Cell membrane-coated nanoparticles for immunotherapy
Hang Liu,Zhaohua Miao,Zhengbao Zha
School of Food and Biological Engineering,Hefei University of Technology,Hefei 230009,China
ABSTRACT Nanoparticle-based disease detection,prevention and therapies have gained increased interests in biomedical applications,owing to their significant advantages in therapeutic efficacy and safety.Nonetheless,suffering from the challenges including fast recognition and clearance of foreign nanoparticles by innate immune system before arriving at diseased regions,clinical applications of nanoparticles are usually intercepted.Among various strategies for reducing non-specific phagocytosis and enhancing diseasetargeting efficiency of nanoparticles,membrane coating nanotechnology exhibits great potential in the disease diagnosis and therapeutics due to both the structural and functional preservation of membrane proteins from source cells.Benefiting the inherited immune-regulation capacities,this review mainly summarized the latest development of such biomimetic nanoparticles for immunotherapy in treating immune-related diseases including microbial infections,inflammation,tumor and autoimmune diseases.
Keywords:Biomimetic nanoparticles Immunotherapy Nanovaccine Cell membrane
1.Introduction
In the past few decades,nanomedicine has attracted extensive attentions due to their multifunctionality which have great potential for disease prevention,detection and treatments[1–3].Despite nanomaterials generally offer improved efficacy and less side effects in comparison to traditional diagnostics and treatments,unremitting efforts are still needed to overcome the challenges including the fast recognition and clearance of foreign nanoparticles by innate immune system before arriving at diseased regions[4–6].Reducing non-specific phagocytosis of nanoparticles by addition of specific targeting groups is hence known as an effective strategy to improve the aggregation efficiency at desired sites[7,8].Therefore,optimizing the delivering efficiency of nanoparticles with various strategies is highly pursued.
Due to the important role of biological interface in determining thein vivofate of nanoparticles,surface engineering is then considered to be the key way for improving the therapeutic effi-cacy of nanoparticles[9].Benefiting from its abundant hydrophilic groups,polymer polyethylene glycol(PEG)was favored to enhance the hydrophilicity of nanoparticles’surface with prolonged circulation time and reduced phagocytosis by the reticuloendothelial system[10].Moreover,in order to increase the active targeting capacities of nanomedicine,numerous ligands,such as antibodies,peptides and aptamers,are further anchored to the surface of nanoparticles[11].Although effective to some extent,such nanoparticles with extended circulation time and active targeting capacities still suffered from inefficient aggregation in diseased regions and/or endocytosis by targeted cells[12].Alternative strategies,including development of stimuli-responsive synthetic polymers[13],were subsequently utilized,but these methods did not significantly improve their immune compatibility and therapeutic efficacy[14].
Recently,biomimetic nanoparticles have become increasingly prevalent in medical applications[15–17].Among them,biomimetic stealthy strategies have been gradually explored to avoid body clearance which are mainly comprised of bottomup and top-down forms[18].Unlike PEG-modified nanoparticles,biomimetic nanoparticles disguise them as endogenous species instead of hiding foreign elements behind the hydration layers[4].In the bottom-up surface functionalization methods,ligands are bound to particle surfaces generally by covalent coupling or noncovalent interactions which are easily suffer difficulties in largescale synthesis and reproducibility[19,20].In contrast,the whole cell membrane is used to cover nanoparticles and the resulting cell membrane-coated nanoparticles inherit intact surface antigens and functions of the source cells as well as the highly adjustable physical and chemical properties of core nanoparticles[21–23].Satisfyingly,the cell membrane-coated nanoparticles can reveal the similar behaviors to the source cells,not only has a wide range of functions,including extended circulation timein vivoand active targeting efficiency,but also escaping from immune clearance as well as a vaccine for immunotherapy[24–28].
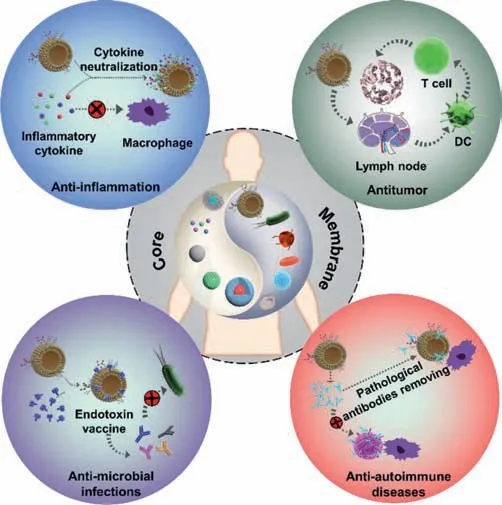
Fig.1.Various biomedical applications of cell membrane-coated nanoparticles.
