NiCu bimetallic catalysts derived from layered double hydroxides for hydroconversion of n-heptane
Yanru Zhu,Minghuan Yang,Zhen Zhang,Zhe An,Jian Zhang,Xin Shu,Jing He
State Key Laboratory of Chemical Resource Engineering,Beijing Advanced Innovation Center for Soft Matter Science and Engineering,Beijing University of Chemical Technology,Beijing 100029,China
ABSTRACT Supported NiCu bimetallic catalysts have been produced in-situ on commercial Al2O3 by using layered double hydroxides as precursors.The resulting catalysts show a uniform Ni and Cu distribution,thus providing good activity and selectivity in the reforming reaction of n-heptane.The catalytic performance has been found to depend on the Cu/Ni ratio,revealing the synergic catalysis between homogeneously dispersed Ni and Cu sites.The good catalysis of NiCu bimetallic catalysts makes it possible to partly or even completely replace Pt with NiCu bimetallic catalysts.
Keywords:n-Heptane reforming NiCu bimetallic catalysts Synergic catalysis Uniform distribution Layered double hydroxides
Supported Pt is a common catalyst for the catalytic reforming reaction[1–4].Considerable efforts have recently been dedicated to the development of low-cost and highly efficient nonplatinum reforming catalysts,and great progress has been made with tungsten carbide[5],Mo-based catalysts such as Mo2C[6],MoOxCy[7]and MoOx[8–10],as well as supported Ni[11–13]and Co[13]catalysts.The great challenges with non-platinum reforming catalysts originate from substantial hydrogenolysis and cracking reactions[14],which result in low isomerization selectivity.Increasing the dispersion of supported metals has been found to contribute to isomerization selectivity[15].For example,Ni-La/HY catalyst exhibits higher isomerization selectivity than Ni/HY in the hydroconversion ofn-C8because the metal dispersion has been improved by rare earth addition[15].Yet the reforming performance of non-noble metal catalyst has not been reported to reach that of Pt catalyst.The bimetallic catalyst,in which small amount of platinum is used together with non-noble metal components such as Ni[16,17]or Cu[18],is till now a good choice to obtain good activity and selectivity while reduce the cost of catalysts.For example,with proper Pt proportions,the Ni-Pt bimetallic catalysts afford higher activity and are more selective to di-branched alkanes than Pt monometallic catalyst in the hydroconversion ofn-C6reaction[17].The complete replacement of platinum with transition metals in the paraffin reforming process remains a great challenge.
In this work,the feasibility to partly or even completely replace Pt with NiCu bimetallic catalyst has been demonstrated,with Ni and Cu in a highly homogeneous dispersion by using Ni and Cu containing layered double hydroxides as precursors.Layered double hydroxides(LDHs),a hydrotalcite-like compound,have been demonstrated to be excellent precursors for supported metal catalysts[19–23].This work reports the homogeneously dispersed Ni and Cu centers,which affords good activity and selectivity in the reforming reaction ofn-heptane.The NiCu bimetallic catalyst even provides higher activity than Pt catalyst while with comparable selectivity.
The Ni and Cu containing ZnAl-LDHs precursors were preparedin situonγ-Al2O3support according to the reported method[24].The Cu/Ni molar ratios were controlled as 0.61,1.19,2.11 and 3.17 as determined by inductively coupled plasma optic emission spectrometer(ICP-OES)analysis(Table S1 in Supporting information).The Ni and Cu containing ZnAl-LDHs precursors were activated in the reactor by first calcining in the air at 550 °C and then reducing with H2flow at 500 °C.The resulting sample was denoted NiCux-ZnAl-LDO@Al2O3(x represents the Cu/Ni molar ratio).The reaction was initiated by introducingn-C7into the reactor,and the products were analysed on-line with a gas chromatograph.For comparison,the supported NiCu bimetallic catalyst with a Cu/Ni molar ratio of 1.14 was also prepared by incipient wetness impregnation method(denoted NiCu1.14/ZnAl-LDO@Al2O3),followed by the same calcination and reduction process as for NiCux-ZnAl-LDO@Al2O3.
