Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
Pengli Go,Zhigng Xie,Min Zheng,*
a School of Chemistry and Life Science,Advanced Institute of Materials Science,Changchun University of Technology,Changchun 130022,China
b State Key Laboratory of Polymer Physics and Chemistry,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
ABSTRACT As a new type of carbon-based fluorescent nanomaterials,carbon dots(CDs)are provided with the advantages of small size,excellent photoluminescence(PL)property,easy surface modification,robust stability,good water solubility and biocompatibility,which endow them with great potential in sensing.In this review,we first describe the preparation of CDs from different starting materials via various techniques,and pre-/post-modification strategies to modulate their PL properties.Second,we outline the optical properties of CDs,including UV-vis absorption and PL,especially the PL mechanisms of CDs are presented in detail from the size effect,molecular state,surface state and defect state.Third,we summarize the research progress of CDs in sensing environmental pollutants,bioactive substances,biological microenvironments,bacteria and viruses via different mechanisms.In addition,we envision the future development trends and prospects for CDs-based nanosensors.We believe that this type of small nanoparticles will bring about big prospect in the near future.
Keywords:Carbon dots Synthesis Modification Mechanisms Sensing
1.Introduction
Optical nanomaterials have high sensitivity and selectivity for sensing variety of substances(such as ions[1–4],biological substances[5–9]and organic pollutants[10–13]),microenvironments(pH[14–16]and temperature[17,18])and fungus[19–22].In the early stage of the development of nanomaterials,semiconductor quantum dots were first prepared[23–25],but their intrinsic toxicity has become a stumbling block for the further biomedical applications[24].With the continuous research of nanomaterials,low toxic nanomaterials have been developed,such as metal nanoparticles[26,27],silicon quantum dots[28]and carbon-based nanomaterials[29–31].Especially,carbon dots(CDs)have been widely concerned and studied,due to their unique properties,performances and diverse applications[32–38].Generally,CDs are monodispersed spherical nanoparticles with the average size of 1–10 nm.CDs are generally composed of carbon core withπ-conjugated domain as well as amorphous region that is constructed by various functional groups on the surface(such as carboxyl,hydroxyl,and amino groups),which endow CDs with various advantages including:superior stability,high water solubility,tunable photoluminescence,exceptional biocompatibility and low toxicity.Based on the above advantages,CDs can be employed as promising probes for sensing.
Since CDs were discovered by accident in 2004[39],their diverse properties have attracted more and more attention.In recent years,some reviews on the preparation,properties and applications of CDs have been published[40–44].Among them,though several outstanding reviews have discussed about CDs as probes in the field of sensing.For example,Liuet al.summarized the sensing of CDs in solution and solid state[42],Sharmaet al.summarized the sensing application of different substances based on green CDs[43].However,there are no reviews focusing on summarizing the research progress of CDs nanosensors that based on different sensing mechanisms in recent years.In this review,first,the PL mechanisms of CDs are briefly discussed,which lay solid foundation for the development of CDs-based sensing systems.Next,we focus on introducing CDs-based nanosensors for the detection of various substances,microenvironments and fungus through different mechanisms.Finally,we look forward to the future research and development of CDs.
2.Preparation of CDs
2.1.Starting materials
The starting materials of CDs can be generally divided into three categories:inorganic,organic and biomass materials.
The main paragraph text follows directly on here.Most inorganic precursors for preparing CDs are limited to carbon-based materials,such as carbon fibers,carbon nanotubes,candle soot,and graphene[45–47].These large-sized carbon materials have a perfect sp2hybrid carbon structure,but lack effective transition energy levels to produce fluorescence.In order to confer luminescent property to CDs,it is required to modify their size,symmetry and surface chemistry.The most common method is to treat these carbon-based materials in strong oxidizing acidic environment.
It is universal to synthesize fluorescent CDs by using organics as starting materials.Commonly used organic precursors include small organic molecules,biological macromolecules,polymers,amino acids and carbohydrate compounds,which undergo dehydration polymerization and carbonization in the process of forming CDs[38,48–50].Since these precursors themselves contain abundant functional groups,such as amino,carboxyl and hydroxyl groups,which endow the as-prepared CDs with interesting properties and potential applications.Among them,citric acid is the most commonly used organic precursor[50].
In addition to inorganic and organic precursors mentioned above,some environmentally friendly materials have been utilized as carbon sources to prepare CDs.CDs synthesized from natural products have been reported,including milk,eggs,fruits,honey,green leavesetc.[51–53].Because this type of natural products is common in nature,CDs obtained from biomass are very popular.Moreover,biomass materials usually contain a large amount of nitrogen and oxygen elements,which are very effective for preparing heteroatom-doped CDs with high PL efficiency.
2.2.Synthetic methods
The synthetic methods of CDs have gone through two phases.The first phase was in 2004–2010,researchers are mainly committed to the development of top-down synthetic routes.The other stage was from 2010,CDs were frequently synthesized from different starting materialsviabottom-up routes.These researches not only simplified the preparation process,but also significantly improved optical properties of CDs.Generally,the preparation methods of CDs fall into two broad categories:top-down and bottomup routes(Fig.1).The top-down technique is“breaking down”bulk carbon sources into smaller-sized CDs through arc discharge[39],laser ablation[54]or electrochemical oxidation[55,56].CDs prepared by this method usually have a perfect sp2hybrid structure,but they have poor PL performance and strict preparation conditions.In order to obtain exceptional PL CDs,it basically involves complicated purification,separation procedures and subsequent modification processes,which are not conducive to largescale production.Therefore,it is imperative to modify the surface of CDs to improve their physical,chemical and optical properties.In order to overcome the disadvantages of the top-down method,researchers have proposed bottom-up route,which includes hydrothermal[57],solvothermal[58,59],microwave[60]and pyrolysis methods[61,62].The bottom-up route has the advantages of relatively simple preparation process,mild reaction conditions and low equipment requirements,which is widely used for preparing CDs from organics or biomass.
3.Modification of CDs
3.1.Pre-modification
The biggest advantage of using the top-down method to synthesize CDs is that carbonization and surface modification can be completed by one step to achieve special customized CDs.Generally,pre-modified CDs are synthesizedviaselecting specific starting materials by means of pyrolysis,microwave,hydrothermal or solvothermal one-pot synthetic method.Zhenget al.successfully prepared multicolor fluorescent CDs that can cross the blood-brain barrier and target glioma cells(C6)by pyrolysis using D-glucose and L-aspartic acid as raw materials[63].After that,our group synthesized a series of CDs by changing the molar ratio of Dglucose and L-aspartic acid,and determined the optimal synthesis ratio for targeting C6 cells[61].Beforehand,our group used cyanine dyes(CyOH)[58],diketopyrrolopyrrole(DPP)[64],porphyrin[65]or dopamine[35]as starting materialsviaone-pot synthetic method,to synthesize CDs with high photothermal or photodynamic efficiency.Heteroatom doping is also an effective way to tune the PL performance of CDs and endow CDs with special functions.Heteroatom-doped CDs are prepared from the precursors that contain heteroelements,including non-metal elements(N[66],S[67],P[68],B[69],F[70],Cl[71],Br[72],Si[73],Se[74])and metal elements(Cu[75],Zn[76],Mn[77,78],Fe[79],Al[80],Zr[50]).Among them,N and S have become common doped elements[81–84],because they have rich valence electrons.Recently,our group reported concentration-modulated PL of CDs[50]which were synthesized by using citric acid and thiourea as starting materials.With the increase of concentrations,the band gap of CDs gradually decreases,resulting in the red-shift of fluorescence(Fig.2).Quet al.successfully prepared N-doped CDs from citric acid and ethylenediamineviahydrothermal method and the PL quantum yield(PQY)of CDs reached up to 94%[85].During the reaction,citric acid is dehydrated to form CDs,and the adjacent amide and carboxylic acid groups form pyrrole-N in the carbon skeleton through intramolecular dehydrogenation,and further converted into graphite-N under heating conditions,thus improving the PQY of CDs.At the same time,they also proved that different types of amino groups can achieve different doping effects.Some studies reported that surface defects that caused by the passivation of heteroatoms can act as excitation energy traps and further influence the PL property of CDs.For example,Xuet al.synthesized S-doped CDs with PQY of 67% using citric acid and sodium thiosulfate as starting materials[86].S atoms play a role in catalyzing redox during the formation of doped CDs and introduce deeper degree of surface defects on S-CDs surface.With the development of research on CDs,researchers are not satisfied with the preparation of single element doped CDs.Co-doping of N and S or other elements can effectively enhance the PL of CDs.Donget al.used citric acid and cysteine as precursors to prepare N,S co-doped fluorescent CDs with PQY of 73% by hydrothermal method[87].N,S-CDs have excitation-independent behavior,and they proved that the PL of CDs originates from the radiation recombination of holes and electrons that were trapped by the surface of CDs.N atoms introduce a new surface state,while the introduction of S atoms enhances the new surface state.Dinget al.prepared S-CDs and N,S-CDs,which have similar sizes but different optical properties[88].The PQY of S-CDs is 5.5%,while the PQY of N,S-CDs is 54.4%.With prolonging the reaction time to increase N content,the PQY of N,S-CDs also gradually increased.They proved that N existed in the form of C=N and C-N,which significantly improved PQY.At the same time,the doping of S element has a synergistic effect in N,S-CDs.Therefore,heteroatom doping can effectively modify the PL of CDs.So far,heteroatoms doping has become an effective method to modulate the properties of CDs,and the development of heteroatom-doped CDs has become a hot topic in the field of CDs.
