Ag-single atoms modified S1.66-N1.91/TiO2-x for photocatalytic activation of peroxymonosulfate for bisphenol A degradation
Tin Wng,Jinjun Zhou,Wenjun Wng,Yunqing Zhu,*,Junfeng Niu,b
a School of Environmental Science and Engineering,Shaanxi University of Science and Technology,Xi’an 710021,China
b School of Environment and Civil Engineering,Dongguan University of Technology,Dongguan 523808,China
ABSTRACT In this study,Ag0.23/(S1.66-N1.91/TiO2-x)single-atom photocatalyst was synthesized by in-situ photoreducing of silver on S,N-TiO2-x nanocomposite and used to degrade bisphenol A(BPA)through heterogeneous activation of potassium peroxymonosulfate(PMS)under visible-light illumination.The structure,physicochemical property,morphology,and electronic property were evalutated by X-ray diffraction(XRD),Raman spectrum,X-ray photoelectron spectra(XPS),high-resolution transmission electron microscopy(HR-TEM),UV–vis diffuse reflectance spectra(UV-vis DRS),electron paramagnetic resonance(EPR)spectrum.Ag0.23/(S1.66-N1.91/TiO2-x)single-atom photocatalyst exhibited 2.4 times higher activity for the synergetic degradation of BPA than that of its counterpart,and 48.73% mineralization rate of BPA also achieved.It was ascribed to the uniformly-dispersed metallic Ag atoms as the active site for accelerating the migration rate of photo-generated carrier for generation of high reactive radicals.The EPR experiments indicated that SO4·– and ·OH was jointly involved in BPA degradation.
Keywords:Photo-Fenton Single-atomic photocatalyst Sulfate radical Bisphenol A PMS
Bisphenol A(BPA)is well-known as an endocrine-disrupting chemical(EDC)that can mimic estrogen and interact with its receptors.It has great potential to cause hazardous health and ecologic effects even at doses lower than the safety level(50 mg kg-1day-1by FDA[1-3].In the past two decades,BPA has been widely used in manufacturing plastics,paper,medical equipment,electronics,and clothes,and every year more than 6.0 × 109pounds of BPA are produced[4,5].Therefore,it is necessary to find an efficient and environmentally friendly treatment method to eliminate BPA.
Fenton-like process is promised as an efficient technology for treatment of refractory organic pollutants because of its strong oxidation capability and environmental benignness[6,7].Different from the traditional·OH based Fenton technology,sulfate radicals(SO4·–)for its high redox potential(E0= 2.5-3.1 eV)and selectively oxidation capability,has attracted plenty of attention in recent years[8,9].In addition,for sulfate radical based Fenton-like technology,the 2-9 pH adoptive range and 30-40 μs half-life also made it more efficient in environmental remediation[10].Furthermore,to increase its catalytic efficiency,several methods,like UV irradiation,transition metal ions(like Co2+,Cu2+,or Fe2+)and metal oxides,have been proposed as approaches for accelerating the reaction of SO4·–generation[11-13].
Recently,photocatalytic Fenton process by coupling of potassium peroxymonosulfate(PMS)with photocatalysts for enhancing the SO4·–radicals generation has been studied as a new and efficient approach.TiO2,Co3O4,BiVO4,C3N4,and manganese oxide molecular sieve were all investigated as photo-Fenton catalysts for contaminants degradation under visible light irradiation[14-16].However,the development of efficient and stable photocatalysts for the generation of SO4·–is still highly desired.Single-atom catalysts(SACs)by engineering the nanoparticle size to extremely small as single atom to achieve a maximum amount of unsaturated coordinated atoms have been widely studied in recent years[17,18].The isolated atoms in SACs are unsaturatedly coordinated and exhibited unique physicochemical properties[19,20].In the photocatalytic reaction,the atomically dispersed atoms are exposed in the reaction situation and participated in the reaction as high reactive sites[21].So the atomic efficiency would be 100%,a maximum value in theory.