With the deep understanding of interactions between immune system and diseases,immunotherapy has become the crucial treatment for many diseases.Immunotherapy refers to the intervention or adjustment of the body’s immune function based on the principle of immunology and the occurrence mechanism of diseases,so as to achieve the purpose of treating diseases.For instance,monoclonal antibodies,immune adjuvants and vaccines that against viruses are becoming promising medicaments for treatments of cancer and infections[29].Our insight into the molecular interactions between tumors and the immune system has triggered numerous studies about exploring creative therapies and continues to motivate the design of more effective treatments[30,31].A novel approach to immunity-based cancer therapy is to enhance the anti-tumor immune response through enlarging the amounts of tumor-responsive T cells,providing exogenous immune activation as well as countering regulatory pathways which induce immune tolerance[32–34].The utilization of cell membrane-coated nanoparticles in immunotherapy is thus emerging as an inspiring research field.Numerous studies have shown that cell membranecoated nanoparticles can insulate the cytotoxic factors,including endotoxins,which are beneficial forin vivosafe and effective immunotherapy[35].The cell membrane-coated nanoparticles can be also used as an antigen to activate dendritic cells,and then further induce humoral and T cell immune responses[36].Later,more and more studies have found that nano-vaccines based on cancer-cell membrane-coated nanoparticles also show promising anti-tumor efficacy[37–39].The corresponding antigens and immune adjuvant on the cancer cell membranes can be effectively delivered to antigen-presenting cells for boosting the anti-tumor immune responses.Nanoparticles coated with immune cell membranes play important roles as vaccines in treating tumor,inflammatory,microbial-infections and autoimmune diseases[40–42].
As membrane-coated nanoparticles have made great progress in drug delivery[43,44],molecular imaging,detoxification[21,45]and immunoregulation[42],this review mainly summarized the latest development of such biomimetic nanoparticles for immunoregulation(Fig.1).We first introduced the versatile preparation strategies of cell membrane-coated nanoparticles and then illustrated their corresponding immune-regulation effects in treating inflammation,infections,autoimmune diseases and tumor,respectively.Finally,the prospects of the opportunities and challenges in clinical transformation of cell membrane-coated nanoparticles were also discussed.
2.Synthesis strategies
The preparation of cell membrane-coated nanoparticles commonly consists of two important steps,membrane separation and nanoparticle fusion,as shown in Fig.2[46].In the case of red blood cells(RBCs),which are devoid of nuclei,the isolation procedure is relatively straightforward.After separating from plasma and serum,the red blood cells were centrifugally washed with PBS and then followed with hypotonic treatment to obtain the purified erythrocyte membranes[47,48].In the presence of protease inhibitors,fragments of cell membranes should be extracted as gently as possible to minimize protein denaturation.Besides hypotonic lysis,other methods for cell membrane extraction have also been developed,including repeated freeze-thaw cycles,physical homogenization,and electroporation.As for the repeated freeze-thaw method,the cells were placed in a frozen state with liquid nitrogen or at-80 °C,and then thaw them at 37 °C or in room temperature along with ultrasonication.The ice crystals formed during the cooling process would destroy the cellular structures by expansive effects[49].Electroporation,which needs the exposure of cells to an electrical field,pores are formed in the cell membranes and make the membranes semi-permeable[50,51].As the separation of cell membranes is basically in these ways,and then,the next step is to fuse the membrane vesicles with nanoparticles[52].
Cell membrane-coated nanoparticles can be prepared by membrane fusion in many different ways.For instance,similar to the synthesis of liposomes,the membrane-coated nanoparticles are often prepared through physical extrusion by using the porous membrane with uniform pore diameters[53].Ultrasonic treatment would also drive the nanoparticles spontaneous formation of core-shell structures with cell membranes,which present similar morphologies with the products of the physical extrusion method.Moreover,the destruction of cell membrane structure is greatly reduced in comparison to physical extrusion while it is not suitable to use ultrasonic treatment for massively producing cell membrane-coated structures[54].Recently,microfluidic systems,which combined rapid mixing and electroporation,have been reported to synthesize cell membrane coated-nanoparticles with enhanced structure and function reservation as well as more efficient production.For instance,a reported microfluidic device which consisted of a Y-type channel,S-shaped channel mixer and electroporation area could generate transient pores in the cell membranes through the electronic pulse to facilitate the encapsulation of nanoparticles by cell membranes.In comparison to other strategies,this method has obvious advantages in maintaining membrane integrity and reducing membrane surface protein loss[15].