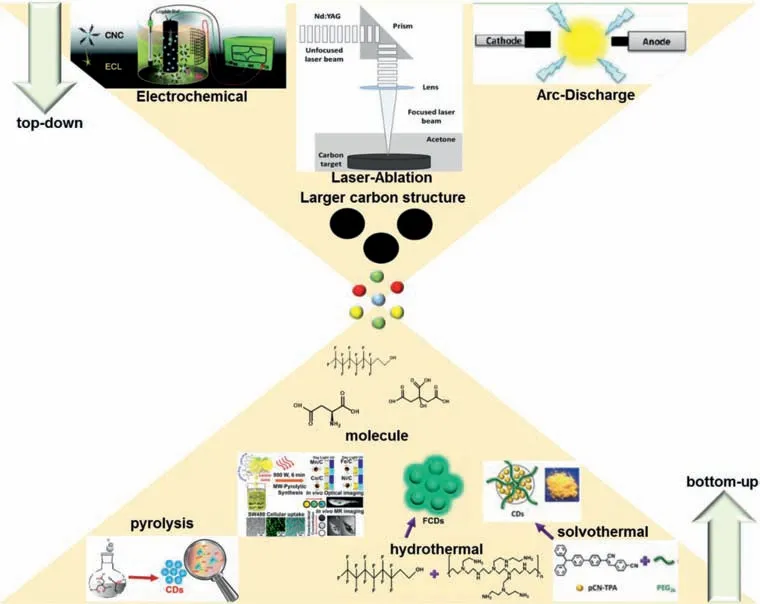
Fig.1.Top-down and bottom-up synthetic methods of CDs.
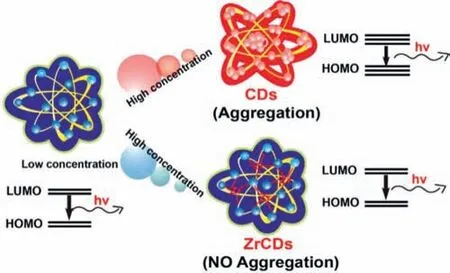
Fig.2.Schematic illustration for the varying band gap transitions of CDs and Zr-CDs at low and high concentrations.Copied with permission[50].Copyright 2020,Elsevier.
3.2.Post-modification
The surface of CDs contains prolific active groups(such as amino,carboxyl and hydroxyl groups),which can be passivated with various agents and functional polymers through covalent or non-covalent interactions.Post-modification can reduce the defects and non-radiative recombination on the surface of CDs and improve the performance of CDs.Polyethylene glycol(PEG)is the most common surface passivators.Sunet al.prepared CDs with no visible fluorescence.After decorated with PEG at 120 °C for 72 h,the passivated CDs emitted bright fluorescence[54].Peng and Travas-Sejdic passivated CDs with 4,7,10-trioxa-1,13-tridecanediamine to enhance the fluorescence of CDs(Fig.3a)[89].The above results demonstrate that the PL property of CDs can be adjusted by surface passivation.After that,plenty of researches have shown that organic molecules(such as PEG[90],mercaptosuccinic acid[91],polyethyleneimine[92])can act as passivating agent to improve the optical property of CDs.CDs with abundant amine groups were successfully conjugated with oxaliplatin to construct theranostic nanomedicine(CD-Oxa,Fig.3b)[93].The PL of blue fluorescent CDs(CDs-B,Fig.3c)could be modulated by modification with vitamin C and acetaldehyde to obtain green(CDs-V)and red emissive CDs(CDs-A),respectively[94].CDs can also be modified CDs with(Fig.3d)drugs[70],organic dyes(Fig.3e)[95,96],and proteins[97,98]vianon-covalent interactions.Postmodification opens up an avenue to improve the performance of CDs and broaden their applications.
4.Optical properties of CDs
4.1.UV-vis absorption
Generally,CDs have strong absorption in the ultraviolet region and can extend into the visible light range[99].In the UV-vis absorption spectrum,the absorption peak at 210–230 nm is ascribed to theπ-π*electron transition of the C=C bond in carbon core,while the absorption peak at 330–360 nm may be due to n-π*electronic transition of C=N/C=O bond[100,101].The absorption spectrum of CDs can generally reflect the optimal PL excitation wavelength.It is worth noting that surface passivation or heteroatoms doping will change the surface groups and intrinsic structures of CDs,and ultimately adjust the absorption property of CDs.Correspondingly,different UV-vis absorption spectra reflect the different structures and compositions of CDs.
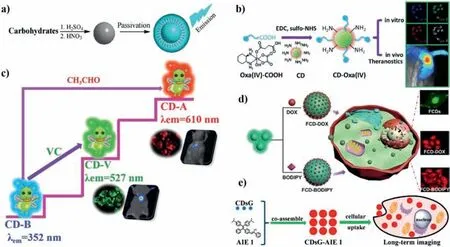
Fig.3.(a)Schematic diagram of improving the photoluminescence of CDs by passivation.Copied with permission[89].Copyright 2009,American Chemical Society.(b)Synthetic scheme for CD-Oxa and its applications in theranostics.Copied with permission[93].Copyright 2014,Wiley.(c)Schematic syntheses and applications of CD-B,CD-V and CD-A.Copied with permission[94].Copyright 2019,Royal Society of Chemistry.(d)Fabrication of FCD-DOX and FCD-BODIPY and the cellular uptake of FCD-DOX.Copied with permission[70].Copyright 2019,American chemical society.(e)Schematic illustration of CDsG-AIE preparation and cellular imaging.Copied with permission[96].Copyright 2016,American Chemical Society.