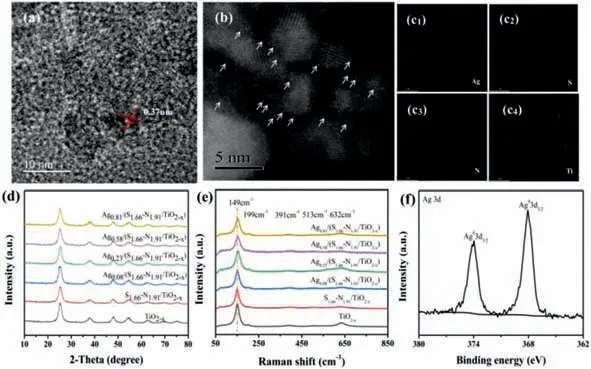
Fig.1.(a)HRTEM diagram of S1.66-N1.91/TiO2-x.(b)HAADF-STEM diagram of Ag0.23/(S1.66-N1.91/TiO2-x).(c)Corresponding EDS mapping of Ag,S,N,Ti elements,respectively of Ag0.23/(S1.66-N1.91/TiO2-x).(d)XRD pattern of Agz/(S1.66-N1.91/TiO2-x).(e)Raman spectra of Agz/(S1.66-N1.91/TiO2-x).(f)XPS spectra of Ag0.23/(S1.66-N1.91/TiO2-x):Ag 3d.
Herein,defective S,N-TiO2-xwith a large number of uniformly distributed surface oxygen vacancies was adopted as substrate for preparation of single-atom Ag photocatalysts.As shown in Fig.1a,the S1.66-N1.91/TiO2-xexhibits a lattice spacing of 0.37 nm corresponding to(101)facet of TiO2[22].Compared with TiO2-x,the lattice spacing is slightly increased(Fig.S1 in Supporting information)[23],which may be ascribed to the doping of sulfur and nitrogen.Fig.1b is the HAADF-STEM diagram of Ag0.23/(S1.66-N1.91/TiO2-x).It can be seen that Ag atoms exist as single atoms on the surface of the S1.66-N1.91/TiO2-x,and the dispersion is uniform.From the EDS mapping images of Ag0.23/(S1.66-N1.91/TiO2-x)(Fig.1c),it can be seen that the four elements are uniformly distributed in the material.The Ag element was isolatedly dispersed on the surface,which further proves that the silver atoms are loaded successfully and are evenly distributed as silver atoms on S1.66-N1.91/TiO2-xsurface.The elements that S and N,Ag can be clearly observed in the EDS diagram of Ag0.23/(S1.66-N1.91/TiO2-x),which proves the successful preparation of this material(Fig.S2 in Supporting information).As shown in Fig.1d,the characteristic diffraction peaks of the prepared material Agz/(S1.66-N1.91/TiO2-x)are located at 25.3°,37.8°,48.1°,53.9°,55.1°,62.7° and 75.0° respectively,corresponding to the(101),(004),(200),(105),(211),(204)and(215)crystal planes of the anatase phase TiO2-xproved that the anatase phase photocatalytic material was successfully prepared[24,25].Fig.1e shows the Raman spectra of TiO2-xand S1.66-N1.91/TiO2-xwith different Ag loadings.The characteristic Raman signal peak position of TiO2-xwas marked in Fig.S3(Supporting information).It can be seen from the figure that the characteristic Raman signals are located at 149 cm-1,196 cm-1,391cm-1,513 cm-1,632 cm-1[26].There is the strongest Raman signal at 149 cm-1,indicating that the prepared material exhibits the Raman characteristic band of anatase phase TiO2-x.This result is consistent with X-ray diffraction(XRD)analysis.The Raman vibration mode of anatase TiO2-xis 3Eg+2B1g+A1g,and the oscillation peaks at 149 cm-1,196 cm-1and 632 cm-1correspond to the Egvibration mode,the oscillation peak at 391 cm-1and 513 cm-1corresponds to the B1goscillation mode[27].Compared with the S1.66-N1.91/TiO2-xRaman signal,the Raman band of Agz/(S1.66-N1.91/TiO2-x)shows no red shift or blue shift with the increase of Ag loading suggesting that the loading of Ag did not change the structure of S1.66-N1.91/TiO2-x.Fig.1f shows the high-resolution XPS peaks of Ag in Agz/(S1.66-N1.91/TiO2-x),as shown in this figure,the binding energies of Ag 3d5/2and Ag 3d3/2orbitals are 368.1 eV and 374.1 eV respectively[28].The difference in binding energy between the two orbitals is 6 eV,indicating that Ag exists as zero valence state.