3.Immunotherapy
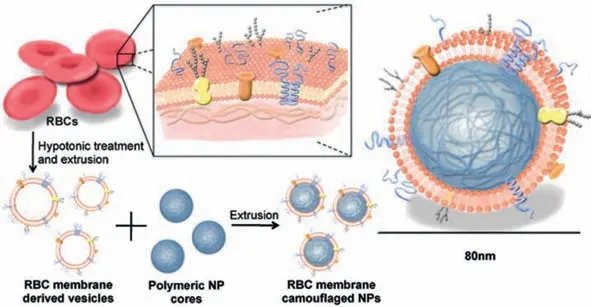
Fig.2.Schematic depicting the fabrication of red blood cell(RBC)membrane–coated nanoparticles.Copied with permission[46].Copyright 2011,National Academy of Sciences.
Since the erythrocyte membrane coated nanoparticles were first reported by Che-Ming J.Hu and colleagues[46],the research of cell membrane coated-nanoparticles have experienced flourish developments in many applications.In addition to regulate the nanoparticle core compositions,the membranes can also be extracted from different cells which would endow resulted cell membrane-coated nanoparticles with unique properties.For instance,nanoparticles coated with platelet membranes inherit not only the abilities to target inflamed sites and damaged blood vessels[53,55-57],but also acting as a decoy to clear pathological antibodies and thus treating platelet-related autoimmune diseases[58].Satisfyingly,nanoparticles coated with cancer cell membranes could arrive the tumor region through homologous-targeting mechanism[59,60].In addition,these biomimetic nanoparticles are reported to have the ability for activating cellular immunity for anti-tumor treatments by retaining specific antigens on tumor cell membranes[42].Similarly,packaging with leukocyte cell membranes would assist nanoparticles to inherit the capacity of sepsis inhibition from source cells due to the reservation of various toxins and cytokine receptors on the surface of isolated leukocyte membranes[61].Other cell membranes,such as stem cell membrane[22],erythrocyte membrane[62,63],endothelial cell membrane[64],and even hybrid membranes of different cell types[65],have been also developed for different disease treatments.Moreover,benefiting from the existence of most proteins of the source cells,the combination of unique properties from various core materials and superficial cell membranes endows the cell membrane-coated nanoparticles with good potential for vaccine design[18,66].
3.1.Anti-microbial infections
According to the epidemiologic studies,around 700,000 people worldwide died each year due to microbial infections,which have become one of the most serious threatens to human health.Although various vaccines have been rapidly developed and successfully used in prevent some specific pathogenic microorganisms,there are still much room for improvement for effectively eliminate many serious infections caused byE.coli,Helicobacter pyloriandStaphylococcus aureus[67].
Recently,vaccines based on outer membrane vesicles(OMVs)have attracted increasing attentions in the prevention and treatment of microbial infections.OMVs are immunogenic spherosomes which are secreted by Gram-negative bacteria carrying with the parent bacteria lipoprotein,lipopolysaccharide(LPS)and so on[68].These inherited substances could perform as pathogen associated molecular patterns(PAMPs)with the host cell surface expression pattern recognition receptors(PRRs,such as toll-like receptors(TLRs)and intracellular receptor response,to launch numerous immune responses[69].OMVs generated from various bacteria includingPseudomonas aeruginosa[70],Salmonella typhimurium[71],Klebsiella pneumoniae[72],andShigellae[73]are thus widely considered as candidates for the new generation of vaccines due to their highly biocompatible nanostructures and naturally abundant immunogenic components[74].For instance,vaccines derived fromNeisseria meningitidis-OMVs have been licensed worldwide for the control of meningococcal B disease in human[75].UsingE.colias the model pathogen,Gao and co-workers have successfully developed gold nanoparticles(BM-AuNPs)with an average diameter of about 30 nm which were coated with OMVs secreted fromE.coli(Fig.3)[76].After being subcutaneously injected,the as-prepared BM-AuNPs were able to move to neighboring drainage lymph nodes as well as induce rapid activation and maturation of dendritic cells in mouse lymph nodes.In addition,BM-AuNPs enhanced the secretion of interferon-gamma(INFγ)and interleukin-17(IL-17).The results showed that BM-Au NPs could effectively induce the immune responses of bacteria-specific B and T cells in inoculated animals.Experimental studies showed that mice injected with BM-AuNPs coated with OMVs had significantly higher number of dendritic cells separated from lymphocytes than mice treated with PBS,suggesting that an increased concentration of dendritic cells in these lymph nodes is occurred.At the same time,the upregulation of co-stimulators CD40,CD80 and CD86 indicated that the isolated dendritic cells in the lymph nodes changed from immature to mature.Subsequently,the induction of specific antibodies toE.coliwas examined to evaluate the bacteria-specific B cell response.The obtained results showed that the mice treated with BM-AuNPs showed continuous elevation of corresponding antibodies,implying that the BM-AuNPs injection also significantly enhanced the specific B-cell immune response.Last,they also confirmed the T cell activation effect of BM-AuNPs to stimulate the body’s immune response to bacteria,as the levels of T cell based immune cytokines including IL-17 and IFN-γwere significantly increased.The results revealed that the as-prepared BM-AuNPs which were synthesized by a top-to-bottom method could preserve the immunogenic antigens with immunoadjuvant properties on the bacterial membranes to perform as bionic bacteria for triggering specific antibacterial immune responses.