4.2.Photoluminescence
Since the advent of CDs in 2004,researchers have successfully prepared CDs with different PL,which covers almost the entire spectral range from UV to near infrared region.There is no doubt that the development of CDs with various PL has become one of the research hotspots that researchers are most concerned on.The PL of CDs can generally be divided into two categories.The first type is excitation-dependent PL.With the change of excitation wavelength,the emission peak will red-shift or blue-shift.Other inorganic or organic luminescent materials are not provided with this type of PL property.Arcudiet al.prepared CDs by microwaveassisted methods[101].They purified the crude product through dialysis and low-pressure gel permeation chromatography to obtain four types of CDs with different fluorescent properties.The other type is excitation-independent PL behavior,where emission peak will not move with the change of excitation wavelength.Jianget al.reported red,green,and blue fluorescent CDs derived fromophenylenediamine,p-phenylenediamine,andm-phenylenediamine,which have excellent excitation-independent PL properties[100].
5.Photoluminescence mechanism
Abundant starting materials,diverse synthesis methods,varied reaction conditions make the PL of CDs present diversity and complexity[102].Until now,explanation on the PL mechanisms of fluorescent CDs has been an open topic that is debated by researchers.According to the previous literature,the PL of CDs mainly includes several mechanisms:size effect,molecular state,surface state,defect state,environmental effect and cross-linking enhance emission effect.
5.1.Size effect
Some researchers believe that the PL of CDs is determined by the sp2hybrid structure that distributes in carbon cores,which can be explained by size effect.Changing the sizes of CDs will affect the distribution of sp2hybrid structure in carbon core,and further impact the corresponding energy band gap,which eventually results in the changes of CDs fluorescence.Sket al.studied the relationship between the size effect of CDs and PL property through theoretical simulation[103].They constructed CDs by density functional theory to explain that size changes will affect the emission of CDs.With the internal conjugated framework of CDs gradually extends outwards,the size of CDs increases and the emission appears obvious redshift.Meanwhile,multicolor fluorescent CDs derived from glutamic acid andp-phenylenediamine were prepared,by changing reaction solvent,precursors and pH[104].With the aid of transmission electron microscopy(TEM),researchers found that the average size of blue,green,yellow,and red fluorescent CDs sequentially increased from 1.8 nm to 7.6 nm.They believed that the dehydration and carbonization processes were controlled by changing reaction conditions,which can promote the increase of sp2hybrid framework structure in carbon core,causing the emission red shift.Therefore,the above results prove that PL property of CDs is affected by size.
5.2.Molecular state
Molecular state is employed to explain the mechanism of CDs with high PL efficiency.It refers to that the PL property of CDs depends on the molecular residues on their surface.Molecular luminescence generally occurs during the synthesis of CDs[105].In the initial stage of carbonization,due to the low temperature,starting materials undergo an intermolecular or intramolecular dehydration process,in which some small organic molecules with strong fluorescence may be generated.They are free in solution,or participate the formation of carbon core during the subsequent carbonization process and connect to the inside and surface of carbon skeleton.Variety of starting materials and synthetic conditions are capable of producing a plethora of chromophores,leading to multifarious PL[106].Songet al.discovered a fluorescent molecule named IPCA during the synthesis of CDs from citric acid and ethylenediamine[107].They verified that IPCA is the true emissive center of CDs,which connects on the surface and inside of the carbon core to make CDs emit fluorescence.Subsequently,CDs derived from citric acid and mercaptoethylamine were characterized by mass spectroscopy and1H NMR,and a luminophore M1 was found(Fig.4a)[108].Combining the results of density functional theory calculation(Figs.4b and c),they certified that the luminophore M1 is the origin of the CDs fluorescence.
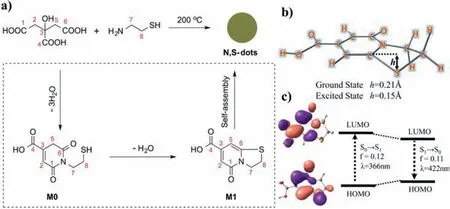
Fig.4.(a)Preparation of CDs and luminescent mechanism diagram.(b)The theoretically simulated optimal configuration of M1 and(c)the HOMO and LUMO energy level arrangement about its ground and excited states.Copied with permission[108].Copyright 2016,American Chemical Society.
5.3.Surface state
Surface state is widely used to explain the mechanism of CDs PL.The abundant surface states of CDs are excited to undergo radiation recombination,which makes CDs emit different fluorescence[109].Therefore,the functional groups or emissive centers on CDs have a great influence on their PL.The surface state of CDs could be modified chemically,which can change or generate new energy levels and further modulate PL property[110].Liet al.utilized hydrothermal methods to change the passivated degree of CDs surface and study the luminescence mechanism of CDs[111].They certificated that the contents of amino groups on the surface of CDs are the factors that affect the luminescence of CDs(Figs.5a–e).CDs were successfully synthesized from thiourea andp-phenylenediamine[110],and purified by column chromatography to obtain multicolor CDs from blue to red.Since the CDs have similar size distribution and graphite structures,size effect was excluded.Through structural analysis of these samples,the researchers found that different extent of surface oxidation caused the band gap of CDs gradually decrease,leading to the fluorescence shift from blue to red(Fig.5f).After that,they also studied the discrepancy of CDs with different surface states,with the aid of single-particle fluorescence imaging technology.The result revealed the rationality about the surface states mechanism of CDs[112].At the same time,model CDs with different contents of amino groups were constructedviathe density functional theory(Fig.5g).It was found that the more amino groups on the surface of CDs,the smaller the energy level,which caused the red shift of emission.The above results demonstrate that luminescence is related to the surface states of CDs.
5.4.Defect state
The PL property of CDs is affected by the dangling bonds of sp3hybrid on the edge of carbon skeleton structure or the defects in the internal structure.In the early development of CDs,bulk carbon materials such as carbon fibers,carbon nanotubes and graphene were flaked by arc discharge or laser ablation to obtain CDs(Fig.6)[54].The as-synthesized CDs have weak fluorescence.After passivated by strong reducing agents,the surface defects of CDs will be more stable to promote effective radiation recombination and get higher PQY.Gencet al.verified that CDs have internal defects by Raman spectroscopy and electron spin resonance spectroscopy[113].They believed that the PL of CDs derived from surface or internal defects of CDs.
5.5.Environmental effects
The environmental factors include temperature,solvent and pressureetc.,which also affect the luminescence properties of CDs.Song’s group discovered that CDs with amino groups[114],can form hydrogen bonds with solvent molecules,which is the main reason for the solvent discoloration of CDs.Our group prepared a kind of CDs with solvent discoloration[115],due to the interaction of CDs with solvents.Besides solvent,temperature and pressure also affect the luminescent properties of CDs.
5.6.Cross-linking enhance emission effect(CEE)
CEE is to explore the origin of strong luminescence of nonconjugated polymer dots with non-emissive or weakly emissive sub-chromophores.Zhuet al.used PEI as a model non-conjugated polymer to prepare polymer dots by cross-linking reaction[116].They found that the decreased vibration and rotation of the crosslinked PEI is the main reason for the luminescence enhancement.In addition,the other three cross-linked polymer dots also proved the universality of CEE.Since then,another literature demonstrated that the luminescent properties of CDs were caused by CEE[117].
6.Application of CDs in sensing
Whether in chemistry or biology field,it is indispensable to detect environmental pollutants,bioactive substances,biological microenvironments,bacteria and viruses(Table 1).Usually,most of analytes exist in food,daily necessities,environment or soil,which are absorbed by human body through the food chain,and ultimately influence human health.Therefore,it is extremely important to develop nanosensors with non-toxicity,excellent biological safety and water solubility.So far,plenty of probes have been developed,such as semiconductor quantum dots[23–25]and fluorescent dyes[118–120].Compared with these materials,CDs have advantages of good water solubility,high stability,low-toxicity and simple preparation[32–35,57–62],which make them great potential for sensing.