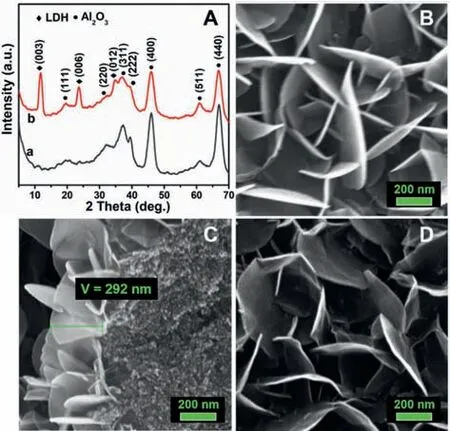
Fig.1.(A)XRD patterns of(a)γ-Al2O3,(b)NiCu1.19-ZnAl-LDHs@Al2O3 with a Cu/Ni molar ratio of 1.19;(B)SEM images of the surface;(C)the cross section of the NiCu1.19-ZnAl-LDHs@Al2O3;and(D)SEM images of NiCu1.19-ZnAl-LDO@Al2O3 obtained from NiCu1.19-ZnAl-LDHs@Al2O3.
The powder X-ray diffraction(XRD)patterns pattern for each NiCux-ZnAl-LDHs@Al2O3sample(Fig.1A and Fig.S1 in Supporting information)clearly illustrates the[003],[006]and[012]reflections characteristic of the structure of hydrotalcites[25],indicating the formation of LDHs on theγ-Al2O3support.No other crystalline phases were detected in addition toγ-Al2O3support and LDHs.Flake-shaped NiCuZnAl-LDHs crystallites can be observed from the SEM image(Fig.1B)as growing in interlaced direction on the surface of Al2O3.As can be seen from the cross section of NiCuZnAl-LDHs@Al2O3(Fig.1C),LDHs phase grows on the Al2O3surface in a thickness of about 292 nm,with the edges of LDH slabs tightly adhered to Al2O3spheres.The growths of LDHs and the corresponding LDO results in the specific surface area increasing from 187 m2/g(forγ-Al2O3)to 218 m2/g(for NiCu1.19-ZnAl-LDHs@Al2O3)or 212 m2/g(for NiCu1.19-ZnAl-LDO@Al2O3),probably because the LDHs or LDO crystallites raise the surface roughness.The calcination and reduction cause no visible destruction of the LDH morphology or change in surface area on Al2O3surface,and the resulting NiCu1.19-ZnAl-LDO@Al2O3well retains the interlaced arrangement of flake-shaped slabs(Fig.1D).
The transmission electron microscopy(TEM)image(Fig.2A)shows that the metal nanoparticles are homogeneously dispersed in NiCu1.19-ZnAl-LDO@Al2O3.The particles are in a narrow size distribution from 1.9 nm to 4.2 nm with the maximum of around 3.0 nm,and the lattice spacing of the exposed NiCu(111)facet is 0.206 nm in the high-resolution TEM(HRTEM)image(inset in Fig.2A).The high angle annular dark-field scanning transmission electron microscopy(HAADF-STEM)image(Fig.2B)indicates a uniform distribution of the metal nanoparticles,just as observed from the TEM image.The STEM-energy dispersive X-ray(EDX)elemental mapping(insets in Fig.2B)displays that the Ni and Cu elements are distributed homogeneously and almost in the same position.The observations from HRTEM image and EDX mapping illustrate a NiCu alloy structure,which is consistent with an earlier report[26].As a comparison,the metal particles in the NiCu1.14/ZnAl-LDO@Al2O3are diverse in morphology(Fig.2C),with a broader size distribution from 1.7 nm to 6.0 nm,as observed in TEM image.Separated Ni and Cu particles with both(111)facet exposed are observed in HRTEM image(inset in Fig.2C).The HAADF-STEM image provides a more obvious difference in the metal distribution between NiCu1.14/ZnAl-LDO@Al2O3and NiCu1.19-ZnAl-LDO@Al2O3).The metal particles are observed to be in a continuous and more chaotic distribution in the HAADF-STEM image of NiCu1.14/ZnAl-LDO@Al2O3,with visible aggregation(Fig.2D).The EDX image(insets in Fig.2D)exhibit a discrepant Ni and Cu element distribution.In the XPS spectra(Fig.3),the binding energies of Ni0and Cu0[27,28]are measured as 852.7 and 932.8 eV for NiCu1.19-ZnAl-LDO@Al2O3(Fig.3A),0.2 and 0.3 eV higher than that for NiCu1.14/ZnAl-LDO@Al2O3(Fig.3B),consistent with better NiCu dispersion in NiCu1.19-ZnAl-LDO@Al2O3.The adjacent binding energy at 855.8-856.9 eV in the Ni 2p3/2XPS spectra originates from a high-spin divalent state of Ni2+in the sample[27].When nickel is alloyed with copper,changes in the electronic properties of nickel result in displacement of BEs to lower values depending on the copper content.
