A novel on-tissue cycloaddition reagent for mass spectrometry imaging of lipid C=C position isomers in biological tissues
Chenglong Sun,Chunxia Ma,Lili Li,Yuhao Han,Daijie Wang,Xiao Wan
a School of Pharmaceutical Sciences,Qilu University of Technology(Shandong Academy of Sciences),Ji’nan 250014,China
b Key Laboratory for Applied Technology of Sophisticated Analytical Instruments of Shandong Province,Shandong Analysis and Test Center,Qilu University of Technology(Shandong Academy of Sciences),Ji’nan 250014,China
ABSTRACT Application of matrix-assisted laser desorption/ionization mass spectrometry imaging(MALDI-MSI)to investigate the spatiotemporal alterations of lipids in biological tissues has brought many significant results.However,the presence of structural isomers varying in C=C double bond(DB)locations makes isomerresolved MSI an urgent need.Herein,we introduce a new type of light-driven on-tissue[2+2]cycloaddition reaction coupled with MALDI-MS/MS imaging to identify lipid DB position isomers and their spatial signatures in biological tissues.3-Benzoylpyridine was introduced as a novel derivatization reagent,and it exhibited great reactivity toward lipid C=C bond to form oxetanes under both ultraviolet light and visible light irradiation.With this approach,DB position isomers of lipids were imaged with highly differential levels in distinct regions of rat brain,providing an accurate and spatially resolved approach to study tissue lipidomics.
Keywords:MALDI-MSI On-tissue cycloaddition Lipid C=C position isomers Biological tissues
Lipids play indispensable roles in constructing cell membranes,transducing biological signals,and regulating cell energy metabolism[1].Accumulating evidence indicates that the alterations of lipids are closely related to the development of cancer,Alzheimer’s disease,and heart disease[2–4].Profiling tissue lipids with spatial signatures not only gives insights into the biochemical heterogeneity but also provides a new approach to uncover the complex lipid metabolic reprogramming of organism in response to a variety of diseases.
Matrix-assisted laser desorption/ionization mass spectrometry imaging(MALDI-MSI)is a powerful method to locate different kinds of lipids directly on the tissue surface[5–7].Lipidomic investigations employing MALDI-MSI techniques have led to the spatially resolved characterization of endogenous lipids from biological tissues[8–10].However,MALDI-MSI data give little structural information on the detected lipids,thus allowing isomeric species,such as C=C double-bond(DB)position isomers,to remain indistinguishable by MALDI-MSI alone.By necessity,electrospray ionization mass spectrometry(ESI-MS)coupled with selective derivatization method,such as ultraviolet(UV)light-induced Paternò-Büchi(PB)reaction[11–19]and ozone-induced dissociation[20–23]have been developed to resolve signals arising from DB-positional isomers of unsaturated lipids.Collision-induced dissociation(CID)MS/MS analysis of the derivatization products can generate isomer-specific diagnostic ions,leading to the assignment of DB positions.
However,characterizing the spatial distribution of lipid in biological tissue is only accessible when using MSI.Investigators have developed different on-tissue derivatization methods to assign DB positions in situ[24–28].Among these methods,UV light-driven PB reaction is often chosen because of its simple operation process and high derivatization efficiency.For example,Bednaˇríket al.proposed an on-tissue PB functionalization approach to visualize DBpositional isomers in mouse cerebellum with a custom-made reaction chamber and MALDI2-MS2I[24].Very recently,benzophenone and 2-benzoylpyridine were developed as PB reactive MALDI matrices by the Heiles group.Benzophenone and 2-benzoylpyridine can not only react with unsaturated lipids in the PB reaction,enabling assignment of DB positions,but also serve as the matrix in MALDI-MSI,facilitating desorption/ionization of lipids[26,27].Nevertheless,it should be noted that using UV light in these reactions may cause health damage and unexpected side reactions[14,29–31].Ouyanget al.[32]and Chenet al.[33]recently reported visible light-activated cycloaddition methods to pinpoint DB locations in unsaturated lipids.However,these reactions were conducted in the liquid phase,which is not suitable for tissue sections.