Oh Youn Kim and co-workers designed an cellular,bioinspired adjuvant-free vaccines(protoplast derived nanovesicles,PDNVs),which removed cytotoxic components from the outer membranes and exhibited effectively against bacterial infections[77].These PDNVs showed higher yield and safety in comparison to the commonly-used vaccine delivery vehicles as well as induced both strong antigen-specific humoral and cellular immune responses in a mouse infectious model,suggesting a protective effect against both gram-negative and gram-positive bacterial sepsis.
Benefiting from inheriting the bacterial membrane associated immunogenicity of antigens,the development of bacterial membrane-based nanoparticles as effective antibacterial vaccines provides new opportunities in anti-infection applications.For example,Vibrio choleraeproteoliposomes could induce the antibody immune responsesin vivoto eliminateV.choleraeinfections after nasal administration[36].And the OMVs developed fromNeisseria meningitidishave been successfully licensed for undergoing clinical trials[78].PertussisOMVs,which are vesicles composed of several kinds of immunogenicity of antigens,includingPertussistoxin,fimbriae,and pertactin,also play an important role in preventing and curing the whooping cough[79].Together,these studies confirmed the potential of bacterial membrane-coated nanoparticles as an emerging and highly effective anti-microbial vaccine platform.
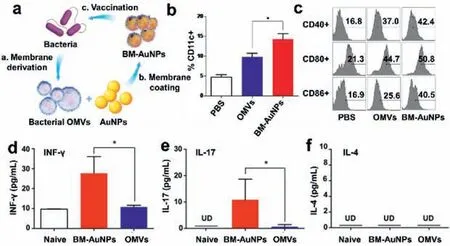
Fig.3.(a)Schematic illustration of the mechanism for modulating antibacterial immunity from bacterial membrane-coated nanoparticles;(b)percentage of CD11c+cells from mice with various treatments;(c)representative flow cytometry analysis of CD40,CD80,and CD86 molecules on CD11c+DCs;levels of(d)IFN-γ,(e)IL-17,and(f)IL-4 from different groups.Data are shown with mean percentage ± SEM.*P <0.05,**P <0.01.Reproduced with permission[76].Copyright 2015,American Chemical Society.
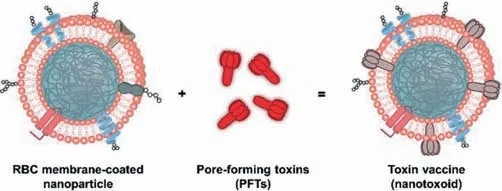
Fig.4.Schematic illustration for the preparation of nanoparticle-detained toxins,denoted ‘nanotoxoid’.Copied with permission[54].Copyright 2013,Springer Nature.
Besides the nanoparticles coated with bacterial membranes,another form of utilization of the anti-bacterial immunity is through co-packaging the nanoparticles with segregated cytotoxic factors to inhibit the release of endotoxins.These endotoxins trapped in the membrane-coated nanoparticles cannot liberate their normal toxic effects,so they can be effectively and safely immunizedin vivo,realizing the prevention and treatment of bacterial infection.