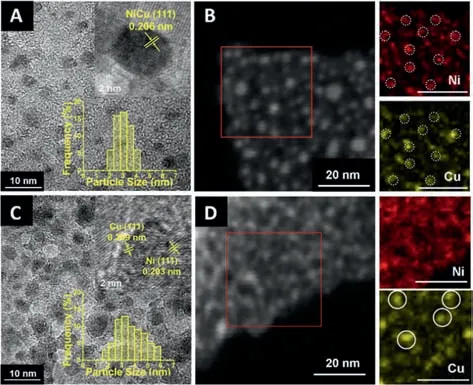
Fig.2.TEM image(A)and HAADF-STEM image(B)of catalyst NiCu1.19-ZnAl-LDO@Al2O3;TEM image(C)and HAADF-STEM image(D)of catalyst NiCu1.14/ZnAl-LDO@Al2O3.Insets in(A)and(C)are the HRTEM images and the particle size frequency distribution histograms.Insets in(B)and(D)are the elemental mapping images of Ni and Cu for the corresponding sample.Scale bar:20 nm.
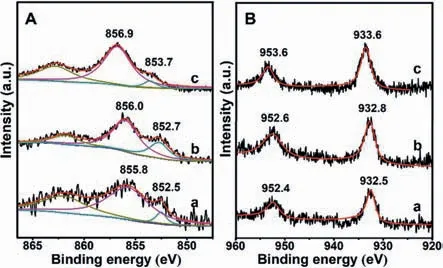
Fig.3.Ni(A)and Cu(B)2p XPS spectra for(a)NiCu1.14/ZnAl-LDO@Al2O3,(b)NiCu1.19-ZnAl-LDO@Al2O3,and(c)0.17%Pt/ NiCu1.19-ZnAl-LDO@Al2O3.
In the hydro-conversion of heptane(n-C7)(Table 1),NiCu1.19-ZnAl-LDO@Al2O3as the catalyst(Table 1,entry 1)shows visibly highern-C7conversion,higher specific activity,higheri-C7selectivity,and lower C1-C4selectivity than NiCu1.14/ZnAl-LDO@Al2O3(Table 1,entry 2).Then-C7conversion over NiCu1.19-ZnAl-LDO@Al2O3is even higher than over Pt/ZnAl-LDO@Al2O3with a 0.32% Pt loading(Table 1,entry 3),while with almost the same selectivity.The better apparent activity and isomerization selectivity of NiCu1.19-ZnAl-LDO@Al2O3is supposed to owe to the well-alloyed structure with NiCu uniform distribution.As revealed by the XPS results,thein situexsolution of NiCu fromLDHs layers provides stronger metal-supports interactions that leads to Ni and Cu centers more electron-deficient,which well accounts for the higher activity of NiCu1.19-ZnAl-LDO@Al2O3than NiCu1.14/ZnAl-LDO@Al2O3.According to previous observation[29],more highly are metal centers dispersed,more is C-C cleavage inhibited.So the better selectivity of NiCu1.19-ZnAl-LDO@Al2O3than NiCu1.14/ZnAl-LDO@Al2O3could also be well explained.The use of Pt in 0.17% loading together with NiCu1.19bimetallic catalyst promotes both ofn-C7conversion andi-C7selectivity,while largely reduces the C1-C4selectivity(Table 1,entry 6).The specific activity on 0.17%Pt/NiCu1.19-ZnAl-LDO@Al2O3is 268% higher than that on 0.32%Pt/ZnAl-LDO@Al2O3.The significant increase in specific activity(normalized by moles of Pt)can be attributed to the contribution of NiCu.The ICP results and TEM/STEM images show that the Pt loading makes no visible impact on components of Ni or Cu and the homogeneous distribution of NiCu elements(Table S1 and Fig.S2 in Supporting information).The NiCu particles retain narrow distribution sized from 3.8 nm to 6.3 nm.In the XPS spectra(Fig.3),a visible increase in the BEs of Ni 2p3/2and Cu 2p3/2is observed with Pt loaded,illuminating the electron transfer from NiCu to Pt atoms.Therefore,the promotion of catalysis observed with 0.17%Pt/NiCu1.19-ZnAl-LDO@Al2O3could originate from the synergies of NiCu and Pt centers.Similar synergy effect of the metal active sites was previously observed for the Ir-Pt/Al2O3catalyst prepared by organometallic grafting method in the ring opening reaction of methyl-cyclopentane[30].
