Synthesis and characterization of poly(p-dioxanone)-based degradable copolymers with enhanced thermal and hydrolytic stabilities
Yi-Teng Yan,Gang Wu,Si-Chong Chen,Yu-Zhong Wang
Collaborative Innovation Center for Eco-Friendly and Fire-Safety Polymeric Materials(MoE),State Key Laboratory of Polymer Materials Engineering,National Engineering Laboratory of Eco-Friendly Polymeric Materials(Sichuan),College of Chemistry,Sichuan University,Chengdu 610064, China
ABSTRACT Herein,we presented a novel biodegradable copolymer via the chain extending reaction of poly(pdioxanone)-co-poly(2-(2-hydroxyethoxy)benzoate)(PPDO-co-PDHB)prepolymer with hexamethylene diisocyanate(HDI)as a chain extender.The structures and molecular weight of PPDO-co-PDHB prepolymer and PPDO-co-PDHB-PU chain-extended copolymer are characterized via hydrogen nuclear magnetic resonance spectroscopy(1H NMR)and viscosity test.The relationship between the molecular structures and properties of the chain-extended copolymers is established.The PPDO-co-PDHB-PU copolymers possess a better thermal stability comparing with the PPDO homopolymer.The study of mechanical properties shows that the elongation-at-break of PPDO-co-PDHB-PU is much higher than that of PPDO.The investigation of hydrolytic degradation behaviors indicates the degradation rate of PPDO can be controlled by adjusting the PDHB compositions,and proves that chain-extended copolymers exhibit an excellent hydrolytic stability being better than that of PPDO.
Keywords:Poly(p-dioxanone)Poly(2-(2-hydroxyethoxy)benzoate)Tunable properties Chain extending Controlled degradation
As a unique biodegradable aliphatic polyester,poly(pdioxanone)(PPDO)has attracted much attention in recent years because of its outstanding biodegradability,biocompatibility and bioabsorbability[1–5].The existence of special ether-ester unit structures also endows it with increased hydrophilicity,excellent flexibility,and so on[6,7].Accordingly,PPDO has been used as ideal biomedical materials and also considered as a candidate for the general-purpose plastics[8–11].However,PPDO has drawbacks of easy to hydrolysis and thermal degradation,which limit its further practical applications.Therefore,various chemical and physical modification methods have been developed to improve the properties of PPDO[10].
As an effective approach,the copolymerization of two monomers or more is often employed to obtain polymer materials with tailored properties which are usually unattainable relative to those of homopolymers[2,12].Shaveret al.presented the aliphatic-aromatic polyester(P2HEB)from ring-opening polymerization(ROP)of a cyclic ester 2,3-dihydro-5H-1,4-benzodioxepin-5-one(2,3-DHB)[13,14].The enzymatic degradation behaviors evidenced by weight loss indicated that degradation rate of P2HEB was much slower than for atactic polylactic acid(PLA),but significantly faster than for poly(ethylene terephthalate)(PET).The current degradation results should be ascribed to the peculiar microstructure that contained both aromatic and aliphatic linkages in the polymer backbone,reducing enzyme access for steric reasons.Therefore,the new 2,3-DHB monomer could be used as component to regulate the degradation process of PPDO.However,it is usually difficult to obtain the desired copolymer with high molecular weight[15].Recently,chain extending reaction has been extensively used in synthesizing high molecular weight biodegradable polymers and improving its performance[16].Though a series of PPDO-based chain-extended polymers with high molecular weight have been reported[17–20],the improvement of properties,especially thermostability and hydrolytic degradability,is still very limited.
In this work,for the first time,we rationally designed and successfully synthesized a PPDO-based chain-extended copolymer,i.e.the poly(p-dioxanone)-co-poly(2-(2-hydroxyethoxy)benzoate)(PPDO-co-PDHB-PU)through a two-steps strategy including the synthesis of PPDO-co-PDHB diol prepolymer and the subsequent chain extending by using hexamethylene diisocyanate(HDI)as a chain extender.This PPDO-based copolymer possessed not only a good mechanical property but also significantly improved thermal and hydrolytic stabilities,indicating a potential application prospect in a wide field.