In recent years,toxoid vaccines,which based on inactivated bacterial toxins,have made brilliant achievements in antitoxin immunotherapy and prevention of bacterial infections.Che-Ming J.Hu and co-workers[54]designed a toxoid vaccine and achieved anti-inflammation immunity by coating nanoparticles with red blood cells and modifying them with pore-forming toxins(PFTs),a membrane-destroying protein that is responsible for the virulence of bacterial infections.As shown in Fig.4,nanotoxoid loaded with haemolysin(Hla)was prepared by usingStaphylococcalA-Hla as a model toxin,while mixing it with red blood cell membranecoated PLGA nanomicelles.The Hla was not released in the beginning 48 h,indicating that the Hla could be securely isolated.After the nanoparticles were first injected subcutaneously with endotoxin,lymphatic drainage was then developed,indicating that the granule vector successfully delivered Hla to the immune system.Subsequently,the therapeutic potential of the nano endotoxin as a vaccine was confirmed by the significant anti-Hla immune responses in the mice inoculated with the nano endotoxin.Meanwhile,after 21 days of treatment,the mice could achieve complete immunity to the toxin and completely eliminate the toxic damage.
3.2.Anti-inflammation
Inflammation is a response that protect the body from exogenous harmful stimuli,including microbial infections,tissue damage,and other unhealthy substances.Sepsis is a life-threatening disease which caused by the maladjustment of body responses caused by infectious factors.Immune cells including macrophage,neutrophils,and white blood cells play important roles in the development of inflammatory process as well as producing abundant inflammatory cytokines,which would further aggravate the inflammatory responses.Despite great advances in the exploration of anti-cytokine biologics,the complexity of cytokine interactions and the diversity of cytokine targets limit the clinical application of most cytokine biologics.The cell membrane-coated nanoparticles retain the antigenicity of the source cells,which enable them to act as decoys capable of absorbing and neutralizing different kinds of complex pathological molecules,without regard to their structural specificity.Therefore,cell membrane-coated nanoparticles can act as highly effective anti-inflammatory agents.
Endotoxins,also known as LPS,are released from bacteria during the process of cell division,death as well as antibiotic treatments which are often recognized by immune cells as PAMPs.Briefly,LPS binds to the binding protein(LBP)through lipid A in the blood,and then LPS-LBP complex binds to CD14 molecules(surface pattern recognition receptor,PRR)on the macrophage membranes to challenge immune cells to release pro-inflammatory cytokines.Based on this mechanism,Soracha Thamphiwatana and co-workers have explored the macrophage membrane-coated PLGA nanoparticles(MΦ-NPs)as macrophage-mimetic decoy to neutralize endotoxin and pro-inflammatory cytokines in sepsis(Fig.5)[36].The experimental results showed that the injection of MΦNPs could effectively inhibit the death rate of pyemic mice by neutralizing LPS and isolating cytokines.
In rheumatoid arthritis,neutrophils participate in receptormediated adhesion to cytokine-activated chondrocytes through the specific interactions between LFA-1 molecules which were expressed on the surface of neutrophil membranes and the intercellular activation molecule 1(ICAM-1)that over expressed on activated chondrocytes,the neutrophil membrane-coated nanoparticles have the capacity to target inflammatory regions.As shown in Fig.6,Qiangzhe Zhang and co-workers designed PLGA nanoparticles coated with neutrophil membranes to alleviate inflammatory arthritis symptoms by acting as neutrophil decoy to inhibit synovial inflammation[80].In vivoandin vitroexperiments proved that neutrophils membrane-coated nanoparticles could not only decrease the levels of IL-1β,TNF-αand other pro-arthritis cytokines,but also penetrate in cartilage to realize protective effect.As immune cells,such as monocytes,macrophages,dendritic cells and T cells,collaborate with each other in the inflammatory responses,their membranes could be derived for coating different nanoparticles for targeted and synergistic treatment of inflammation.