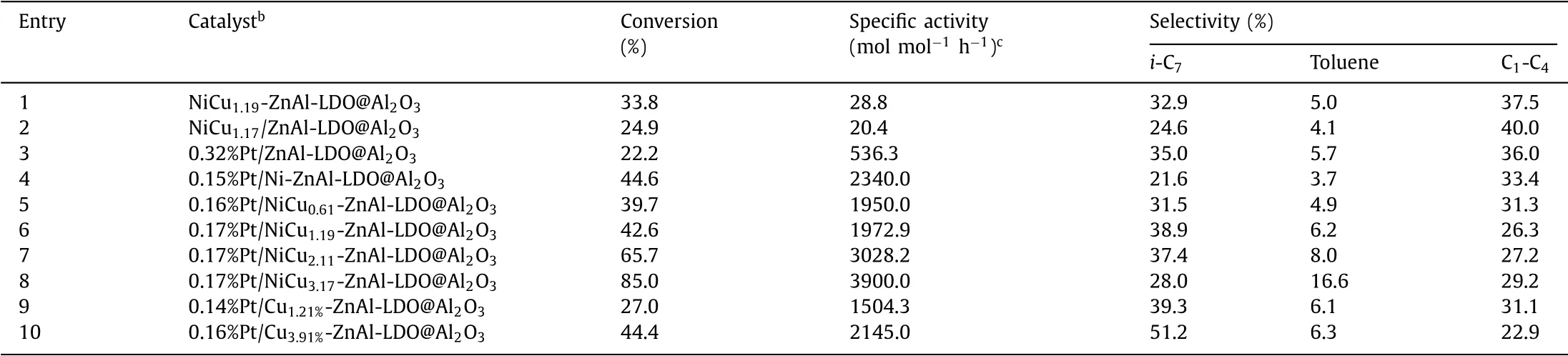
Table 1 Catalytic results for the hydroconversion of n-C7.a
To better understand why the reforming catalysis of Ni and Cu even better than supported Pt,the Cu/Ni molar ratios(Table S1)have been varied in this work.As shown in the XPS spectra(Fig.4),in comparison with Ni-ZnAl-LDO@Al2O3,Cu introduction decreases the binding energy(BE)of Ni 2p3/2(Fig.4A)slightly,and meanwhile the BE of Cu0in NiCu0.61-ZnAl-LDO@Al2O3rises in comparison to Cu-ZnAl-LDO@Al2O3(Fig.4B),consistent with the electron transfer from Cu to Ni.With increasing Cu/Ni ratio,both of Ni0and Cu0BEs successively increases.But the reason for the increase in the BEs needs more investigations.Similar trends are observed on Pt-containing samples(Figs.4C and D).