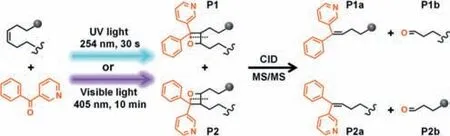
Fig.1.UV and visible light activated[2+2]cycloaddition reaction of 3-BzPy to unsaturated lipids,and MS/MS analysis.
Here,we developed a new type of light driven[2+2]cycloaddition reaction,coupled with MALDI-MS/MS imaging analysis,for the identification of lipid DB-positional isomers and their spatial distributions in biological tissues.3-Benzoylpyridine(3-BzPy)was discovered as a novel on-tissue derivatization reagent.On both UV light(254 nm)and visible light(405 nm)irradiation,3-BzPy can directly react with unsaturated lipids in a[2+2]cycloaddition reaction,enabling precise location of DB positions in MS/MS experiment(Fig.1).The spatial distributions of lipid DB position isomers in distinct regions of rat brain were clearly imaged via the unique combination of on-tissue cycloaddition reaction with MALDI-MS/MS imaging.
BzPys,including 2-BzPy,3-BzPy and 4-BzPy,were selected as efficient cycloaddition reaction reagents from a series of candidate compounds(Fig.S2 in Supporting information)because of their high reaction yield and strong MS signals.The carbonyl group and aromatic groups enable the photocycloaddition reactivity and UV absorption of BzPy,respectively.2-BzPy,3-BzPy and 4-BzPy all presented strong UV absorption around 254 nm(Fig.S3 in Supporting information).Upon 254 nm UV light irradiation,the mixed solutions of unsaturated lipids and different BzPys were directly injected into the Q-TOF mass spectrometer for MS analysis.The results show that although unsaturated lipids could react with all BzPys for[2+2]cycloaddition,their reactivity to 3-BzPy and 4-BzPy were much higher than that of 2-BzPy(Fig.S4 in Supporting information).We further performed tandem MS experiments on the photocycloaddition products.It is worth noting that,compared with 4-BzPy,3-BzPy-derivatized unsaturated lipids gave more structure-related fragment ions with much stronger ion intensity(Fig.S5 in Supporting information).4-BzPy-derivatized unsaturated lipids are more likely to break the newly formed bonds of oxetane ring during CID MS/MS analysis,resulting in the lack of fragments related to the position of lipid C=C bonds.Therefore,3-BzPy was selected as the cycloaddition reaction reagent in this study.Moreover,compared with the commonly used photochemical reagent 2-acetylpyridine(2-AP),3-BzPy can significantly improve the ion intensities of photocycloaddition products(Fig.S6 in Supporting information).
Fig.2A illustrates the mass spectra of fatty acids after 30 s 254 nm UV light irradiation.The carboxyl group in the fatty acid and the pyridine group in 3-BzPy ensure that the photocycloaddition products can be detected in both positive and negative ion modes.This provides the ability to simultaneously detect fatty acids and their metabolic pathway-related metabolites.(Fig.S7 in Supporting information).
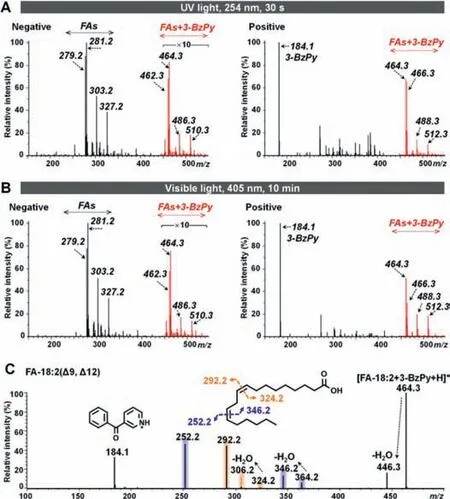
Fig.2.(A)Mass spectra of fatty acids following UV light-activated cycloaddition.(B)Mass spectra of fatty acids following visible light-activated cycloaddition.(C)CID MS/MS spectrum of[FA-18:2(Δ9, Δ12)+3-BzPy]after visible light irradiation.