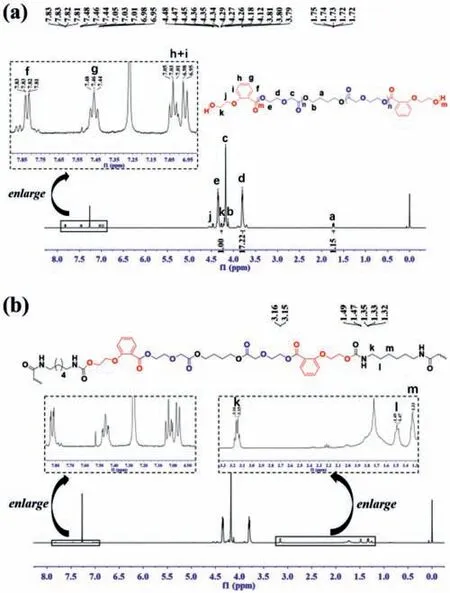
Fig.1.1H NMR of(a)PPDO-co-PDHB5.4% prepolymer and(b)PPDO-co-PDHB5.4%-PU chain-extended copolymer.
As shown in Scheme 1,the PPDO-co-PDHB-PU were synthesized through two steps:firstly,ROP ofp-dioxanone(PDO)with 2,3-DHB using 1,4-butanediol(BDO)as initiator and stannous octoate[Sn(Oct)2]as catalyst at 120 °C to synthesize PPDO-co-PDHB prepolymer;secondly,chain extending reaction of PPDO-co-PDHB with HDI at 140 °C to synthesize PPDO-co-PDHB-PU.To investigate the effect of the chemical compositions on the properties of the chain-extended copolymers,the PPDO-co-PDHB-PU with different PDHB compositions were obtained by varying the feed molar ratio of 2,3-DHB to PDO from 1/20 to 1/5(Table S1 in Supporting information).
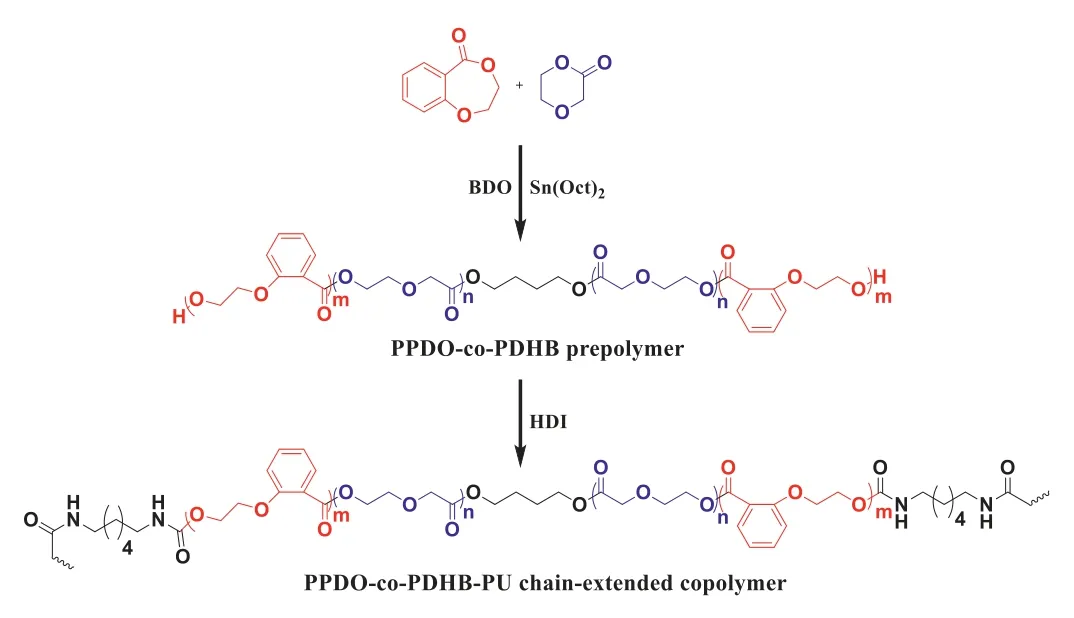
Scheme 1.The synthetic route of PPDO-co-PDHB prepolymer and PPDO-co-PDHBPU chain-extended copolymer.