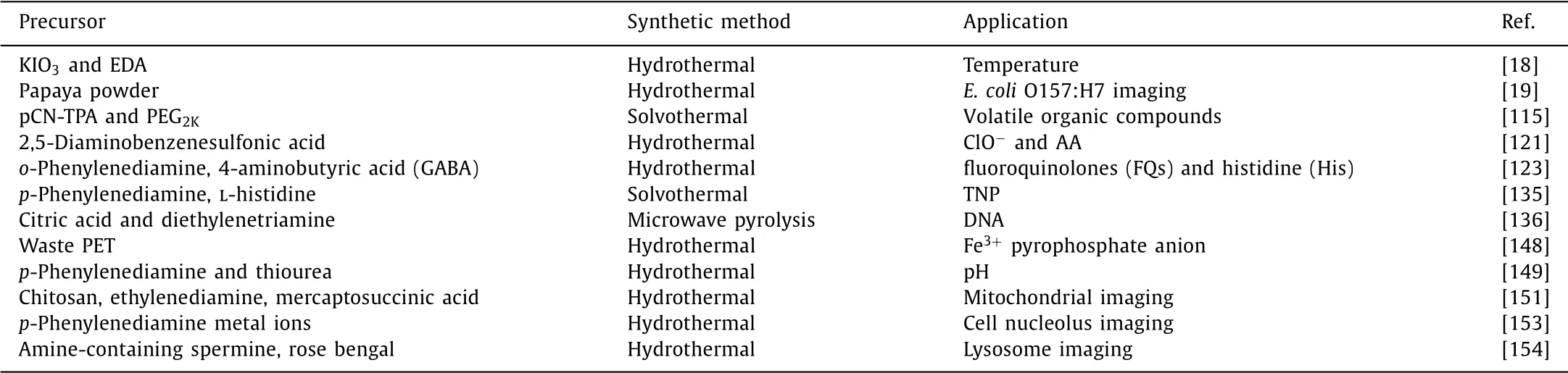
Table 1 Summary of representative CDs used in various sensing applications.
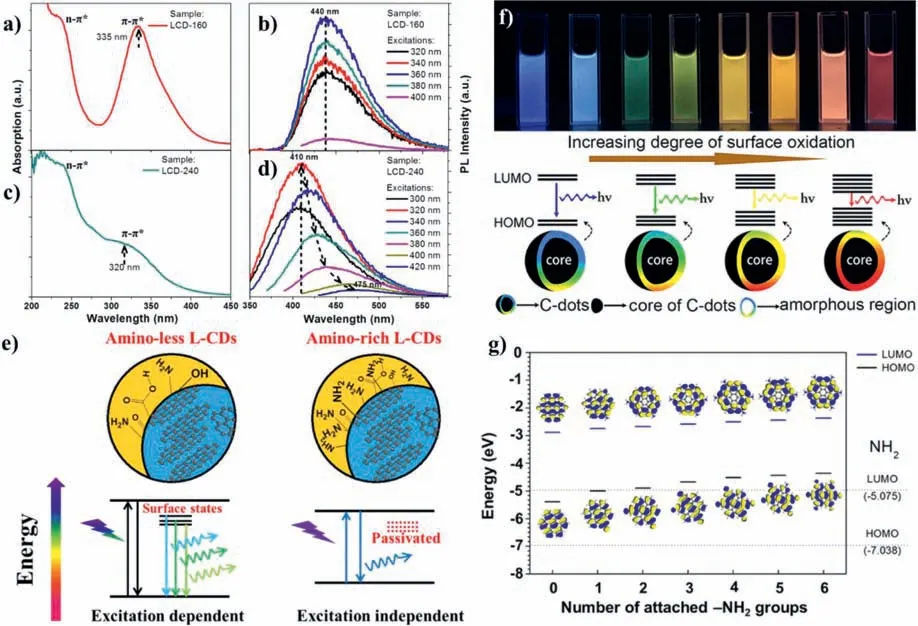
Fig.5.(a)UV-vis absorption and(b)PL spectra of CDs prepared at 160 °C.(c)UV-vis absorption and(d)PL spectra of CDs prepared at 240 °C.(e)Surface-state engineering of CDs with excitation-dependent and excitation-independent luminescent properties.Copied with permission[111].Copyright 2014,Springer Nature.(f)Models for the tunable PL of CDs with different degrees of oxidation.(g)Density functional theory simulation calculation of the relationship between the structures of CDs containing different amounts of amino groups and the energy band gap.Copied with permission[112].Copyright 2013,American Chemical Society.
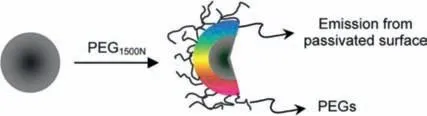
Fig.6.The emission of CDs from passivated surface by PEG1500N.Copied with permission[54].Copyright 2006,American Chemical Society.
The surface of CDs contains abundant functional groups.These functional groups act as active sites to react with analytes,and further change the PL of CDs to a large extent.Based on the changed PL signal,CDs can be employed as an effective fluorescence probe for sensing.In summary,the quenching or suppressed quenching mechanisms of CDs can be divided into eight types:static quenching,dynamic quenching,aggregation-induced quenching(AIQ),Förster energy resonance transfer(FERT),inner filter effect(IFE),photoinduced electron transfer(PET),oxidation/reduction and coordination reaction.
6.1.Different mechanisms-based sensing
6.1.1.Fluorescence quenching
Static quenching-based sensing:The complexes between CDs and analytes are formed through intermolecular interactions,resulting in the fluorescence quenching of CDs.Static quenching has several obvious characteristics:(1)The fluorescence lifetime of CDs remains changed before and after quenching,(2)the absorption spectrum of CDs changes after adding analytes,(3)temperature affects the stability of complex,leading to reduced quenching efficiency.
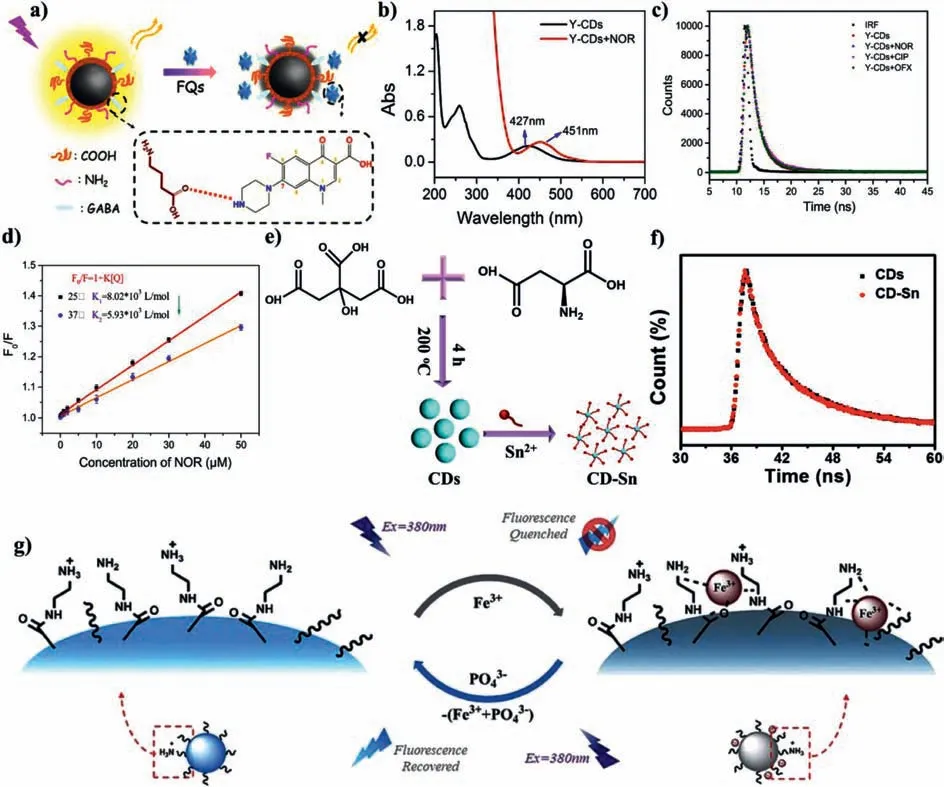
Fig.7.(a)Schematic illustrating the sensing mechanism of Y-CDs to FQs.(b)UV-vis absorption.(c)Fluorescence decay curves of Y-CDs before and after adding FQs.(d)The quenching efficiency at 25 °C and 37 °C.Copied with permission[123].Copyright 2018,American Chemical Society.(e)Schematic illustration for fabricating chiral CDs-based nanoprobes for assaying Sn2+.(f)Fluorescence decay curves of chiral CDs before and after adding Sn2+.Copied with permission[126].Copyright 2020,Elsevier.(g)Schematic representation of the sensing process of CD to Fe3+.Copied with permission[122].Copyright 2018,American Chemical Society.
