Self-sacrificial template synthesis of Fe,N co-doped porous carbon as efficient oxygen reduction electrocatalysts towards Zn-air battery application
Tianfang Yang,Ye Chen,Yang Liu,Xupo Liu,Shuyan Gao
School of Materials Science and Engineering,Henan Normal University,Xinxiang 453007,China
ABSTRACT Designing highly efficient non-precious based electrocatalysts for oxygen reduction reaction(ORR)is of significance for the rapid development of metal-air batteries.Herein,a hydrothermal-pyrolysis method is employed to fabricate Fe,N co-doped porous carbon materials as effective ORR electrocatalyst through adopting graphitic carbon nitride(g-C3N4)as both the self-sacrificial templates and N sources.The g-C3N4 provides a high concentration of unsaturated pyridine-type N to coordinate with iron to form Fe-N active sites.Through adjusting the Fe doping amounts,it is proved that appropriate Fe doping content is conducive to the construction of abundant defects and active sites of Fe-N.The as-prepared catalyst exhibits superior electrocatalytic ORR performance in alkaline media with half-wave potential(E1/2 = 0.82 V)and onset potential(Eonset = 0.95 V),equivalent to the commercial Pt/C catalyst.Moreover,there is almost no activity loss after 10 k continuous cyclic voltammetry cycles and methanol tolerance,indicating the excellent durability and superior methanol tolerance.Remarkably,when assembled as the cathode in a Zn-air battery,the device displays a power density of 99 mW/cm2,an open-circuit potential of 1.48 V and long-term discharge-charge cycling stability,indicating the promising potential to substitute the Pt catalyst for practical application.
Keywords:Electrocatalyst Oxygen reduction reaction Fe N co-doped porous carbon Fe-N active sites Zn-air batteries
In view of the current energy dilemma and environmental pollution,the progress of sustainable and clean energy is the key research direction at present[1,2].Zn-air batteries(ZABs)are identified as one of the most promising next-generation energy equipment on account of the high safety factor,inexpensive,excellent durability and impressive theoretical specific energy density[3,4].For ZABs,the oxygen reduction reaction(ORR)is a fundamental reaction occurred on the cathode[5].However,the sluggish kinetics of ORR has restricted the extensive implementation of ZABs[6].Currently,platinum-group-metal(PGM)catalysts are widely utilized as the electrocatalysts for ORR owing to the excellent catalytic performance[7].Unfortunately,the high cost,serious scarcity and long-term instability have hindered the large-scale application of PGM catalysts[8,9].Therefore,the investigation of inexpensive and effective non-PGM catalysts for ORR is significant for promoting the rapid development of ZABs[10].
Recent advances have demonstrated that some non-PGM catalysts including metal oxides,sulfides,carbides and nitrides can be adopted as high-performance ORR catalysts,especially transition metal-nitrogen-carbon catalysts(M-N-C)[11].The M-N-C composite catalysts(especially Fe-N-C)have been reported as the potential alternatives to the PGM for boosting ORR due to the relatively low cost,prominent catalytic activity and excellent durability[12,13].More importantly,the nitrogen dopant atoms(particularly pyridinic-N and pyrrolic-N)feature high affinity to the transition metals,thereby forming the M-N active sites as the oxygen adsorption and reduction centers,which drastically improves the electrocatalytic performance[14].In this regard,it is of critical importance to manipulate the electronic interaction by adjusting the type of nitrogen.Therefore,choosing appropriate nitrogen sources is beneficial for regulating the ORR property of MN-C catalysts[15].Besides,the strategies of optimizing the pore structure and constructing high specific surface area are also conducive to improving the ORR activity[16].Generally,the template method is usually used to prepare porous materials,such as applying the templates of SiO2nanospheres,styrene spheres and metal oxides.However,this pore-making process is always cumbersome and toxic and the employed hydrofluoric acid could etch and poison the crucial M-N active sites[17,18].Therefore,it is necessary to develop the new facil approach to fabricate efficient porous M-N-C materials for promoting the progress of ORR electrocatalysts.