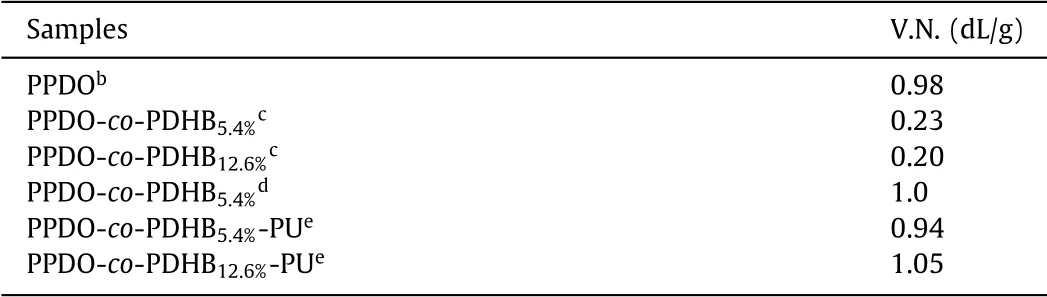
Table 1 Results of molecular weights of the synthesized PPDO,PPDO-co-PDHB and PPDOco-PDHB-PU.a
The molecular structures of the synthesized PPDO-co-PDHB and PPDO-co-PDHB-PU were characterized by1H NMR and13C NMR.As shown in Fig.1a,it can be seen that resonances located at 4.36 ppm(δHe),4.19 ppm(δHc),and 3.81 ppm(δHd)belong to the three methylene groups of PPDO,respectively.The signals at 4.12 ppm(δHb)and 1.73 ppm(δHa)correspond to the methyleneprotons of BDO initiator.The signals at 7.82 ppm(δHf),7.46(δHg)and 7.05 ppm(δHh+i)are assigned to the aromatic rings protons of PDHB,and the signals at 4.47 ppm(δHj)and 4.27 ppm(δHk)belong to the methylene protons of PDHB.According to the following Eq.1,the amount of PDHB incorporations in the prepolymer can be calculated from the integral areas ratio of characteristic signals of respective PPDO and PDHB.

whereI4.27andI3.81are the peak intensities of methylene protons in PDHB and PPDO,respectively.It can be found that PDHB incorporations in PPDO-co-PDHB prepolymers are almost consistent with the initial monomer feed ratio(Table S1).
Furthermore,from the data of1H NMR,the degree of polymerization(Dp)for PPDO-co-PDHB prepolymer can be calculated according to the following equation:

whereI1.73is the peak intensity of the methylene of BDO in copolymer chain.It can be seen that the experimentalDpof prepolymer(36)are close to the theoretical value(40),suggesting a controllable polymerization process.
As shown in Fig.S2(Supporting information),further analysis of the monomer sequencing in the PPDO-co-PDHB by quantitative13C NMR spectroscopy indicates the lack of a high quality of 2,3-DHB*-PDO and PDO*-2,3-DHB resonances(where*denotes the observed carbonyl),suggesting copolymer produced is likely to be blocky copolymer.Similarly,no distinctly separated cross-peaks are observed in the methylene carbon and hydrogen adjacent to the oxygen atom in ring-opened structures of both PDO and 2,3-DHB.The 2,3-DHB*-PDO and PDO*-2,3-DHB carbonyl diad resonances with low integration are presented,which is the evidence of a short gradation between blocks.Current results are consistent with the previous reports[21-23],that is a block-like copolymer is formed.
For the PPDO-co-PDHB-PU copolymer,all characteristic signals for both PPDO and PDHB repeat units are visible in Fig.1b.The further evidence for the implementation of chain extending reaction is the signals occurring at 1.33 ppm(δHm),1.49 ppm(δHl)and 3.16 ppm(δHk),which are assigned to the three kinds of methylene protons derived from HDI chain extender.The viscosity numbers(V.N.)of PPDO-co-PDHB prepolymers and PPDO-co-PDHB-PU chain-extended copolymers were measured by Ubbelohde viscosimeter.It can be seen from Table 1 that the V.N.values of PPDO-co-PDHB-PU obviously increase in comparison with the prepolymers,demonstrating again the success of the chain extending reaction to effectively elevate the molecular weight.The molecular weight is one of critical factors that influence the properties of polyester materials.For a better comparison,PPDO homopolymer and PPDO-co-PDHB copolymer with similar viscosity(viscosity number of about 1.0)were also synthesized.
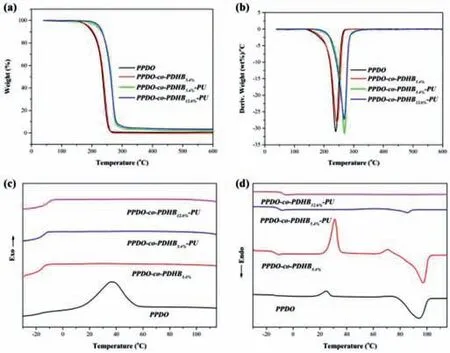
Fig.2.(a)TG and(b)DTG curves of PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU.DSC curves of PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU:(c)the first cooling scans and(d)the second heating scans.