Based on the mechanism of static quenching,the fluorescence of CDs is quenched by various analytes[121–125].Luet al.synthesized bright yellow fluorescent CDs(Y-CDs)and used them as a multi-functional nanosensor for the detection of fluoroquinolones(FQs)(Fig.7a)[123].The fluorescence of Y-CDs can be effectively quenched by FQs.The addition of FQs leaded to the absorption peak of Y-CDs at 427 nm disappeared,while a new absorption peak appeared at 451 nm(Fig.7b),indicating that FQs formed complexes with the functional groups on the surface of Y-CDs.The fluorescence lifetime did not change in the presence of FQs(Fig.7c).At the same time,they also studied the quenching efficiency at different temperature(Fig.7d),and the results proved that the higher the temperature,the lower the quenching effi-ciency.The results verified that the quenching mechanism was static quenching.Our group designed a kind of chiral CDs from L-aspartic acid and citric acid[126].The chiral CDs have good selectivity on Sn2+ions(Figs.7e and f).The addition of Sn2+had no effect on the fluorescence lifetime of CDs,demonstrating that the fluorescence quenching of chiral CDs induced by Sn2+was static quenching.CDs prepared by Liuet al.have the property of strong acid-induced fluorescence enhancement.Fe3+could coordinate with amino groups or amide groups on the surface of CDs to form CDs/Fe3+hybrids(Fig.7g)[122].The fluorescence lifetime of CDs and CDs/Fe3+hybrids is 8.06 ns and 7.85 ns,respectively,certifying that the quenching mechanism of CDs belonged to static quenching.Maet al.prepared B-CDs by using citric acid and sodium tetraphenylborate as starting materialsviahydrothermal method.When theorthohydroxyl groups of catechol cross-linked with the boron hydroxyl groups of B-CDs,the fluorescence of BCDs was quenched[127].The bimodal quenching constant proves that fluorescence quenching of B-CDs is a static quenching process.When the concentration of catechol is 1–50 nmol/L,the limit of detection(LOD)is 0.25 nmol/L.
Dynamic quenching-based sensing:When the excited state of CDs collides with quenchers,it returns to the ground state through energy or charge transfer,this process is dynamic quenching.The characteristics of dynamic quenching are the exact opposite of static quenching:(1)The fluorescence lifetime of CDs will change upon the addition of quenchers,(2)the UV-vis absorption spectrum of CDs is not affected,and(3)quenching efficiency is enhanced by increasing temperature.
Huet al.prepared N,S,Cl-CDs from salty foodviaacid hydrolysis,(Fe(CN)6)4-can effectively quench the fluorescence of N,S,Cl-CDs by dynamic quenching(Figs.8a–c)[128].Thus,N,S,Cl-CDs can be successfully used to detect(Fe(CN)6)4-in food samples with high sensitivity.Liuet al.prepared N-CDs by oxidizing cork materials.Fe3+can effectively quench the fluorescence of N-CDs[129].They suggested that the excited electrons of CDs were transferred to the 3d orbit of Fe3+(Fig.8d),and these non-radiative electron holes cause fluorescence quenching.At the same time,after adding Fe3+,the fluorescence lifetime of CDs was attenuated,which further proved the dynamic quenching mechanism.Songet al.also demonstrated the multicolor N-CDs have similar quenching mechanism for detecting Fe3+in living cells(Figs.8e and f)[130].Pb2+ions could effectively quench the fluorescence of CDs synthesized from bamboo[131],with LOD less than 0.14 nmol/L(Fig.8g).
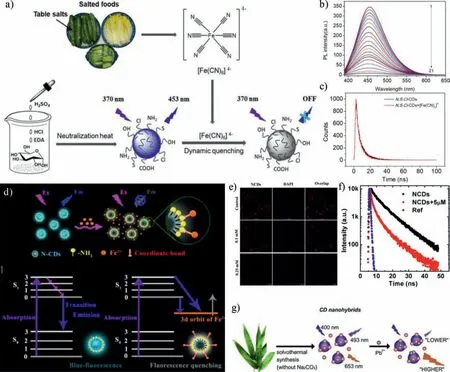
Fig.8.(a)Preparation and schematic illustration of N,S,Cl-CDs-based nanosensors for detection of[Fe(CN)6]4- in food samples.(b)The photoluminescence spectra of the N,S,Cl-CDs-based sensing system containing various concentrations of[Fe(CN)6]4-.(c)The fluorescence lifetime of N,S,Cl-CDs before and after adding[Fe(CN)6]4-.Copied with permission[128].Copyright 2020,Elsevier.(d)Mechanism diagram of N-CDs coordinated with Fe3+ ions.Copied with permission[129].Copyright 2020,Elsevier.(e)Images of cells incubated with different concentrations of NCDs/Fe3+(f)The fluorescence lifetime of NCDs before and after adding Fe3+.Copied with permission[130].Copyright 2017,American Chemical Society.(g)Schematic illustration of CDs for the detection of Pb2+.Copied with permission[131].Copyright 2019,Elsevier.
AIQ-based sensing:The AIQ effect is almost common for most fluorophores.When the fluorescent probe aggregates,the excited state energy of the probe is transferred to the ground state through a non-radiative transition,resulting in sharp decrease or even complete disappearance in the fluorescent signal.The coordination of CDs with analytes makes CDs aggregate,which causes the fluorescence quenching.The main manifestation is that the average size becomes larger after adding quenchers.
Yueet al.prepared ethylenebis-(oxyethylenetriaza)tetraacetic acid(EGTA)modified CDs by hydrothermal method for the detection of Ca2+[132].After adding Ca2+,EGTA on the surface of CDs can capture Ca2+(Figs.9a–c)and form the aggregates,leading to the fluorescence quenching of CDs.CDs synthesized from L-threonine and P2O5have high sensitivity to Au3+[133].The addition of Au3+makes the average size of CDs increase from 0.62 ± 0.4 nm to 21.1 ± 0.8 nm(Fig.9d),proving that Au3+induces the aggregation of CDs and fluorescence quenching.Sunet al.successfully prepared CDs that have different fluorescence quenching to Ce3+,Fe3+and Cu2+(Figs.9e–h)[134].
FRET-based sensing:FRET needs to satisfy the following conditions between the excited state CDs and the ground state quencher:(1)The emission spectrum of CDs(energy donor)overlaps with the absorption spectrum of quencher(energy accepter),(2)the distance between CDs and quencher range from 1 nm to 10 nm.When the energy resonance transfer occurs,it is often accompanied by the attenuation of the fluorescence lifetime of CDs.