Herein,a facile hydrothermal-pyrolysis method is employed to fabricate Fe,N co-doped porous carbon materials(NPC-Fex)as high-performance ORR electrocatalyst.The graphitic carbon nitride(g-C3N4)that is non-metallic semiconductor polymer material with graphite structure has been adopted as both the selfsacrificial templates and N sources.The high concentration of unsaturated pyridine-type N in g-C3N4provides rich lone pair electrons trap metal ions in the ligands to form abundant and uniform Fe-N active sites.In addition,using g-C3N4as templates cannot only enlarge the specific surface area of carbon materials,but also eliminate the complicated steps of traditional template methods.The as-prepared NPC-Fe0.3catalyst exhibits splendid electrocatalytic property with the half-wave potential(E1/2= 0.82 V)and onset potential(Eonset= 0.95 V),superior stability as well as high methanol tolerance.Moreover,the assembled ZAB with the NPC-Fe0.3catalyst contains the maximum power density of 99 mW/cm2and extraordinary durability,demonstrating better property than the Pt/C-based device.It reveals the promising application of the ORR catalyst as supersedes for Pt/C within energy conversion equipment.
The Fe,N co-doped porous carbon materials(NPC-Fex,x represents the mass of iron precursors)were synthesized through a facile hydrothermal-pyrolysis process(Scheme 1).For comparison,the PC-Fe sample was synthesized without g-C3N4,while NPC was synthesized without iron(III)2,4-pentanedionate.Firstly,the g-C3N4was achieved by the thermal condensation of melamine in air.The XRD pattern of g-C3N4exhibits the in-plane repeating motifs of the continuous heptazine networks(100)and interlayer stacking of aromatic segments(002)at 12.9° and 27.5°,respectively(Fig.S1 in Supporting information)[19].Then,the hydrothermal treatment was carried out after thoroughly stirring with glucose as the carbon sources,g-C3N4as the nitrogen sources and iron(III)2,4-pentanedionate as the metal sources.Therein,Fe atoms were concomitantly immobilized in the frameworks to form Fe/g-C3N4@C precursors since the g-C3N4could provide the rich unsaturated N atoms as the anchoring sites.Finally,the Fe,N co-doped porous carbon materials(NPC-Fex)were obtained by pyrolysis of Fe/g-C3N4@C precursors at 900°C in N2atmosphere.Notably,the g-C3N4could also be employed as the templates for pore-making.The TGA curve shows that the thermal decomposition of g-C3N4begins at 550 °C until the complete disintegration at 750°C(Fig.S2 in Supporting information),consistent well with the previous literature[20].During this process,the gas molecules produced by the g-C3N4escape from the carbon materials,which are expected to generate abundant pores in the carbon materials.
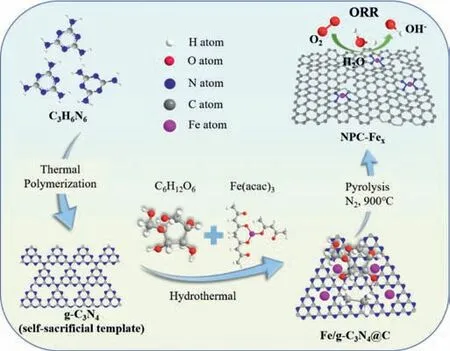
Scheme 1.Schematic illustration of preparing NPC-Fex catalysts through a facile hydrothermal-pyrolysis process.
The prepared samples of NPC-Fe0.3and NPC show a broad characteristic diffraction peak at 22.6°,which is assigned to the(002)plane of graphitic carbon.There is no peak corresponding to metallic Fe or other iron compounds(Fig.1a).Meanwhile,some obvious signals in the ranges of 20°-30° and 40°-50° are determined for PC-Fe,matching well with the characteristic peaks of FeC and Fe3C phases.It is speculated that the absence of Fe3C and FeC characteristic peaks in NPC-Fe0.3is attributed to the fact that Fe is mainly coordinated with the N species in g-C3N4.Raman spectroscopy was performed to characterize the chemical structure and the degree of structural defects of NPC-Fe0.3.There are two typical peaks located at 1350 cm-1(D band)and 1580 cm-1(G band)(Fig.1b).The D band corresponds to the disordered sp3carbon(on behalf of defects and disorder-induced features),whereas the G band denotes the graphitic sp2carbon.Moreover,The defect level of carbon-based materials was studied by the intensity ratio of D band to G band(ID/IG)[21].As revealed in Fig.1b,the relative intensity ratio of NPC-Fe0.3(ID/IG= 1.31)is much higher than that of PC-Fe(ID/IG= 1.18),indicating that more defects are generated in the graphitic carbon lattices owing to the addition of nitrogen atoms[22].Actually,the