The thermostabilities of PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU with different 2,3-DHB incorporations were studied by thermogravimetric analysis(TGA).The typical TGA and DTG curves are shown in Figs.2a and b,and the relevant data are listed in Table S2(Supporting information).As shown in Fig.2a,all chainextended copolymers exhibit essentially a one-step degradation profile with much higher decomposition temperature at 5% weight loss(T5%)(about 220 °C)than those of PPDO(185.0 °C)and PPDOco-PDHB5.4%(188.1 °C).The thermal decomposition of PPDO mainly proceeds through the unzipping depolymerization initiated from the reactive hydroxyl group at the chain end[13,24,25],the chainend capping with isocyanate restrains the reaction and therefore improves the thermostability of PPDO-based chain-extended copolymers.In addition,a slight increase of theT5%from 216.7 to 223.2 °C is observed with the increase of PDHB.Overall,such results demonstrate that PPDO-co-PDHB-PU has much better thermal stability than PPDO and PPDO-co-PDHB.
As shown in Figs.2c and d,the thermal transition behaviors of PPDO-co-PDHB-PU chain-extended copolymers,PPDO homopolymer and PPDO-co-PDHB copolymer were researched by differential scanning calorimetry(DSC),and the relevant data were summarized in Table S3(Supporting information).Owing to the very different crystalline behaviors of PDHB in the nascent form and after melting[13,14],the crystallization of chain-extended copolymers are predominantly contributed by PPDO segments.No crystallization peak is observed during the first cooling scans for PPDO-co-PDHB-PU chain-extended copolymers and PPDO-co-PDHB5.4%copolymer(Fig.2c),while PPDO-co-PDHB5.4%and PPDOco-PDHB5.4%-PU shows the weak melting endotherms signals at the second heating scans(Fig.2d),suggesting that the chain-extended copolymer and PPDO-co-PDHB5.4%copolymer are semicrystalline polymers with a slow crystallization rate.The further evidence for the weakened crystalline property is that the degree of crystallinity calculated byΔHmdecreases with the introduction of PDHB,suggesting PDHB polymeric segment in the PPDO-co-PDHB destroys the regularity of polymer chains,decreasing the crystallization rate of PPDO.It also can be found that the degree of crystallinity of PPDO segment in the chain-extended copolymers further decreased through the chain-end capping.These results clearly show that the introduction of both PDHB segment and chain extender structure units in the PPDO-co-PDHB-PU not only slow down the crystallization process but also depress the crystallinity of PPDO.
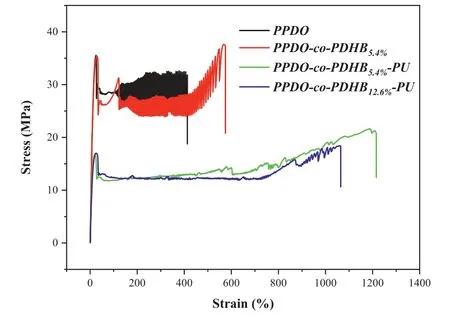
Fig.3.Stress-strain curves of PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU.
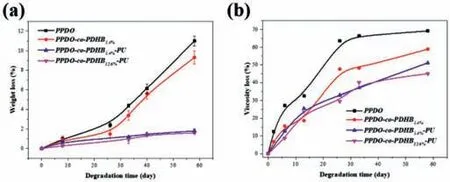
Fig.4.Plots of(a)weight loss and(b)viscosity loss for PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU in deionized water at 25 °C as a function of time.
The mechanical properties of polymers are one of the important indexes for the practical applications.Fig.3 shows the representative stress-strain curves of PPDO homopolymer,PPDO-co-PDHB copolymer and PPDO-co-PDHB-PU chain-extended copolymers,and the results are listed in Table S4(Supporting information).It can be seen that elongation-at-break and tensile strength of PPDO-co-PDHB are higher than PPDO,which should be ascribed to the comprehensive influence of the weakened crystalline property as well as the aliphatic and aromatic structures[16].It also can be found that the elongation-at-break of PPDO-co-PDHB-PU chain-extended copolymers is 2 to 2.5 times higher than that of PPDO,but their tensile strength which still meet the application requirements is lower than that of PPDO.Meanwhile,the elongation-at-break of PPDO-co-PDHB-PU decreases slightly with the increase of PDHB,which should be ascribed to the introduction of the more rigid aromatic structures[16,19].It could be concluded that the chain extending reaction improves the flexibility,and destroys the crystallization of polymer chains,thereby increasing the elongation-atbreak and decreasing the tensile strength of chain-extended products[16].