FRET is also one of the common quenching mechanisms for CDs-based nanosensors.For example,our group developed amphiphilic CDs for sensing environmental nitro-aromatic explosives(Fig.10a)based on FRET[135].Compared with other nitroaromatics(Figs.10b and c),we observed that the fluorescence spectrum of CDs had a better overlap with the UV-vis absorption spectrum of 2,4,6-trinitrophenol(TNP),which resulted in the fluorescence of amphiphilic CDs was effectively quenched.Huet al.designed red dual-emission CDs that can sensitively detect methyl blue[125].They found that fluorescence spectrum of CDs partially overlapped with absorption spectrum of methyl blue,which promoted nonradiative recombination and quenched the fluorescence of CDs.Kudret al.used microwave pyrolysis to prepare fluorescent CDs from citric acid and diethylenetriamine for detecting DNA damage[136].They explored the competition experiment of the binding of CDs and ethidium bromide(EtBr)with DNA.CDs acted as energy donors,while EtBr acted as energy acceptors,promoting the binding of CDs with DNA though FRET.Effective energy resonance transfer occurred between 3-aminophenylboronic acid-derived CDs and MnO2,as evidenced by quenched fluorescence[137].With the concentration of MnO2increased,the quenching efficiency of CDs gradually increased,but the fluorescence lifetime of CDs markedly decreased.
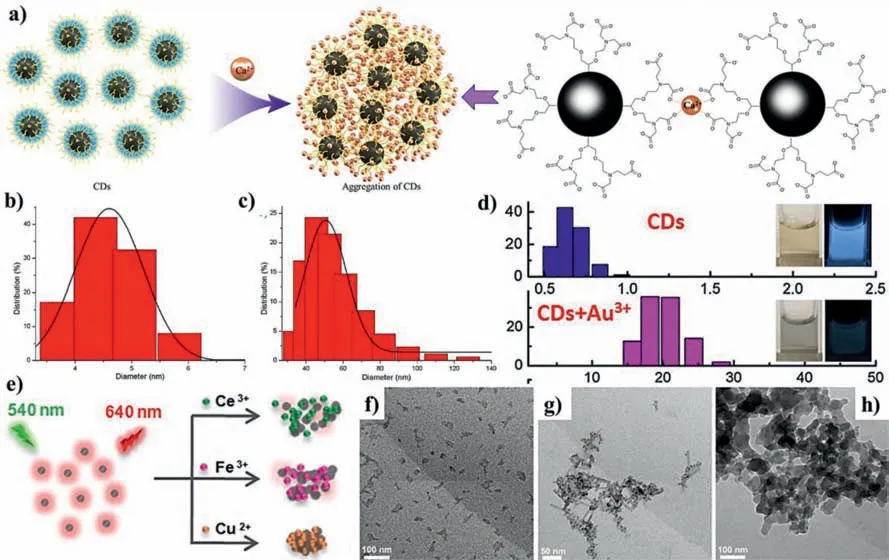
Fig.9.(a)Schematic diagram of Ca2+ ions induce CDs aggregation.The hydrodynamic diameters of CDs before(b)and after(c)the addition of Ca2+ ions.Copied with permission[132].Copyright 2019,American Chemical Society.(d)The hydrodynamic diameters of CDs and CDs+Au3+.Copied with permission[133].Copyright 2018,Elsevier.(e)Schematic illustration of CDs for detection of Ce3+,Fe3+ and Cu2+.TEM images of CDs with the addition of(f)Ce3+,(g)Fe3+,and(h)Cu2+.Copied with permission[134].Copyright 2018,American Chemical Society.
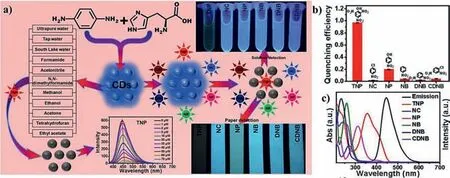
Fig.10.(a)Schematic diagram for synthesizing amphiphilic fluorescent CDs and their application in TNP detection.(b)Fluorescence quenching efficiency of CDs upon the addition different nitroaromatics.(c)Absorption spectra of various nitroaromatic compounds and the emission spectrum of CDs.Copied with permission[135].Copyright 2019,Elsevier.
IFE-based sensing:When the UV-vis spectrum of quencher overlaps with the excitation or emission spectrum of CDs,inner filter effect(IFE)will occur.Quenchers can not only shield the excitation light of CDs,but also can absorb the emission of CDs.The enhanced absorption of quencher indicates that the fluorescence of CDs is quenched.Most importantly,the IFE-based fluorescence quenching mechanism does not affect the fluorescence lifetime of CDs.
CDs sensing application that based on IFE for the detection of Cr2O72-was first developed by Zhenget al.in 2013[138].After adding Cr2O72-,the fluorescence of CDs was completely quenched under 365 nm excitation(Fig.11a),and because the excitation spectrum of CDs completely overlapped with the absorption spectrum of Cr2O72-(Fig.11b),which met with the condition of IFE.Xuet al.prepared CDsviahydrothermal method by using(3-aminopropyl)triethoxysilane and glycerin as starting materials.CDs were employed as a ratiometric nanosensor for the detection of cholinesterases[5].Huanget al.developed fluorescent N,Co-CDs that can be sensitively quenched by 2,3-diaminophenazine through IFE[139].N,Co-CDs can be employed as a ratiometric fluorescent probe to effectively detect cholesterol and uric acid in human serum with LOD of 3.6 nmol/L and 3.4 nmol/L,respectively.
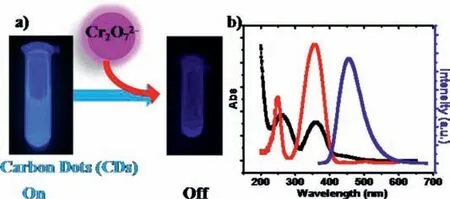
Fig.11.(a)Schematic illustration of fluorescence assays of CDs for the detection of Cr2O72- based on IFE.(b)Fluorescence excitation(red solid line)and emission(blue solid line)spectra of CDs and UV-vis absorption spectrum of Cr2O72-(black solid line).Copied with permission[138].Copyright 2013,American Chemical Society.
PET-based sensing:In the process of PET quenching,CDs act as electron donors or acceptors,quenchers cause fluorescence quenching of CDs through electron transfer,this quenching manner is defined as PET quenching.
Wuet al.constructed a mitochondrial-targeted CDs-based nanosensor to detect peroxynitrite(ONOO-)in living cells[140].The addition of ONOO-caused the fluorescence of CDs drop off gradually.When its concentration was in the range of 0.2–10 μmol/L,the LOD is 13.5 nmol/L.Experiments showed that ONOO-reacted witho-diaminobenzene on the surface of CDs to generate benzotriazole,and the surface electrons of CDs were transferred to benzotriazole,resulting in the quenched fluorescence(Fig.12a).Liet al.developed red-emissive CDs for detecting organophosphorus pesticides(OPs)(Fig.12b)[141].In the presence of dopamine(DA),acetylcholine(ATCh)and acetylcholinesterase(AChE),OPs inhibited the activity of AChE that blocked the hydrolysis of ATCh,which promoted the polymerization of DA to form polydopamine(PDA).PDA could effectively absorb the electrons of CDs,and then transfered them from the highest occupied molecular orbital(HOMO)to the lowest unoccupied molecular orbital(LUMO).The results indicated that the quenching was attributed to PET mechanism.Heet al.synthesized blue fluorescent CDs by using citric acid and EDTA as starting materials[142].A series of experiments were carried out to prove that Hg2+quenched the fluorescence of CDs through PET mechanism(Fig.12c).In addition to the analytes mentioned above,testosterone[143],water[144],hydrogen peroxide[145],and rhodamine B were detectedviaPET mechanism.