index(ID/IG)of NPC-Fe0.3is lower than that of NPC(ID/IG= 1.33)due to the catalysis effect of iron can promote the graphitization of carbon[23].Furthermore,NPCFe0.3exhibits a typical 2D peak at about 2700 cm-1belonging to the typical peak of graphene-based material,further indicating the graphene structure of catalysts[24].The specific surface area and pore size distribution were characterized by nitrogen gas adsorption/desorption isotherms.All the samples reveal a classic type-IV curve with an H4-type hysteresis loop at the relatively higher relative pressure,demonstrating the existence of a large amount of mesopores(Fig.1c).The curves of pore size distribution further reveal the mesoporous structure and the pore sizes of NPCFe0.3are mainly centered at ~11.4,~15.6 and ~26.1 nm(the insert in Fig.1c).Generally,the mesopores can improve specific surface area,afford numerous active sites and facilitate the transfer and diffusion of ORR related species(O2and*OH,etc.)[25].Compared to PC-Fe,NPC-Fe0.3exhibits more abundant porous structures toward large sizes and broad distributions,further confirming the favorable pore-making role of g-C3N4.The Brunauer–Emmett–Teller(BET)specific surface area of NPC-Fe0.3is 224.4 m2/g,which is much larger than the specific surface area of PC-Fe(162.9 m2/g).It is speculated that the large specific surface area and hierarchical porous structure of NPC-Fe0.3are attributed to the following reason:the thermal decomposition of g-C3N4releases corrosive gas to etch the carbon matrix,which acts as the templates to create abundant mesopores,thus increasing the specific surface area[26].
FT-IR spectra were conducted to analyze the functional groups of catalysts(Fig.1d).The peak located at ~3433 cm-1corresponds to the-OH stretching vibration[24],while the peaks of C-H stretching vibration are located at ~2920 and ~1460 cm-1,respectively.The peaks at ~1626,~1548,~1420,~1084 and ~1052 cm-1are attributed to C=O,C-N,C-N,C-O and C-O-C vibrations,respectively[27].Moreover,a new peak appears for NPC-Fe0.3at ~880 cm-1after the introduction of iron precursors,which can be attributed to the Fe-N stretching vibration[28].However,in the absence of metal precursors or g-C3N4,there is no Fe-N bonds for NPC and PC-Fe.The above results indicate that nitrogen and oxygen doping is obviously observed in the NPC-Fe0.3,which is beneficial for improving the contact performance between the electrode materials and electrolytes.In addition,the existence of Fe-N bonds can effectively improve the ORR performance.The sample of NPC-Fe0.3has been selected to explore the structure of catalysts.The SEM image reveals that NPC-Fe0.3presents a gossamer lamellar structure with rich wrinkles(Fig.1e),which is profitable for the exposion of active sites and mass transportation[29].In addition,PC-Fe sample without g-C3N4has a large number of uniform nanospheres due to the hydrothermal reaction of glucose(Fig.S3a in Supporting information),while NPC shows a similar appearance to NPC-Fe0.3(Fig.S3b in Supporting information).The above results clearly indicate that the presence of g-C3N4in the synthesis process promotes the formation of lamellar structure with wrinkles.The lamellar frameworks of NPC-Fe0.3were further ascertained by the TEM image(Fig.1f).The HR-TEM image(Fig.1g)demonstrates the lattice fringes of 0.34 nm matching well with the(002)crystal plane of graphitic structure[30].The selected area electron diffraction(SAED)pattern exhibits a blurred ring resulted from the poor crystallinity of the N-doped carbon substrate(the insert in Fig.1g).The EDS element mappings of NPC-Fe0.3show that the C,N,O and Fe atoms are uniformly distributed throughout the structure(Fig.1h),revealing that Fe and N elements are successfully incorporated into carbon materials.The above results sufficiently prove that NPC-Fe0.3is rich of N-and O-functional groups and Fe-N bonds,which is conducive to improving the hydrophilicity.The water contact angle is 2.5° for NPC-Fe0.3by the water contact angle test(Fig.1i and Fig.S4 in Supporting information),which is significantly much smaller than that of PC-Fe(8.2°)and NPC(13.6°),indicating a super hydrophilicity[25].