The hydrolytic degradability of PPDO,PPDO-co-PDHB and PPDO-co-PDHB-PU was comparatively studied.The weight loss is a simple and intuitive evaluation for the hydrolysis process of polymer during the exposure to the hydrolytic medium.Fig.4a shows the weight loss of the samples as a function of time in deionized water at 25 °C.It can be seen that the PPDO-co-PDHB5.4%copolymer exhibits a slower weight loss than that of PPDO throughout the degradation period,demonstrating that the incorporation of hydrophobic PDHB chains could reduce the hydrophilicity of PPDO chains and enhance the hydrolytic stability of PPDO.Meanwhile,it can be found that the PPDO-co-PDHB-PU copolymers exhibit a much slower weight loss comparing with the PPDO and PPDO-co-PDHB throughout the degradation period.Specifically,the weight loss values of PPDO and PPDO-co-PDHB severally reach to about 11% and 9% at 58 days,while the weight loss of PPDO-co-PDHB-PU is no more than 2% at 58 days,demonstrating that the PPDO-based chain-extended copolymers possess the better hydrolytic stability in comparison with PPDO and PPDO-co-PDHB.In addition,it also can be found that the weight loss of PPDO-co-PDHB-PU further decreases with the increase of PDHB.The above results could be attributed to the introduction of hydrophobic PDHB chains to reduce the hydrophilicity of PPDO chains as well as the role of chain extending reaction on decreasing the content of terminal hydroxyl groups.
To better understand the degradation process,the Ubbelohde viscosimeter was used to trace the variation of the viscosity numbers(V.N.)of samples with degradation time,and their viscosity loss values were plotted in Fig.4b.The viscosity losses of PPDOco-PDHB and PPDO after 26 days severally reach to over 45% and 65%.By contrast,PPDO-co-PDHB-PU shows a slower viscosity loss in keeping with the result of weight loss,and its value for PPDOco-PDHB-PU5.4%at 26 days is only about 35%,which demonstrates again that the hydrolytic degradation of PPDO could be retarded by our approach.
Fig.S5(Supporting information)is the1H-NMR spectra of samples after hydrolytic degradation of different times.It can be found that the characteristic peaks of PDO monomers occur with the degradation for PPDO and PPDO-co-PDHB.The resulted monomers should be ascribed to the back-biting reaction of the low molecular weight degradable products with terminal hydroxyl groups[26].However,no obvious monomers peaks are observed in the PPDOco-PDHB-PU copolymers,confirming that the back-biting reaction of terminal hydroxyl groups to generate the PDO is suppressed severely.
The current degradation results should be ascribed to the comprehensive influences of the hydrophobicity and the steric hindrance from PDHB chains as well as the modification method of chain extending on the hydrophilicity of PPDO and the diffusion rate of water molecules in PPDO,so as to suppress the hydrolytic degradation of PPDO and improve the hydrolytic stability.Overall,these results suggest that the hydrolysis rate of the PPDO-based chain-extended copolymers could be controlled by regulating their composition.
In summary,the PPDO-co-PDHB-PU copolymers with adjustable compositions were synthesized through the two-steps strategy of copolymerization and chain extending reaction.The molecular structures of the synthesized prepolymers and chain-extended copolymers were characterized by means of1H NMR.The molecular weights of resulting chain-extended copolymers increased significantly comparing to the prepolymers.In comparison with the PPDO homopolymer,the chain-extended copolymers possessed the better thermostability.The results of mechanical properties showed that the introduction of PDHB chains improved observably the elongation-at-break of PPDO,but decreased slightly the tensile strength.More importantly,the results of the hydrolytic degradation suggested that introducing the hydrophobic,steric hindraned PDHB chains into PPDO and combining with chain extending method suppressed significantly the hydrolytic degradation of PPDO,and thus improved greatly its hydrolytic stability.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.U19A2095),the Sichuan Science and Technology Program(No.2017SZDZX0015),and the Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.124.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