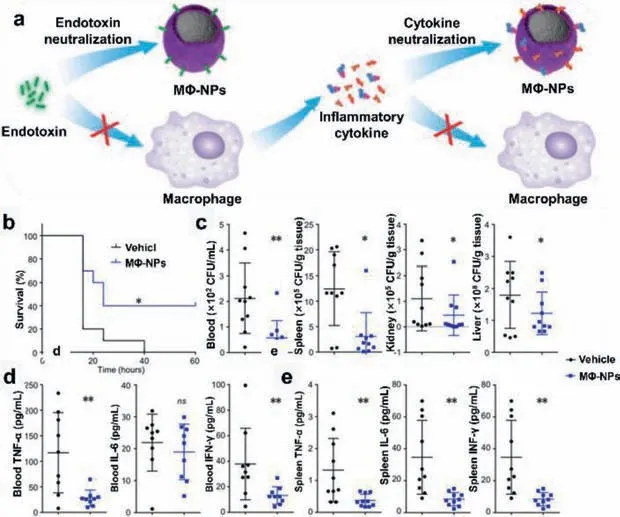
Fig.5.(a)Schematic representation of therapeutic mechanism of MΦ-NPs;(b)survival curve of mice(n = 10);(c)bacteria enumeration in blood,spleen,kidney,and liver at 4 h after MΦ-NPs administration;levels of IL-6,TNF-α,and IFN-γ in the(d)blood and(e)spleen.ns,not significant;*P <0.05,**P <0.01.Reproduced with permission[36].Copyright 2017,National Academy of Sciences.
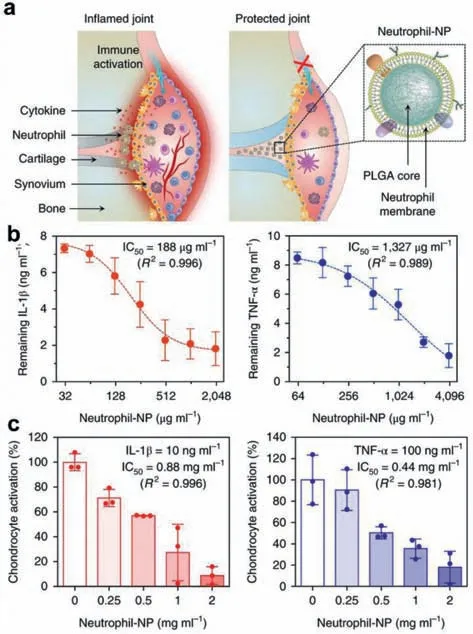
Fig.6.(a)Schematic illustration of neutrophil-NPs designed for suppressing inflammation;(b)binding capacity of human neutrophil-NPs with IL-1β(left)and TNFα(right);(c)dose-dependent inhibition of chondrocyte activation which were induced by IL-1β(left)or TNF-α(right).Data presented as mean±SD.Reproduced with permission[80].Copyright 2018,Springer Nature.
3.3.Anti-autoimmune diseases
Autoimmune diseases which were resulted from the failure of self-tolerance are among the leading causes of death in the world[78].Traditional treatments for autoimmune diseases included the use of cytotoxic drugs,administration of antibodies,and glucocorticoid agents.However,these extensive,nonspecific immunosuppression approaches often bring great risks and even lead to more serious diseases,such as autoimmune hemolytic anemia(AIHA)and immunogenic thrombocytopenia purpura(ITP).Among various emerging therapeutic strategies,the utilization of cell membrane-coated nanoparticles to act as decoy for targeting the autoimmune disease has been emerged as immunotherapies.For instance,Jonathan A.Copp and co-workers developed erythrocyte membrane-coated PLGA nanoparticles as alternative targets for anti-erythrocyte antibodies[81].In comparison to traditional immunotherapy,the biomimetic nanoparticles did not cause the inhibition of either normal lymphocytes or immune effector cells,while could disrupt disease progression in a minimized toxic manner by selectively depleting disease-causing antibodies.The experimental results showed that the erythrocyte membranecoated nanoparticles could effectively bind to the red blood cell antibody through typical antigen-antibody interactions and competitively inhibit the integration of the red blood cell and antibodies,so as to realize the effective treatment of AIHA.Meanwhile,the results showed that the autoantibodies of mice treated with RBC-ANS did not enhance the humoral immune response to nanoparticle-associated membrane antigens,and had good biocompatibility compared with the control group.
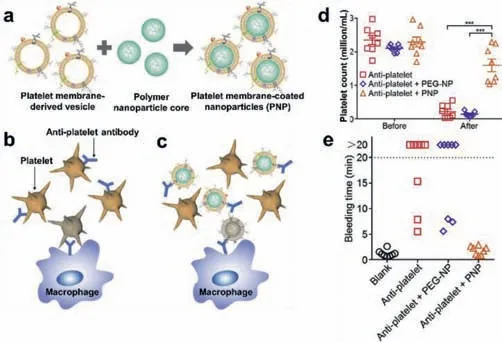
Fig.7.Schematic illustration of(a)the PNPs fabrication process and ITP treatment without(b)or with(c)PNPs administration;(d)platelet counts;(e)bleeding time. n= 8;mean ± SEM.***P <0.001.Reproduced with permission[58].Copyright 2016,Elsevier Ltd.