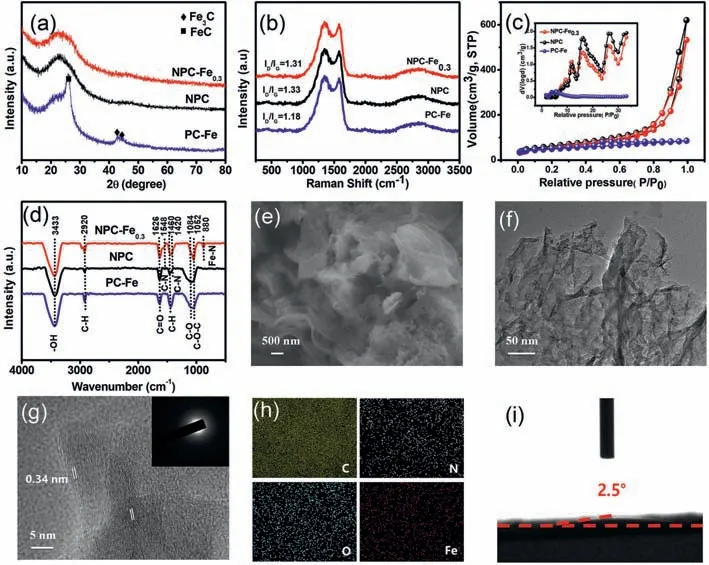
Fig.1.(a)XRD patterns.(b)Raman spectra.(c)nitrogen gas adsorption-desorption isotherms(the insert is pore-size distribution).(d)FT-IR spectra of NPC-Fe0.3,NPC and PC-Fe.(e)FE-SEM image.(f)TEM image.(g)HR-TEM image(insert is SAED pattern).(h)EDS mapping images of C,N,O and Fe elements.(i)contact angle of NPC-Fe0.3.
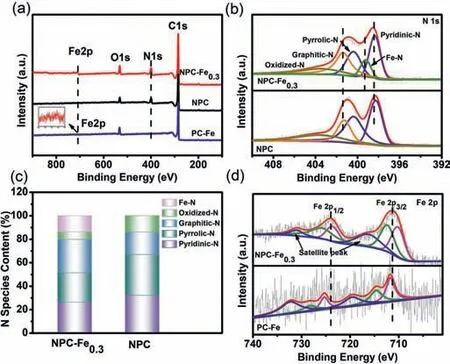
Fig.2.(a)XPS survey spectra of NPC-Fe0.3,NPC and PC-Fe.(b)N 1s spectra of NPCFe0.3 and NPC.(c)The contents of various N configurations for NPC-Fe0.3 and NPC.(d)Fe 2p spectra of NPC-Fe0.3 and PC-Fe.
XPS determination was carried out to analyze the compositions and chemical states.The survey spectra(Fig.2a)have indicated that Fe and N elements are successfully doped into the carbon skeleton for NPC-Fe0.3.For comparison,the sample of PC-Fe was synthesized without the addition of g-C3N4.However,due to the lack of N element coordination with iron ions in PC-Fe,the metal cannot be anchored effectively and part of Fe combines with C to form FeC and Fe3C nanoparticles.Some nanoparticles are easily removed in the pickling process,resulting in a low content.Fe signal of PC-Fe is much weaker than that of NPC-Fe0.3according to XPS spectra.The high-resolution C 1s spectrum of NPC-Fe0.3in Fig.S5a(Supporting information)can be fitted into C=C,C–C,C–N and O–C=O bonds at 284.6,285.3,286.5 and 289.1 eV,respectively[27].The high-resolution O 1s spectrum of NPC-Fe0.3(Fig.S5b in Supporting information)exhibits four different O species,which are assigned to C=O(531.0 eV),C–OH(532.3 eV),C–O–C(533.2 eV)and O–C=O(534.7 eV),respectively[31].The N 1s XPS spectrum of NPC-Fe0.3in Fig.2b can be separated into five peaks of pyridinic-N(398.3 eV),pyrrolic-N(400.6 eV),graphitic-N(401.5 eV),oxidized-N(405.0 eV)and the apparent peak at 399.2 eV corresponding to Fe-N bonds[12,28],which is an available evidence for the presence of Fe-N bonds in NPC-Fe0.3.Importantly,a positive shift of pyridinic-N and graphitic-N relative to NPC in NPC-Fe0.3is about 0.2 eV due to the modified Fe atoms and Fe-N bonds.It suggests that the electronic metal-support interaction between Fe atoms and g-C3N4.The g-C3N4provides a high concentration of unsaturated pyridine-type N to coordinate with iron to form Fe-N active sites.The high concentration of unsaturated pyridinic-type N in g-C3N4affords rich electron lone pairs for trapping metal ions in the ligands,leading to the relevant charge transfer and changes of the binding energy of Fe and N species[32,33].Moreover,the relative contents of Fe-N,pyrrolic-N,oxidized-N,pyridinic-N and graphitic-N species of NPC-Fe0.3and NPC are calculated from the XPS spectra(Fig.2c and Table S1 in Supporting information).Although the N content decreases after doping Fe atoms,the percentage of Fe-N increases up to 13.8%.In addition to forming Fe-N bonds,the doped Fe atoms also facilitate the conversion of pyrrolic-N and oxidized-N to graphitic-N,which is