH.
3.7.ICD induction by OVs
OVs are natural or recombinant viruses that selectively induce tumor cell lysis without harming normal cells.Currently used OVs vectors include poxvirus,adenovirus,and herpes simplex virus.Different viruses have different dominant modes.Most oncolytic adenoviruses induce autophagy in tumor cells.Coxsackie B3 virus induces immunogenic apoptosis in non-small cell lung cancer cells,and the measles virus induces the release of inflammatory cytokines and HMGB1 and activates DCs.Currently,OVs approved for cancer treatment are Rigvir,Oncorine,and Imlygic[91].The virus can cause tumor inflammatory reactions,affecting the TME,tumor blood vessels,tumor-associated fibroblasts,and extracellular matrix.OVs kill tumor cells by releasing tumor-associated antigens,pathogen-associated molecular pattern signals of the virus,cell hazard-related molecular pattern signals,and cytokines.These molecules promote the maturation of antigen-presenting cells and activate effector T cells.These functions can be enhanced and manipulated by targeted addition,deletion,modification,or specific expression of certain genes[92].
OVs can express immune checkpoint antibodies by gene recombination,which provides a new method for ICD administration.Hamiltonet al.modified the influenza virus expressing a singlechain antibody to antagonize CTLA4(IVA-CTLA4)and obtained a transgenic virus that can achieve the combination of OVs therapy and ICB therapy[93].The results showed that intratumoral injection of IVA-CTLA4 could effectively inhibit the growth of the tumor,inhibit the growth of B16-F10 tumor,inhibit the growth of the distal untreated tumor,and prolong the overall survival of mice.
3.8.ICD induction by multipronged approaches
Multiple functional nanoparticles may maximize ICD induction[94–97].Zhouet al.designed acidic and matrix metalloproteinase(MMP)-2 dual reaction prodrug vesicles loaded with OXA prodrug and polyethylene glycol photosensitizer,which can combine chemotherapy and PDT to eliminate the tumor and promote ICD[98].In the experiment,the drug can effectively inhibit the growth of primary and secondary tumors,inhibit tumor metastasis and prevent tumor recurrence.A hybrid nanoreactor(Cu-DMONs)was designed to serve as a Fenton catalyst,consume glutathione in cells,amplify the immunogenic cell death caused by DOX and activate immune cells.In combination with ICB,this strategy can inhibit primary tumors and untreated distant tumors[99].Kuaiet al.designed a sHDL mimicking nanodisc for codelivery of antigen peptides and adjuvants to lymphoid organs and maintaining antigen expression on DCs[100].The new antigen-specific CD8αT cells induced by nanodiscs is 47 times more potent than soluble vaccines and 31 times higher than the strongest adjuvant in clinical trials(i.e.,CpG in Montanide).When used in combination with anti-PD-1 and anti-CTLA-4 therapies,sHDL effectively eliminate established MC-38 and B16F10 tumors.
3.9.Strategies to enhance tumor targeting
Improving tumor therapy targeting is currently an important research direction.High-target therapy can improve the local curative effect of tumors and reduce the toxicity and side effects of drugs.Appropriate nanomedicine carrier design can be conducive to enhance drug targeting[101].Ultrasound microbubble controlled-release chemotherapy has become a promising method for the treatment of malignant tumors.Huanget al.prepared DOX liposome microbubble complex(MbDOX)to control the targeted drug release by ultrasound(MbDOX+ultrasound treatment)to achieve the targeted drug aggregation in the tumor[102].More DOX accumulation was observed in tumor cells and tissues,and the induction of ICD was enhancedin vivoandin vitro.
The characteristics of hypoxia in the TME can also be formulated to enhance tumor targeting.AQ4N is a kind of hypoxiaactivated prodrug with cancer cytotoxicity only in a hypoxia environment.Fenget al.prepared a multi-purpose liposome,in which hydrophilic AQ4N and hydrophobic cetylamine conjugated chloride e6(Hce6)were encapsulated in its water cavity and hydrophobic bilayer,respectively[103].During PDT treatment,reactive oxygen species are consumed,resulting in local hypoxia of the tumor.At this time,AQ4N is activated in the hypoxia environment,causing toxicity to cancer cells and achieving targeted tumor attack[103].
Nanodrugs can enhance the targeting of drugs to tumors by modifying drugs and combining chemotherapy and other therapies[104].Auet al.utilize fully synthetic antibody mimic selective high-affinity ligand(SHAL)-functionalized doxorubicinencapsulated nanoparticles(DOX NPs)to treat human leukocyte antigen-D related(HLA-DR)antigen-overexpressing tumors by the strategy of translatable concurrent chemo-immuno-radiotherapy[105].The specific antibody mimics the selective binding of functional NPs to human lymphoma cells with high expression of HLADR,enhances tumor uptake,reduces the systemic toxicity of DOX,and augments ICD,enhancing the cell killing efficiency of radiotherapy by more than 100% and eradicating more than 80% of lymphoma tumors[105].