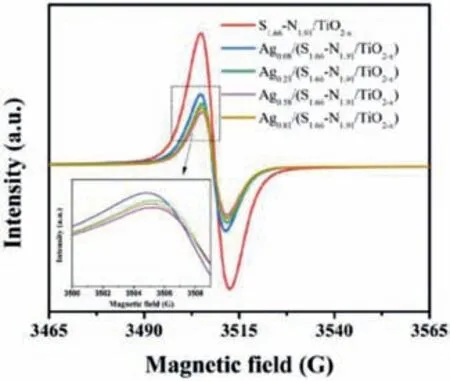
Fig.2.EPR spectra of Agz/(S1.66-N1.91/TiO2-x).
The g factor of the electron paramagnetic resonance(EPR)signal for all the samples(Fig.2)is 2.003,which is classified into EPR signal of oxygen vacancies[29].The S1.66-N1.91/TiO2-xhas the highest intensity of EPR signal,which indicated that the concentration of oxygen vacancies is highest in all the samples.After loading Ag species,the EPR signal intensity of oxygen vacancies gradually decreased with the increase of the loading amount of Ag.But when the loading amount of Ag is higher than 0.81%,it no longer follows the above-mentioned rules.This is due to the formation of Ag nanoclusters or nanoparticles on the surface of S1.66-N1.91/TiO2-x,only a small amount of Ag occupies oxygen vacancies.
The transient photocurrent(15 μA/cm2)of S1.66-N1.91/TiO2-xis greater than that of pure TiO2-x(12 μA/cm2),this is due to the fact that S6+can be reduced to more stable S4+[30].Doped S6+can increase the separation rate of photo-generated electrons and holes.N replaces O2-by bonding or enters into the crystal lattice to cause distortion of the TiO2-xlattice.When S and N co-doping interaction in the TiO2-xband gap,a new energy level is formed,which improves the separation efficiency of the catalyst for photo-generated carriers[31].After Ag loading,Ag0.08/(S1.66-N1.91/TiO2-x),Ag0.23/(S1.66-N1.91/TiO2-x),Ag0.58/(S1.66-N1.91/TiO2-x)and Ag0.81/(S1.66-N1.91/TiO2-x)show the transient photocurrents of 28,42,23,14 μA/cm2,respectively(Fig.3a).It was found that the transient photocurrent of Ag0.23/(S1.66-N1.91/TiO2-x)was the largest,which was about 3.5 times that of TiO2-xand 3 times that of S1.66-N1.91/TiO2-x,indicating that single atom Ag can improve the separation rate of photo-generated electrons and holes[32],and in the photo-Fenton process,it can also increase the transport and capture photo-generated electrons.The DRS spectrum can further prove that the material Ag0.23/(S1.66-N1.91/TiO2-x)has the highest light absorption(Fig.S4 in Supporting information).However,the increasing of Ag loading amount resulted a decreasing of transient photocurrent,which is due to the excessive Ag prior to form Ag particles with lower dispersity and low-density of carrier separation centers.It can be seen from Fig.3b that the radius of the electrochemical impedance spectroscopy curve of the S1.66-N1.91/TiO2-xmodified by co-doping of TiO2-xwith S and N is small,indicating that the electrochemical impedance is small and the interface charge transfer is faster,and the separation effect of photo-generated electrons and holes is better[33].Compared with pure S1.66-N1.91/TiO2-xelectrochemical impedance,the Ag-loaded S1.66-N1.91/TiO2-x(Agz/(S1.66-N1.91/TiO2-x))light Fenton catalytic materials have low electrochemical impedance,relatively fast interface charge transfer,and long carrier lifetime[34].The electrochemical impedance of the single-atom catalytic material Ag0.23/(S1.66-N1.91/TiO2-x)is the smallest,which is nearly 1/4 of the electrochemical impedance of S1.66-N1.91/TiO2-x,is maximized the atomic utilization rate,it can make the separation effect of photo-generated electrons and holes best,which is conducive to the improvement of catalytic activity.
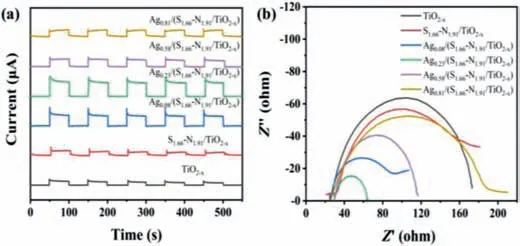
Fig.3.(a)Transient photocurrent response.(b)EIS Nyquist plots of Agz/(S1.66-N1.91/TiO2-x).