conductive to promote the ORR activity[34].Furthermore,the high proportions of pyridinic-N and pyrrolic-N have been confirmed to increase theπstate density,spin density and current density of C atoms around the Fermi level,consequently promoting the ORR performance[35].Recent advances have proved that the N configuration(pyridinic-N,pyrrolic-N or graphitic-N)is the key aspect to the electrocatalytic activity of nitrogen-doped carbon materials.The pyridinic-N and graphitic-N possess superior electron-accepting abilities to absorb oxygen,while the pyrrolic-N and pyridinic-N tend to form the metal coordination structure(Fe-N),which is reported to be the effective active sites of Fe,N co-doped porous carbon materials[28,36].
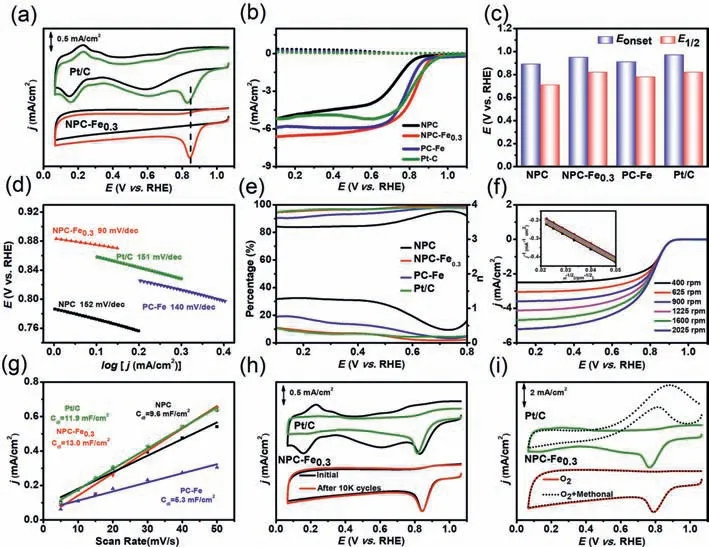
Fig.3.Electrocatalytic behavior in 0.1 mol/L KOH:(a)CV curves of NPC-Fe0.3 and Pt/C.(b)LSV curves.(c)comparison of E1/2 and Eonset.(d)Tafel plots.(e)H2O2 yields and electron numbers of NPC,NPC-Fe0.3,PC-Fe and Pt/C.(f)LSV curves of NPC-Fe0.3 with a sweep rate of 10 mV/s at different rotating speeds ranging from 400 rpm to 2025 rpm(the insert is K-L plots in different potentials).(g)plots extraction.(h)stability tests.(i)resistance of methanol crossover of NPC-Fe0.3 and Pt/C.
The spectra of Fe 2p(Fig.2d)can be deconvolved into two main peaks,which are assigned to Fe 2p3/2and Fe 2p1/2,respectively[11].It is found that the Fe 2p3/2binding energy region shows two peaks at 710.1 and 712.2 eV,which are assigned to the 2p3/2of the Fe(II)and Fe(III)ions,respectively[13].Meanwhile,the peaks at 723.7 and 725.8 eV are attributed to the binding energy of the 2p1/2orbitals of the Fe(II)and Fe(III)species,respectively[37].The peaks at 716.2 eV and 731.2 eV stand for the corresponding satellite peaks[38].Based on the analysis,the peak at 710.1 eV in the Fe 2p3/2XPS spectrum of NPC-Fe0.3indicates that Fe ions are coordinated with pyridinic-N to form Fe-N bonds,which can accelerate O2adsorption and 4e-reduction to enhance ORR activity[39].As expected,the high-resolution Fe 2p XPS spectrum also confirms the absence of metallic Fe in the NPC-Fe0.3and Fe mainly exists in the ionic states of Fe(III)and Fe(II).Additionally,it is obvious that the slight negative shifts of Fe 2p3/2and Fe 2p1/2are observed for NPC-Fe0.3compared with PC-Fe.According to the d electron catalysis theory,the d electron orbital property of transition metals is one of the core factors affecting the intrinsic activity of catalytic active sites[40].The transition metal Fe has a strong ability to form coordination compounds with ligands due to the existence of empty orbitals in the outer d orbital.Therefore,Fe is easy to coordinate with the unsaturated pyridine type N in g-C3N4,which is stable in the cavity formed by the three heptazine rings[41].The results are consistent with the N 1s spectra analysis,indicating that the interaction between Fe and N is conducive to the stable adsorption of O2and intermediates(*OH)[42].