Similar to AIHA,ITP is also ascribed to the production of body’s own pathological anti-platelet antibodies,leading to a decrease in platelet count and increase in bleeding.Furthermore,nonspecific immunotherapy often resulted in serious side effects than the disease itself.Xiaoli Wei and co-workers used platelet membranecoated nanoparticles as platelet-bionic nanoparticles(PNPs)to specifically remove anti-platelet antibodies[58].Bothin vivoandin vitrostudies had shown that PNPs could specifically and stably bind to anti-platelet antibodies,and exhibit as an alternative target of anti-platelet antibodies for the treatment of ITP and protection of circulating platelets.(Fig.7).In comparison to control groups,treatment with PNPs could prevent common immune side effects as well as alleviate ITP symptoms in established ITP mouse models.Therefore,the cell membrane-coated nanoparticles could be used as promising and effective biomimetic decoys for the treatment of autoimmune diseases.
3.4.Anti-tumor
In the past few decades,tumor immunotherapy has received extensive attentions in the field of tumor treatment.Although some clinical anti-tumor vaccines have been successfully developed,several challenges including the lack of versatility and tumor-specificity are still existed in their clinical applications[82].In addition,immunosuppression in the tumor environment is often difficult to break.From another perspective,numerous studies have focused on developing biomimetic nanoparticles coated with cell membranes which could realize effective anti-tumor immunotherapy.It has been shown that cell membrane delivery,together with either immune stimulation adjuvant or checkpoint blocking therapy,would promote antigen presentation and activate tumorspecific immune responses which were benefit to break tumor immunosuppressive microenvironment[83].Jun Xu and co-workers used tumor antigens derived from cancer cell membranes of resected tumors and fluoroalkane-grafted polyethyleneimine(F13-PEI)as the antigen carrier(F13-PEI/Mem)to fabricate the cell membrane-based nanovaccine,which was then used to combine with ICB therapies to treat residual tumors after surgery[84].In their work,two types of B16F10 melanoma were subcutaneously inoculated on the left and right sides of C57BL/6 mice.After one side of the tumor was surgically removed,the tumor cell membranes were then extracted to develop F13-PEI/Mem nanoparticles.Each mouse was injected intradermally with F-PEI/Mem nanovaccine at the base of the tail.The obtained results showed that tumor growth was significantly delayed in mice immunized with F13-PEI/Mem nanovaccine compared with untreated mice and the mice that treated with F13-PEI alone.Moreover,anti-PD1(programmed cell death protein 1)therapy could further improve the anti-tumor effect of F13-PEI/Mem nanovaccine.
Mature antigen-presenting cells(APCs)can process specific antigens which expressed on cancer cell membrane vesicles(CCMV)to trigger cytotoxic T lymphocyte(CTL)-based anticancer immunity.Despite its fundamental role in vaccine design,single CCMV still lacks effective immune stimulation against APC,which requires a more comprehensive CCMV-based strategy[85].Bacterial OMVs are prokaryotic vesicles shed by Gram-negative bacteria,which contain many natural adjuvant ingredients that inherited from the source bacteria[86].Thus,bacterial OMVs have the ability to stimulate immune maturation and trigger inflammatory responses[87],thus,OMVs have great potential in vaccine design as a natural adjuvant.Qi Chen and co-workers[88]designed and constructed eukaryotic-prokaryotic vesicles(EPV)by fusing melanoma cytomembrane vesicles(CMVs)and attenuatedSalmonellaOMVs,and the fusion vesicles were implanted by the poly(lactic-co-glycolic acid)-indocyanine green(ICG)moiety(PI)to achieve synergetic antitumor effects(Fig.8).The as-prepared EPV with average diameter of around 100 nm could be used as a preventive vaccine to activate the maturation of dendritic cells and increase the amount of effector T cells.In addition,the implanted photosensitive PI moiety endowed EPV with the ability of transforming NIR light into cytotoxic heat,which would generate supplementary tumor antigens presented to the immune system.