Although the absorption of 3-BzPy in the visible light region is much lower than that in the UV light region,our study showed that visible light can also induce[2+2]cycloaddition reaction between 3-BzPy and lipid C=C bond.As shown in Fig.2B,on 405 nm visible light irradiation for 10 min,3-BzPy-derivatized unsaturated fatty acids were successfully detected.Structurally,3-BzPy has a large conjugated system,and the benzene ring and the pyridine ring are connected by a carbonyl group.We further irradiated acetophenone(APh,with benzene ring and acetyl group)and 3-acetylpyridine(3-AP,with pyridine ring and acetyl group)with unsaturated lipids under visible light,but the results showed that both APh and 3-AP could not form oxetanes with lipid C=C bond under visible light.The large conjugated aromatic ring system of 3-BzPy may be the main reason leading to the formation of oxetanes under visible light irradiation.
Fig.2C shows the CID mass spectrum of 3-BzPy-derivatized FA-18:2([M+H]+,m/z464.3).Two pairs of diagnostic ions atm/z292.2/324.2 andm/z252.2/346.2 allow for the identification of C=C locations atΔ9 andΔ12.Similarly,derivatized FA-20:4(Δ5,Δ8,Δ11,Δ14)and FA-22:6(Δ4,Δ7,Δ10,Δ13,Δ16,Δ19)can generate four pairs(m/z268.1/372.3,308.2/332.2,348.2/292.2,and 388.2/252.2)and six pairs(m/z254.1/410.3,294.1/370.3,334.2/330.2,374.2/290.2,414.2/250.2,and 454.3/210.1)of diagnostic ions,respectively(Figs.S8 and S9 in Supporting information).However,for phospholipids,diagnostic ions usually appear in groups of three.For example,two groups of diagnostic ions atm/z252.2/690.5/841.5 andm/z292.2/650.4/801.5 were observed in 3-BzPy-derivatized phosphatidylcholine(PC)16:0_18:2(Δ9,Δ12);two groups of diagnostic ions atm/z252.2/714.5/865.5 andm/z292.2/674.4/825.5 were observed in 3-BzPy-derivatized PC18:2(Δ9,Δ12)_18:2(Δ9,Δ12)(details are shown in Figs.S10 and S11 in Supporting information).
To achieve MS imaging of lipid C=C location isomers in biological tissues,we further evaluated the spray deposition of liquid-phase 3-BzPy as a means of on-tissue[2+2]cycloaddition.The standard solutions of unsaturated lipids and 3-BzPy were sprayed on ITO-coated slide and exposed to UV or visible light.After coating matrix,3-BzPy-derivatized lipids can be directly detected on the slide by MALDI-TOF/TOF mass spectrometer.Fig.S12(Supporting information)shows the typical on-tissue MALDIMS/MS spectra of 3-BzPy-derivatized lipids.
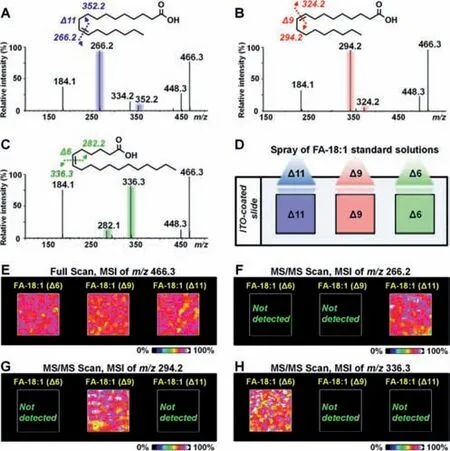
Fig.3.(A–C)CID MS/MS spectrum of 3-BzPy-derivatized FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)after UV light irradiation.(D)Standard solutions of FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)were sprayed on the slide.(E)MALDIMS images of[FA-18:1+3-BzPy]in sprayed samples.(F-H)MALDI-MS/MS image of diagnostic ions of FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)in sprayed samples.