The ORR performances were determined through a common three-electrode system in 0.1 mol/L KOH.As shown in Fig.3a and Fig.S6(Supporting information),there is a quasi-rectangular without O2reduction peak in N2condition,while a distinct O2reduction peak appears at about 0.845 V in the CV curves of NPC-Fe0.3in O2-saturated electrolyte,which is 18 mV more positive than that of the commercial Pt/C.The electrochemical impedance spectra(EIS)are analyzed based on the equivalent circuit diagram(Fig.S7 in Supporting information).The equivalent series resistance(Rs)and interfacial charge transfer(Rct)values of NPC-Fe0.3are 1.9Ωand 60.7Ω,respectively.The low inherent resistance of electrolyte facilitates ions diffusion.The Warburg resistance(Rp)for NPC-Fe0.3is only 0.36Ω,demonstrating the fast ion diffusion velocity[43,44].The LSV curves were recorded on RRDE to further illustrate the ORR activity.Fig.3b shows that NPC-Fe0.3displays the prominent ORR activity in terms ofEonset(0.95 V)andE1/2(0.82 V),which are superior to those of NPC(Eonset= 0.89 V,E1/2= 0.71 V)and PC-Fe(Eonset= 0.91 V,E1/2= 0.78 V),confirming that the introduction of iron and nitrogen is imperative to facilitate the ORR activity of carbon materials.It is worth noting that theE1/2values of NPC-Fe0.3is comparable to Pt/C(Eonset= 0.97 V,E1/2= 0.82 V)and exceeds most of the reported catalysts(Fig.3c and Table S2 in Supporting information).Moreover,the limiting current density(jL)of NPC-Fe0.3is 6.5 mA/cm2,which is 1.3-fold higher than that of Pt/C,evidencing a superior ORR activity.
Tafel slope is a crucial parameter to evaluate the ORR activity.The Tafel slope of NPC-Fe0.3is 90 mV/dec,which is the smallest among all the determined samples(Fig.3d),indicating a rapid ORR kinetic process.Meanwhile,the corresponding RRDE measurements were also employed to calculate the yields of hydrogen peroxide(H2O2%)and the electron transfer numbers(n)(Fig.3e).In the potential range from 0 to 0.80 V,the H2O2yield remains below 10% and the n values of NPC-Fe0.3are 3.80–4.00,similar to that of Pt/C.Therefore,the prepared NPC-Fe0.3catalyst exhibits superior ORR activity with a desirable four-electron reaction pathway.Furthermore,the RDE measurements were conducted at various rotating speeds changing from 400 rpm to 2025 rpm to investigate the catalyst kinetics of NPC-Fe0.3(Fig.3f).As the rotational speed increases,the diffusion-limiting current density of NPC-Fe0.3gradually increases with the reduction of O2diffusion distance.The KL plots of NPC-Fe0.3in the inset of Fig.3f display good linearity and parallelism betweenj-1andω-1/2,suggesting the first-order reaction kinetics toward the dissolved O2concentration.According to the K-L plots,the electron transfer number is calculated to be 3.8–4.0,further proving the 4e-dominant process.Besides,the electrochemical active surface area(ECSA)was calculated by the electrochemical double-layer capacitance(Cdl),which was obtained from the CV curves in the absence of O2(Fig.3g and Fig.S8 in Supporting information).TheCdlof NPC-Fe0.3is 13.0 mF/cm2,which is much higher than those of NPC(9.6 mF/cm2),PC-Fe(5.3 mF/cm2)and Pt/C(11.9 mF/cm2),indicating the excellent accessibility[45,46].