Ashley V.Kroll and co-workers[89]reported a PLGA nanoparticles loaded with CpG which were then coated with B16-F10 mouse melanoma cell membranes(CpG-CCNP)to act as a bionic anti-tumor nanovaccine.The immunogenic antigens retained on the melanoma cell membranes were ingested firstly by antigenpresenting cells,and then CpG was used to activate and promote the maturation of antigen presenting cells,and the presentation of tumor antigens as well as the secretion of typical pro-inflammatory cytokines including interleukin-6(IL-6)and interleukin-12(IL-12).In the meantime,antigen-presenting cells are also promoted to activate a variety of specific T cells to produce anti-tumor immune effect.The results demonstrated the great potential of CpG-CCNP to act as a nanovaccine for anti-tumor immunotherapy.Moreover,Ravi B.Patelet al.also used bacterial membranes to package PC7A/CpG nanoparticles for realizing effective anti-tumor treatment[90].
Rong Yang and co-workers wrapped the immune adjuvant with mannitol modified tumor cell membranes[59].Typically,the Tolllike receptor 7 agonist,imiquimod(R837)was loaded in PLGA nanoparticles,and the obtained nanoparticle(NP-R)was then coated with B16-OVA cancer cell membranes(NP-R-M).Cell membrane surface protein was used as tumor specific antigen and NPR-M was further modified with mannitol(NP-R-M-M).The resulted NP-R-M-M nanovaccine showed enhanced uptake by antigen presenting cells and effective anti-tumor immune responses.Similarly,Ronnie H.Fanget al.also reported the coating PLGA nanoparticles with B16F10 melanoma cell membranes to generate biomimetic nanoparticles named CCNPs[18].Due to the preservation of membrane surface antigens,CCNPs could deliver tumor-associated antigens to antigen-presenting cells and then promote anti-tumor immune responses.Bothin vitroandin vivostudies showed that CCNPs,especially when used in combination with immune adjuvant,would significantly promote the maturation of antigen presenting cells,stimulate T cell immunity,and realize anti-tumor immunotherapy.
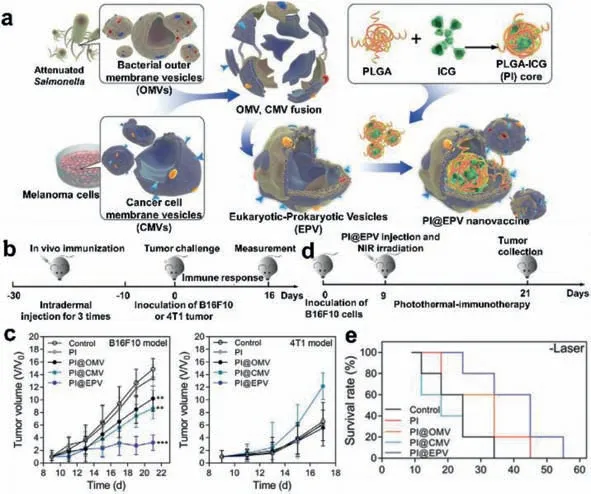
Fig.8.(a)Schematic illustration of PI@EPV nano-vaccine;(b)experimental design for tumor occurrence assay;(c)tumor growth curves of B16F10 or 4T1 tumors;(d)protocol of the therapeutic assay;(e)survival curves of mice.Reproduced with permission[88].Copyright 2020,WILEY-VCH.
In addition to the application of anti-tumor vaccines with cell membrane coating technology,there have also been a large number of studies on the use of immune cell membrane-coated nanoparticles to target tumors,by inhibiting or enhancing proinflammatory effects,and produce anti-tumor immune effects[91].Particularly,the membrane coating nanoparticles modified by Slayer protein on the bacterial membrane were also developed to protect the antigens from degradation and activate anti-tumor immunity[92].The research of membrane coating technology in activating antitumor immunity have shown great potential in effective tumor treatments.
4.Summary and outlook
Due to the inherited properties from source cells,the cell membrane-coated nanoparticles could be utilized for the treatment of numerous diseases.Until now,this emerging technology covers the red blood cells,platelets,cancer cells,immune cells as well as various microbes,and plays an important role in disease surveillance and prevention.Although plenty of pioneered studies have been carried out,cell membrane coating technology still encounters obstacles including limited therapeutic effect in practical application,which are mainly ascribed to the complicated fabrication process and relatively low yield.Therefore,optimizing the fabrication process with improved yield of cell membrane-coated nanoparticles has become one of the mainstream research fields to achieve interesting biomedical applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China(No.31800834),the University Synergy Innovation Program of Anhui Province(Nos.GXXT-2019–045 and GXXT-2020–063).
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
- Applying nanotechnology to boost cancer immunotherapy by promoting immunogenic cell death