Oxidation/reduction reaction-based sensing:The fluorescence of 2,5-diaminobenzenesulfonic acid-derived RD-CDs can be quenched by hypochlorite(ClO-)by means of oxidation reaction as well as forming nonluminescent complex.Then the oxidant of RD-CDs was deoxygenized by ascorbic acid(AA),leading to the recovery of RDCDs fluorescence(Fig.13a)[121].Zhenget al.prepared CDs from citric acid and diethylenetriamineviapyrolysis.The fluorescence of CDs was quenchedviaIFE.After adding AA,Cr(VI)was reduced to Cr(III),IFE was eliminated,and the fluorescence of CDs was restored(Fig.13b)[138].Xuet al.constructed a nonluminescent pCDs-MnO2for detecting GSH[146].After adding GSH to pCDs-MnO2solution,pCDs were released through a series of reduction processes,and their fluorescence was restored.
Coordination reaction-based sensing:Gaoet al.constructed CDs/Fe3+complex through PET mechanism,which could sensitively detect phytic acid(PA).Since PA has a higher affinity with Fe3+than CDs,Fe3+preferentially coordinated with PA,causing the remove of Fe3+from the surface of CDs.Thus,the fluorescence of CDs was restored[147].Similarly,Huet al.developed CDs/Fe3+nanosensors to effectively detect pyrophosphate.The fluorescence of CDs was restored by the coordination between Fe3+and pyrophosphate[148].In the concentration range of 2–600 μmol/L,the LOD of pyrophosphate reached 0.86 μmol/L.Meanwhile,our group constructed chiral CD-Sn hybrids for sensing lysine enantiomers.L-Lysine(L-Lys)could complex with Sn2+to prompt L-Lys to flee from the surface of CDs,recovering the fluorescence of chiral CDs[126].However,D-Lys had no effect on the fluorescence of chiral CDs.Thus,CD-Sn was used for sensing Lys enantiomers and the LOD of L-Lys was 3.34 μmol/L.
6.2.Other type sensing
6.2.1.CDs for microenvironment sensing
It is essential to develop nanosensors for detecting microenvironmental conditions.Since chemical processes and major diseases are closely related to abnormal pH fluctuations,it is essential to develop CDs-based pH nanosensors.Our group constructed two kinds CDs(CD-A and CD-B)derived from BODIPY dyes A/B with PEG1500.Both CD-A and CD-B can be used for pH sensing in extreme acidic environments in bacteria(Fig.14a)[14].Guoet al.prepared N,S-CDs fromp-aminobenzenesulfonic acid andophenylenediamine by solvothermal method.The pH titration experiment proved that N,S-CDs could act as a ratiometric fluorescent probe to detect pH with good reversibility[15].The protonation and deprotonation of pyridine N and pyrrole N in CDs manifested the response to pH.When pH dropped from 3.0 to 1.0,a new emission peak appeared at 634 nm,which could be attributed to the charge transfer of functional groups on the surface of CDs in an acidic environment.Our group has developed lysosome-targeting probes based on CDs to detect pH changesin vitroandin vivo(Fig.14b)[149].CDs have a good pH-sensitivity in pH range from 4.0 to 8.0.CDs exhibit good performance in quantitatively measuring pH changes in living cells and monitoring pH fluctuations in organisms.In short,the design and exploitation of CDs-based pH nanosensors has opened up a new path for disease diagnosis.
Whether in chemical process or living system,temperature is also one of the important parameters.Accurately detecting the temperature of various environments has also become top priority.Compared with other nanomaterials,CDs have greater advantages for temperature sensing.Kalytchuket al.constructed N,S,I-CDs from C3N3S3,potassium iodate(KIO3)and ethylenediamine(EDA),which were successfully employed for sensing the temperature in HT-29 cells(Fig.14c)[17].Yanget al.synthesized N-CDs from C3N4and ethanediamine[16].When temperature increased from 20 °C to 80 °C,the fluorescence of N-CDs gradually decreased(Fig.14d).
6.2.2.CDs for subcellular organelle and bacteria sensing
CDs have the advantages of good biocompatibility,excellent resistance to photobleaching and non-toxicity,and have been employed for sensing subcellular organelles and bacteria.Since organelles play a key role in cell function,the imaging and location of certain subcellular structures have an important guiding role in the origin of some diseases.Wu’s group has studied the imaging of subcellular structures[150–154].For example,Huaet al.prepared red fluorescent CDs that were doped with metals,and they selected nickel-doped CDs to achieve high-resolution imaging of cell nucleus[153].CDs can be employed not only for nucleus imaging,but also for other organelles imaging,such as mitochondria and lysosomes[154].The fluorescent CDs prepared from glycerol and silane possess mitochondrial targeting function,which can distinguish cancer cells from normal cells based on the difference in mitochondrial membrane potential[151].
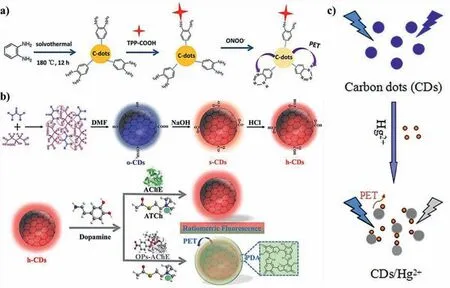
Fig.12.(a)Schematic illustration of preparation of CDs and a suggested PET mechanism of fluorescence quenching of C-dots-TPP in the presence of ONOO–.Copied with permission[140].Copyright 2017,Elsevier.(b)Synthesis route of CDs and schematic illustration of h-CDs/DA-based sensor for OPs detection.Copied with permission[141].Copyright 2020,American Chemical Society.(c)Schematic of CDs as sensors for the sensing process of Hg2+ ions.Copied with permission[142].Copyright 2016,Elsevier.
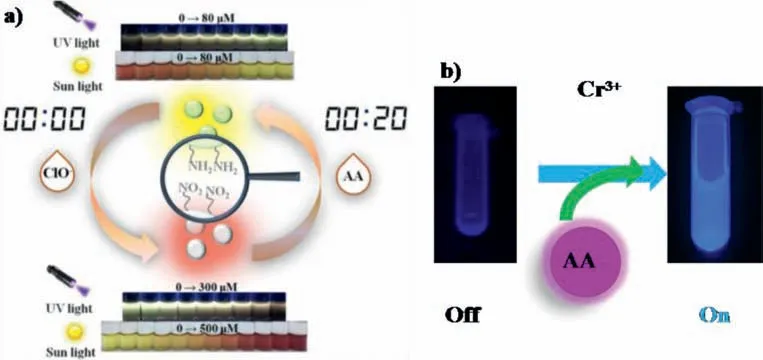
Fig.13.(a)Reversible sensing performance of RD-CDs for ClO– and AA in colorimetric and fluorescence modes.Copied with permission[121].Copyright 2019,American Chemical Society.(b)Schematic illustration of fluorescence assays for AA based on reduction reaction of CD-Cr(VI).Copied with permission[138].Copyright 2013,American Chemical Society.
Bacteria and fungi are widespread in nature,and their infection is one of the biggest challenges to human health globally.Thus,it is urgent to develop sensors for detecting bacteria and fungi with high sensitivity and selectivity.CDs-based nanosensors for rapidly and accurately detecting bacteria or fungi have been reported[155–157].Wanget al.used papaya powder as carbon source to synthesize W-CDs by hydrothermal method.W-CDs exhibit excellent fluorescence response toE.coliO157:H7[19].As the concentration ofE.coliO157:H7 increased,the fluorescence of W-CDs gradually enhanced(Fig.14e),and the LOD is 9.5 × 104cfu/mL.They believed that W-CDs could interact with the FimH protein inE.coliO157:H7,resulting in the fluorescence enhancement of WCDs.On the contrary,the obtained E-CDs from ethanol have no fluorescent response toE.coliO157:H7.Furthermore,Wanget al.constructed a class of aptamer-conjugated CDs(CD-apt).CD-apt can specifically detect Salmonella typhimurium with high selectivity[20].Huaet al.prepared fluorescent CDs from bacteria(S.aureusorE.coli)for the first time[21],which can selectively stain dead bacteria and fungi.It has also been reported that NPS-CDs prepared from yeast extracts can be used to stain dead bacteria[158].CDs-605 synthesized fromL.plantarumcan be able to image biofilm-encased microorganisms(e.g.,E.coli,S.oneidensis,P.aeruginosa,S.aures,andT.reesei)[22].