Excellent durability and methanol tolerance are also crucial for ORR electrocatalysts.The durability of NPC-Fe0.3has been investigated by cycling the electrode in 0.1 mol/L KOH at an accelerated scan rate of 50 mV/s in O2-saturated.As depicted in Fig.3h,after 10 k continuous cyclic voltammetry cycles,the peak potential is almost identical to the original one,much better than that of Pt/C.The unobvious decay demonstrates the brilliant durability of NPC-Fe0.3towards ORR,which is attributed to the large graphitization degree of carbon matrix.It can availably strengthen the carbon corrosion resistance,improving the operating stability of catalysts.In addition,the resistance of methanol crossover has been tested by injecting 1 mL 1.0 mol/L methanol into the electrolyte during the CV test.As shown in Fig.3i,the obvious methanol poisoning effect containing a distinct methanol redox peak and a significant current drop are observed for Pt/C,whereas the current response of NPC-Fe0.3barely decays upon the addition of CH3OH.The consequence demonstrates that NPC-Fe0.3exhibits more excellent tolerance to the methanol than Pt/C catalyst.By making a preliminary summary,NPC-Fe0.3possesses superior stability and selectivity towards ORR in alkaline electrolyte,which can be regarded as a promising electrocatalyst for practical application.
Furthermore,the influence of Fe contents on structures and electrocatalytic performances has been investigated by synthesizing a series of Fe,N co-doped porous carbon materials.XRD patterns in Fig.S9(Supporting information)exhibit that the excess Fe dopants lead to the serious agglomeration of metal sites to form numerous FeC and Fe3C nanoparticles.As revealed in Fig.S10(Supporting information),the calculatedID/IGvalues are 1.27,1.30,1.31,1.15 and 1.08 for NPC-Fe0.1,NPC-Fe0.2,NPC-Fe0.3,NPC-Fe0.4and NPC-Fe0.5,respectively.With the increased iron doping amounts,the defect degree appears a trend of first increase and then decrease.The defects can modulate the surface state of the electronic structure(charge and spin densities)and greatly affect the adsorption of intermediates related to ORR process,thereby enhancing the ORR activity[47].The nitrogen adsorption/desorption isotherms of all samples present a typical IV type curve and the pore size distribution trend is also similar,indicating that the Fe contents have no significant effect on the surface area and pore size distribution(Fig.S11 in Supporting information).The survey XPS spectra(Fig.S12 in Supporting information)indicate that the Fe and N elements are successfully doped into the carbon skeletons.As shown in Table S3(Supporting information),the highest Fe contents are found when the doping amount is 0.3 g.When the doping amounts continue to increase,the Fe contents show a downward trend.The excessive Fe dopants lead to the serious agglomeration of metal sites to form numerous FeC and Fe3C nanoparticles,which can be removed during the acid leaching process.The loss of nanoparticles results in a decrease in the Fe contents of NPC-Fe0.4and NPC-Fe0.5.Therefore,the higher Fe contents are conducive to forming abundant Fe-N active sites,further promoting the oxygen reduction activity.Furthermore,the ORR activity of electrocatalysts with different Fe contents was evaluated by the CV measurements.NPC-Fe0.3shows a smaller semicircle than other catalysts in the Nyquist plot(Fig.S13 in Supporting information),demonstrating a fast charge transfer efficiency.As depicted in Fig.S14a(Supporting information),the oxygen reduction potential of NPC-Fe0.3is the most positive among these catalysts,which is much higher than those of other catalysts.As can be observed from the LSV curves in Fig.S14b(Supporting information),NPCFe0.3affords the best catalytic activity withEonsetof 0.95 V andE1/2of 0.82 V,much higher than those of NPC-Fe0.1(Eonset= 0.91 V,E1/2= 0.79 V),NPC-Fe0.2(Eonset= 0.92 V,E1/2= 0.80 V),NPCFe0.4(Eonset= 0.90 V,E1/2= 0.79 V)and NPC-Fe0.5(Eonset= 0.90 V,E1/2= 0.78 V).In addition,NPC-Fe0.3manifests the greatest advantage in H2O2yield,electron transfer number,Tafel slope and electrochemical active area(Figs.S14c–e and S15 in Supporting information).Remarkably,the effect of Fe doping contents on ORR activity follows a“volcano plot”trend(Fig.S14f in Supporting information),which is consistent with the trend of the defect degree of the carbon materials(Table S3).It further proves that the defects influence the ORR activity of carbon-based catalysts.The activity is continuously accelerated as the Fe doping increases up to 0.3 g and then decreases with the larger Fe doping.This phenomenon manifests that the lower doping generates insufficient active sites,whereas the higher doping results in the formation of FeC and Fe3C nanoparticles as well as unfavorable carbon structure(i.e.,fewer defects).Therefore,it proves that the active sites of iron species serve as a significant role in the process of promoting ORR activity.