Wanget al.synthesized a MON with spatially packaging ovalbumin antigen(OVA)as a photosensitizer carrier,effectively triggering an immune cascade reaction,and then wrapped the MON with the B16-OVA cancer cell membrane.Because of the cancer cell membrane coating,MONs have homophilic targeting towards tumors.Finally,the tumor was eliminated by laser irradiation,resulting in a long-term antitumor immune memory effect[106].
Nanodrugs can enhance the targeting of tumor cells and intracellular organelles through an appropriate design to achieve enhanced ICD.Denget al.synthesized nanoparticles targeting the ER with photosensitivity[68].Reduction-sensitive Ds-sP NPs(PEG-s-s-1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethyleneglycol)-2000]nanoparticles)were loaded with an efficient ER-targeting photosensitizer TCPPTER(4,4′,4′’,4′’’-(porphyrin-5,10,15,20-tetrayl)tetrakis(N-(2-((4-methylphenyl)sulfonamido)ethyl)benzamide).Under NIR laser irradiation,the resulting Ds-sP/TCPP-T ER NPs could selectively accumulate in the ER and locally generate ROS to induce ER stress,solving the problem of ER stress deficiency caused by short halflife and insufficient diffusion of ROS to enhance ICD,eliminate the primary tumor and inhibit distant tumors through the abscopal effect[68].
4.Conclusion and future perspectives
The molecular mechanism of tumor immune escape and the microenvironment of immunosuppression are the major obstacles to tumor immunotherapy efficiency[3].The application of ICDinducing drugs induces the production of crucial ICD markers,such as exogenous ATP,HMGB1,and ecto-CRT,in dead tumor cells to activate the tumor-killing potential of cancer patients’immune systems[107,108].The OVs is also a potent inducer of type I IFN,which has been defined as the fourth key marker of ICD,causing extensive DAMPs expression[109].Enhancing antitumor immunity by inducing ICD can improve the treatment efficiency of carcinomain situand has a good effect on tumor metastasis and prevention[110–113].The application of nanotechnology in cancer therapy shows advantages in drug delivery,multi-treatment combination,and ICD induction[9].Nanodrug transport can improve the unfavorable transport properties of certain drugs,such as hydrophobicity,and can also increase the tumor targeting of drugs through appropriate drug carrier design,correspondingly improving drug utilization and reducing side effects[114,115].In PTT,nanomedicine facilitates precise dose control to maintain therapeutic temperatures in the appropriate range,boosting the quality and magnitude of an immune response in a predictable and designable fashion[116].NPS provides ultrashort electrical impulses to tumor cells,stimulating adaptive immune responses through immunogenic forms of apoptosis,eliminating tumors,and inhibiting the growth of secondary tumors[85].Several known and novel inorganic and polymeric nanoparticles,such as AuNPs,metal organic frameworks(MOFs),and micelles,can be used in ICD induction models to synergistically improve therapeutic effects with immunotherapy while limiting systemic toxicity[117,118].The application of nanotechnology in tumor immunotherapy can combine multiple therapies,induce ICD to improve immunotherapy,improve targeting and reduce side effects.
To date,the molecular mechanisms for inducing ICD are not comprehensive,and only a few stimuli have been shown to induce ICD.Exploring more compounds or modalities that induce ICD is needed.Because the current ICD-inducing experiments are independent research and there is a lack of dynamic monitoring of ICD indicators,the results of different ICD-induced programs are challenging to quantify and compare their induction efficacy.It is also necessary to explore the synergistic effect of drugs combined with conventional ICD-inducing drugs according to the ICD mechanism to enhance the intensity of ICD induction.Even if these therapies may achieve the best effect in inducing ICD,once the immune stimulation provided by ICD stops,they cannot guarantee the long-term sustainability of tumor-associated antigens-guided T cells with a low-affinity T-cell receptor due to the mechanism of peripheral tolerance.Improved medication is also needed[119].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China[Nos.82072996(Z.Sun.),81874131(Z.Sun),51703187(Z.Xu)],National Key Research and Development Program(No.2017YFSF090107),the Chongqing Talent Plan for Young Top Notch Talents(No.CQYC202005029),Hubei Province Natural Science Funds for Distinguished Young Scholar[No.2017CFA062(Z.Sun)]and Innovative Research Team of High-level Local Universities in Shanghai[No.ZLCX20180500(Z.Sun)].
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