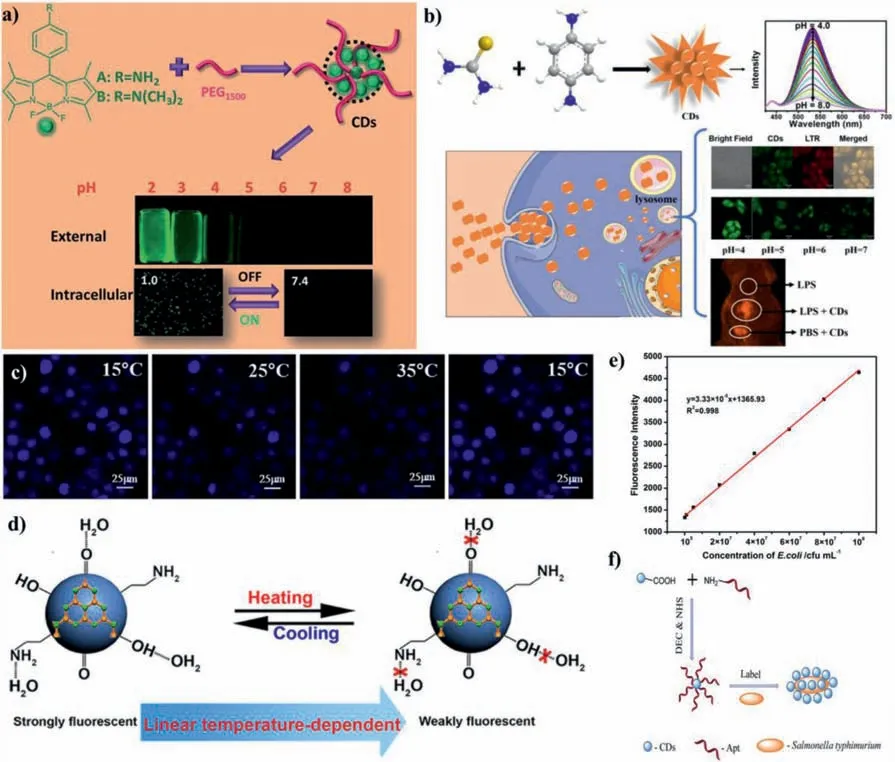
Fig.14.(a)Synthetic scheme for CDs and their application as pH probes.Copied with permission[14].Copyright 2018,Royal Society of Chemistry.(b)Schematic illustrating the fabrication of CDs,their lysosome targeting ability and applications for probing pH changes in vitro and in vivo.Copied with permission[149].Copyright 2020,Elsevier.(c)Confocal microscopy images of N,S ,I-CDs-colon cancer cell HT-29 with corresponding fluorescence field at 15,25,35 and 15 °C,respectively.Copied with permission[17].Copyright 2020,Elsevier.(d)Schematic mechanism for the temperature-dependent fluorescence intensity of N-CDs.Copied with permission[16].Copyright 2015,American Chemical Society.(e)The linear calibration of fluorescence intensities for the W-CDs at 1.17 mg/mL in the presence of various amount of E.coli O157:H7 within a concentration range of 105–108 cfu/mL.Copied with permission[19].Copyright 2016,Elsevier.
6.2.3.CDs for sensing volatile organic compounds
By now we were standing9 in the long Holiday line in the Layaway Department, and I figure it was now or never. I lay down on the ground and began screaming, I want that telephone, over and over again. Weary Christmas shoppers looked as my mother calmly said, Becky, you better get up by the count of three or else. One…Two…Three.
Previously,our group developed hydrophilic CDs with solvatochromism,which can be applied as optical noses for sensing volatile organic compounds(VOCs)[115].We dropped the CDs solution onto the indicator paper to obtain a paper-based sensor for detecting VOCs(Fig.15).Moreover,the color change of indicator paper is reversible,proving that the paper-based sensor has the advantage of fast response.Furthermore,Wanget al.designed fluorescent complexes(MCM/CDs)from mesoporous materials and CDs,which integrated the physical adsorption of mesoporous materials and the fluorescent property of CDs[159].MCM/CQDs could sensitively detect acetic acid gas with the LOD of 0.2 μmol/L.
7.Summary and outlook
Survival of the fittest is not only the law of survival in nature,but also the law in the field of scientific research.With the continuous development of nanotechnology,nanomaterials are constantly being updated.Compared with other organic or inorganic nanomaterials,CDs possess ultrasmall size,remarkable photoluminescence,high water solubility,low toxicity,robust stability,extraordinary biocompatibility,and easy surface functionalization,which make CDs superior in a variety of research fields.
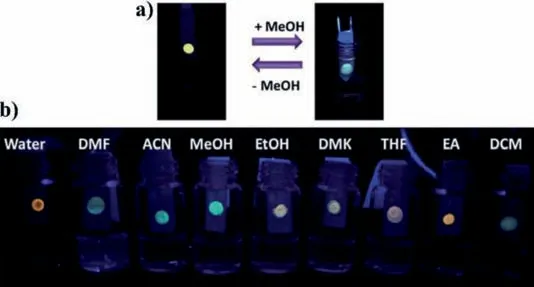
Fig.15.(a)The reversible emission color changes of CDs indicator paper under UV lamp(365 nm).(b)Photographs of the paper sensor printed with CDs after exposed to the VOCs under the UV lamp(365 nm).Copied with permission[115].Copyright 2016,Royal Society of Chemistry.
Here,we emphasized the modification of CDs,including premodification and post-modification.For pre-modification,in the special customization process of CDs,CDs are endowed with multiple luminescent properties and functions by selecting various starting materials and reaction conditions.On the other hand,for post-modification,the surface of CDs contains plentiful functional groups,which can react with a multitude of functional materials through covalent or non-covalent interactions.
Due to the diversity of preparation conditions,the obtained CDs are invested with various PL properties,so the corresponding luminescence mechanisms are also different.According to the currently reported literature,the luminescence mechanism of CDs can be classified into four categories:size effect,molecular state,surface state and defect state.In this review,we have explained the luminescence mechanisms of CDs in detail.However,by changing the reaction conditions during the synthesis of CDs,the corresponding luminescence mechanisms will also be very different,and in many cases they will appear synergistically.Because of the infinite possibilities of CDs,their PL has been continuously exploited by researchers.Continuous exploration the PL mechanism of CDs is a top priority,because CDs may have many interesting and meaningful properties that we need to discover.
When CDs come to sensing,we summarized CDs nanosensors with different mechanisms,including static quenching,dynamic quenching,AIQ,FERT,IFE,PET,oxidation/reduction and coordination reaction.Second,CDs-based nanosensors for probing microenvironments,bacteria and virus,and VOCs were stated.However,the potential applications of CDs are far more than the content of this review.We believe that CDs-based nanosensors still have a long way to go.
Although CDs-based fluorescent nanosensors have made great progress,it is worth noting that there are very few reports on CDs for sensing viruses.However,viruses have seriously affected human health.Therefore,designing and meticulously customizing CDs-based nanosensors for the detection of viruses are still a stupendous challenge.There is no doubt that overcoming this challenge will open up a brand-new research path for the development of sensors,and will further promote the development of CDs.We firmly believe that CDs,such small-sized fluorescent nanomaterials,have a broader development prospect in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The financial support from the National Natural Science Foundation of China(No.51873023),the Jilin Province Science and Technology Research Project(No.20200201088JC).
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
- Applying nanotechnology to boost cancer immunotherapy by promoting immunogenic cell death