Furthermore,threeΔ11,Δ9,andΔ6 isomers of FA-18:1 were used to evaluate the capability of 3-BzPy-functionalized MALDIMS/MS imaging for assigning double bond positions.Figs.3A–C suggest that 3-BzPy-derivatized FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)can produce isomer-specific diagnostic ions during CID analysis.Three spray models containing FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)were prepared on an ITO-coated slide(Fig.3D).After 3-BzPy coating and light irradiation,we conducted MALDI-MSI and MALDI-MS/MS imaging experiments on the spray models.The MALDI-MSI result is shown in Fig.3E,and it suggests that 3-BzPy-derivatized FA-18:1([M+H]+,m/z466.3)can be detected and imaged in all these three spray models.The MALDIMS/MS ion images of diagnostic ions corresponding to FA-18:1(Δ11),FA-18:1(Δ9),and FA-18:1(Δ6)are shown in Figs.3F–H.The fragment ion ofm/z266.2 can only be imaged in FA-18:1(Δ11)(Fig.3F).The ion ofm/z294.2 was only expressed in FA-18:1(Δ9)(Fig.3G).The ion ofm/z336.3 can only be imaged in FA-18:1(Δ6)(Fig.3H).These results indicate that the spray deposition of 3-BzPy on the sample surface is an effective approach for MALDI-MS/MS imaging of lipid C=C location isomers.
Next,we applied our 3-BzPy-functionalized MALDI-MS imaging method to map the spatial locations of lipid C=C position isomers in rat brain.To achieve better on-tissue reaction efficiency,we investigated the impacts of irradiation time on the on-tissue cycloaddition of unsaturated lipids.Fig.S13(Supporting information)shows the MS images of representative 3-BzPy-derivatized lipids in adjacent brain tissue sections under different irradiation times,and the results suggest that the MS signals of 3-BzPy-derivatized lipids are the strongest when exposed to UV light for 1,2 min.However,the ion intensities of many saturated lipids,such as FA-16:0,FA-18:0,LysoPE-16:0,LysoPE-18:0,PS-34:0,and PE-38:0,decreased continuously with increasing irradiation time(Fig.S14 in Supporting information).Some saturated lipids,such as sulfatides(ST),did not change much in 2 min,but their MS signal decreased significantly when the irradiation time increased to 5 min(Fig.S15 in Supporting information).Therefore,the UV light irradiation time was set to 1 min.Similarly,the visible light irradiation time was optimized as 30 min(Fig.S16 in Supporting information).The spraying density of 3-BzPy on tissue sections was also optimized.As shown in Fig.S17(Supporting information),when the spraying density of 3-BzPy was ~100 nmol L-1cm-2,3-BzPy-derivatized lipids exhibited stronger MS imaging signals.
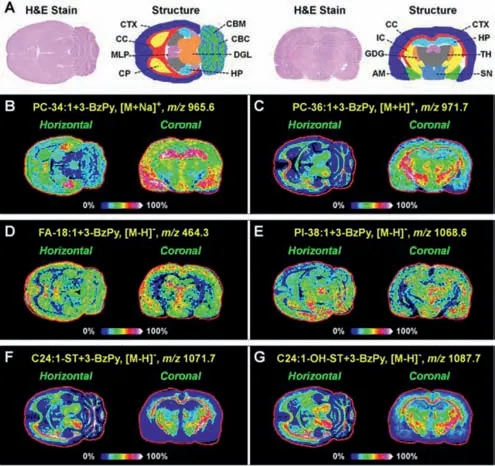
Fig.4.MALDI-MS images of representative 3-BzPy-derivatized lipids in brain tissue sections after UV light irradiation.AM,amygdala;CBC,cerebellar cortex;CBM,cerebellar medulla;CC,corpus callosum;CP,caudate putamen;CTX,cerebral cortex;DGL,deep gray layer of the superior colliculus;GDG,granular layer of the dentate gyrus;HP,hippocampus;IC,internal capsule;MLP,molecular layer of the hippocampus;SN,substantia nigra;TH,thalamus.
Using the optimized on-tissue cycloaddition method,we first performed MALDI-MSI experiment on the horizontal and coronal sections of rat brain.Fig.4 illustrates the MALDI-MSI result,and it suggests that a variety of 3-BzPy-derivatized lipids in the brain can be successfully detected and imaged.