To further explore the practical application of NPC-Fe0.3,the primary ZAB with NPC-Fe0.3as the air cathode and Zn foil as the anode were assembled in 6.0 mol/L KOH and 0.2 mol/L Zn(OAc)2(Fig.4a).Accordingly,the ZAB assembled by the commercial 20 wt% Pt/C was also conducted in the similar condition.As a result,the open-circuit voltage(VOC)of NPC-Fe0.3is 1.48 V,whereas the battery based on Pt/C only exhibits the voltage of 1.45 V(Fig.4b).As demonstrated in Fig.4c,the power density of the NPC-Fe0.3-based ZAB reaches a maximum of 99 mW/cm2at 150 mA/cm2,which exceeds the Pt/C-based device(82 mW/cm2at 125 mA/cm2).Moreover,two series-connected NPC-Fe0.3-based ZABs can power 69 red LEDs(in the inset of Fig.4c),demonstrating the potential application of the prepared catalysts in practical energy equipment.Besides,the current density is further altered between 5 mA/cm2and 100 mA/cm2to assess the stability of cycle discharge performance.The discharge voltages for the NPCFe0.3-based ZAB are maintained higher than that of Pt/C-based battery with the increasing current density,especially at high current density(Fig.4d).Notably,the discharge voltage can also be maintained after lowering the current density to the origin low value,indicating the excellent discharge stability and rate capability of the NPC-Fe0.3derived ZAB[48].Furthermore,to evaluate the stability of the ZABs,the galvanostatic discharge-charge cycling curves are obtained with a constant current density of 5 mA/cm2in Fig.4e.The NPC-Fe0.3-based ZAB exhibits exceptionally high cycle stability with negligible voltage decay over 145 h.Therefore,the as-prepared NPC-Fe0.3sample can be employed within ZABs as a high-efficiency and durable catalyst.
In summary,a facile hydrothermal-pyrolysis approach is employed to fabricate Fe,N co-doped porous carbon materials as highly efficient ORR electrocatalyst through adopting g-C3N4as both the self-sacrificial templates and N sources.The unsaturated pyridinic-type N with a single component in g-C3N4is applied to combine with iron to form rich Fe-N active sites.Employing g-C3N4as templates can also effectively increase the specific surface area of carbon materials.Through systematically studying the Fe doping amounts,we emphasize that the abundant defects and adequate Fe-N active sites are critical for promoting the ORR property of catalysts.Owing to these factors,the as-prepared NPC-Fe0.3catalyst exhibits superior ORR performances in the alkaline solution with onset potential(Eonset= 0.95 V)and half-wave potential(E1/2= 0.82 V),as well as low Tafel slope,low H2O2yield and quick 4-electron transfer ORR pathway.At the same time,NPCFe0.3exhibits prominent durability and methanol tolerance.Furthermore,when adopted as the air electrode,the assembled ZAB delivers a power density of 99 mW/cm2and superior durability over 145 h.This work provides valuable ideas for the research and progress of high-performance non-noble metal-based ORR electrocatalysts in both energy conversion and storage applications.

Fig.4.(a)Schematic diagram of the Zn-air battery.(b)The open-circuit voltage.(c)The polarization curves and corresponding power density plots(inset is the photograph of 69 red LED powered by two ZABs in series with NPC-Fe0.3 as the air cathode).(d)Galvanostatic discharge curves with different current densities from 0 to 100 mA/cm2.(e)The voltage-capacity curves of ZABs at 5 mA/cm2 assembled with NPC-Fe0.3 and commercial Pt/C.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Nos.U1804255 and 51872076),the Program for Innovative Research Team of Henan Scientific Committee(No.CXTD2014033),the Project of Central Plains Science and Technology Innovation Leading Talents,Henan Province(No.194200510001)and the Scientific and Technological Research Project,Henan province(No.212102210651).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.014.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