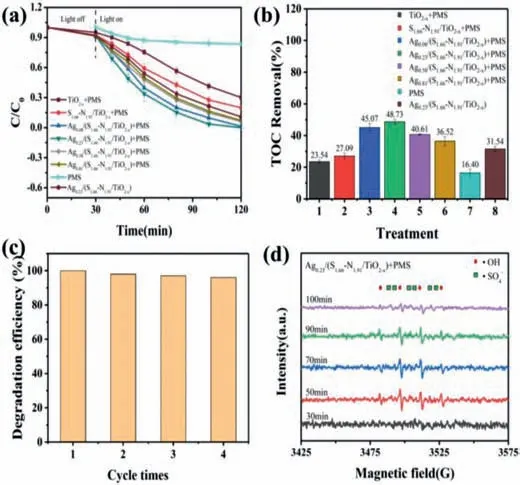
Fig.4.Photo-Fenton degradation of BPA by Agz/(S1.66-N1.91/TiO2-x)activated persulfate.(a)Typical time course of BPA concentration.(b)Histogram of TOC removal.(c)Catalyst recycling for the photodegradation of BPA.(d)EPR spectra of free radical.
The photo-Fenton degradation of BPA at initial concentration of 15 mg/L with molar ratios of Oxone to BPA at 2.2 is shown in Fig.4a.In case of PMS under visible light irradiation,no obvious degradation of BPA was observed.By activation of PMS in dark with Ag0.23/(S1.66-N1.91/TiO2-x),53.88% of BPA and 19.73% of total organic carbon(TOC)were removed after 120 minutes of contact time(Fig.4b).Once visible light irradiation was applied,100% of BPA and 48.73% of TOC were removed at the end of treatment.Based on the data,the degradation of BPA was fitted with the pseudo-first order kinetic model(Fig.S5 in Supporting information),and the kinetic constant was 0.032 min-1which is two to three times higher than its counterpart.While comparing the catalysts with different Ag loading amount,Ag0.23/(S1.66-N1.91/TiO2-x)displayed the highest catalytic efficiency of BPA and TOC removal in the 120 min reaction.Tables S1 and S2(Supporting information)listed the BPA and TOC removal of all samples in the photo-Fentonlike process.It suggested that the high catalytic efficiency was ascribed to the isolated silver atoms in the single atom catalysts.The cycling test(Fig.4c)implied that Ag0.23/(S1.66-N1.91/ TiO2-x)SAC shown high stability in the photo-Fenton degradation of BPA,and after four times cycling experiments,the catalytic efficiency only shown slightly decrease.
The EPR spectrum was used to detect the active radicals during the photo-Fenton degradation of BPA with Ag0.23/(S1.66-N1.91/TiO2-x)using DMPO as spin trapping agent.As shown in Fig.4d,the signals of DMPO-OH and DMPO-SO4adducts were both observed in the reaction.It means that·OH and SO4·–coparticipate in the degradation process of BPA[35,36].With the time increasing,the signal intensity was firstly enhanced and then reduced.It agreed with the reaction trend that during the initial stage of the reaction,the generated active radicals were fast reacted with BPA molecule resulting in a high reaction rate,and EPR spectum showed no singal.After 30 min,the reaction rate decreased and the DMPO trapped radicals were increased.The SO4·–reacted with H2O to form plenty of·OH.So the signal intensity was enhanced correspondingly.Then at the end of reaction,PMS was used up.The signal of the radicals were both reduced,which further confirmed that the·OH was generated from SO4·–[37].
In this work,the single atomic photocatalysts were successfully prepared and tested in the photo-Fenton-like system for BPA degradation under visible-light irradiation.In this system,the SACs labeled as Ag0.23/(S1.66-N1.91/TiO2-x)possessed high-efficiency for photo-generated carrier separation and transportation,which resulted in a high yield of SO4·–radicals and also the conversion to·OH for effective mineralization of BPA.Meanwhile,photocatalytic process promoted the reduction of the isolated Ag atoms from Ag+to Ag0to ensure the efficient activation of PMS.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
Thia work is financial supported by the National Nature Science Foundation of China(No.21876105),Key Research &Development Program Projects of Shaanxi Province(No.2019SF-252),the Startup Foundation for Advanced Talents of Shaanxi University of Science and Technology.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.085.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