MALDI-MS/MS imaging experiments were then performed on adjacent tissue sections to characterize the fatty acyl combinations and C=C position of brain phospholipids.Fig.5A shows the ontissue MALDI-MS/MS spectrum of 3-BzPy-derivatized PC-34:1.The ion ofm/z496.3 appears in the MS/MS spectra of underivatized PC-34:1 and 3-BzPy-derivatized PC-34:1,indicating that there is a lysoPC-16:0 group in the structure of PC-34:1.Since,the two fatty acyl side-chains of PC-34:1 are speculated to be C18:1 and C16:0.Two C=C location diagnostic ions atm/z650.4(red)andm/z678.5(blue)were observed,suggesting the existence ofΔ9 andΔ11 isomers for the C18:1 chain of PC 16:0_18:1.Therefore,PC 16:0_18:1(Δ9)and PC 16:0_18:1(Δ11)are two isomers of PC-34:1 in rat brain.The spatial distributions of PC 16:0_18:1(Δ9)and PC 16:0_18:1(Δ11)in rat brain were imaged using their diagnostic ions,and they are shown in Fig.5B.On the horizontal section,bothΔ9 andΔ11 isomers of PC 16:0_18:1 exhibited stronger ion intensities in the CTX,HP,caudate putamen(CP),and cerebellar cortex(CBC)regions,but the relative abundance ofΔ9 isomer in the CTX and HP regions was significantly higher thanΔ11 isomer.
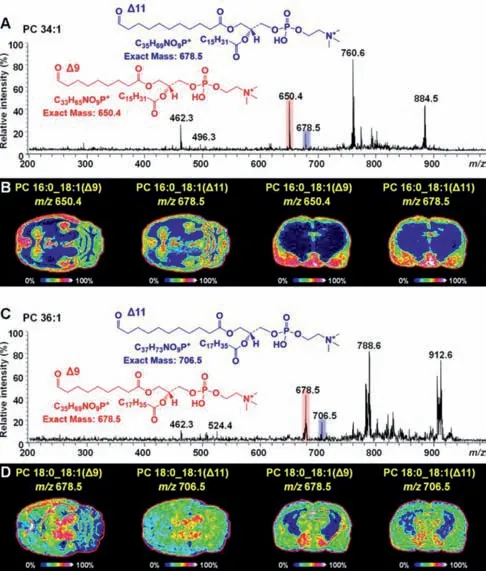
Fig.5.MALDI-MS/MS spectra and MS/MS images of diagnostic ions representing PC 34:1(A,B)and PC 36:1(C,D)C=C position isomers in rat brain after UV light irradiation.
Similarly,the ion atm/z524.4([LysoPC-18:0+H]+)was found in the MS/MS spectra of 3-BzPy-derivatized PC-36:1(Fig.5C)and underivatized PC-36:1,thus identifying the fatty acyl side-chains of PC-36:1 as C18:0 and C18:1.Two C=C location isomers,PC 18:0_18:1(Δ9)and PC 18:0_18:1(Δ11)were further identified from PC-36:1 based on two diagnostic fragment ions atm/z678.5 andm/z706.5(Fig.5C).Fig.5D illustrates the spatial distributions of PC 18:0_18:1(Δ9)and PC 18:0_18:1(Δ11)in rat brain.PC 18:0_18:1(Δ9)was detected in higher abundance in the CC,deep gray layer of the superior colliculus(DGL),and cerebellar medulla(CBM)regions.PC 18:0_18:1(Δ11)was more greatly distributed in the DGL region.
In summary,we present a simple and efficient on-tissue[2+2]cycloaddition method,coupled with MALDI-MS/MS imaging,to visualize the spatial locations of lipid C=C position isomers in biological tissues.3-BzPy,a novel derivatization reagent,can react with C=C bond to form four-membered oxetane ring under both visible light and UV light irradiation.3-BzPy was used for the ontissue cycloaddition of unsaturated lipids.By optimizing 3-BzPy coating density and on-tissue derivatization time,we successfully detected and imaged the spatial distributions of phospholipid C=C position isomers in rat brain.The combination of on-tissue cycloaddition and MALDI-MSI offers an accurate and spatially resolved approach to study tissue lipidomics.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Nos.81903571,21904080),the Taishan Scholars the Taishan Scholars Program(C.Sun,No.tsqn202103096),and the Shandong Province“Double-Hundred Talent Plan”Program.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.034.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