In the hydro-conversion ofn-C7,in comparison with Ni catalyst without Cu(Table 1,entry 4),the introduction of Cu in a Cu/Ni ratio of 0.61 decreases then-C7conversion while improves the isomerization selectivity visibly(Table 1,entry 5).With the Cu/Ni ratio increasing,n-C7conversion,specific activity,and toluene selectivity all increase,while the isomerization selectivity shows a first increasing and then decreasing change(Table 1,entries 5-8).The dependence of catalytic activity and selectivity on the Cu/Ni ratio of NiCu bimetallic catalyst indicates the synergic catalysis between highly homogeneous Ni and Cu sites[31].The introduction of Cu in a Cu/Ni ratio of 0.61 increases the electronic density of Ni site,accounting for the activity reduction on Pt/NiCu0.61-ZnAl-LDO@Al2O3in comparison to Pt/Ni-ZnAl-LDO@Al2O3.The activity enhancement with increasing Cu/Ni ratio is well consistent with the successive decreases in the electronic density of both Cu and Ni sites.
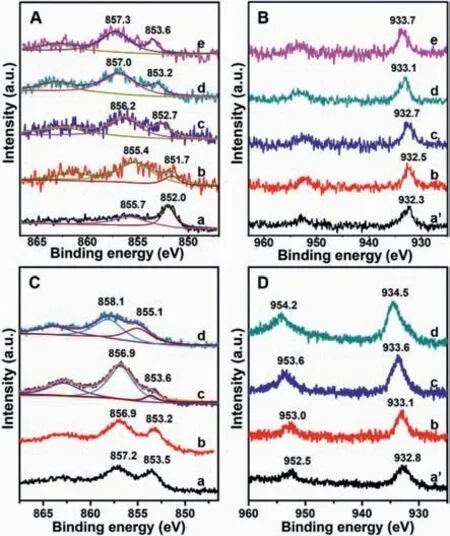
Fig.4.Ni(A,C)and Cu(B,D)2p XPS spectra for(a)Ni-ZnAl-LDO@Al2O3,(a’)Cu-ZnAl-LDO@Al2O3,(b)NiCu0.61-ZnAl-LDO@Al2O3,(c)NiCu1.19-ZnAl-LDO@Al2O3,(d)NiCu2.11-ZnAl-LDO@Al2O3 and(e)NiCu3.17-ZnAl-LDO@Al2O3 without(A,B)and with(C,D)Pt loaded.
The introduction of Cu inhibits the hydrogenolysis and/or cracking reactions,giving a reduced C1-C4selectivity(Table 1,entries 4-8).But a visible inhibition of C1-C4selectivity needs a higher Cu loading when no Ni is present(Table 1,entries 9 and 10).The toluene selectivity increases with Cu/Ni ratio(Table 1,entries 5-8),but not with the amount of single Cu(Table 1,entries 9 and 10),indicating the role of synergic catalysis between Cu and Ni sites in the improvement of toluene selectivity.When a small amount of Cu added,the XPS show that the binding energy of Ni shifts toward a low energy of 0.3 eV,and the binding energy of Cu increases 0.3 eV(Fig.4).The changes of electronic structure of the metals result in a slight decrease in catalytic activity.Further increase the ratio of Cu/Ni,the binding energy of Ni and Cu increases successively.The activities of catalysts show an increasing trend and the selectivity of toluene increase in sequence.The dehydrogenation ability of the metals is enhanced with the binding energy of metals increasing.The selectivity of isomerization increases at first and then decreases,while the cleavage selectivity shows an opposite trend,but the magnitude of the change is relatively small.
In the H2-TPR profiles(Fig.S3 in Supporting information),the reduction temperature for Ni(II)gradually decreases from 596 °C to 481 °C with increasing Cu/Ni ratio,while the reduction temperature of Cu(II)decreases from 341 °C to 254 °C.Additional hydrogen consumptions are observed at 325 °C for NiCu1.19-ZnAl-LDO@Al2O3and 288 °C for NiCu2.11-ZnAl-LDO@Al2O3,which suggests the possible formation of NiCu alloy phase.But the hydrogen consumption at 287 °C almost diminishes for NiCu3.17-ZnAl-LDO@Al2O3.
Theisomerizationselectivityon0.16%Pt/Cu3.91%-ZnAl-LDO@Al2O3is 142% higher than on 0.15%Pt/Ni-ZnAl-LDO@Al2O3.The isomerization reaction rate has been reported highly dependent on the acidic property of catalysts[4].But there is no significant difference in the amount and strength of acidic sites between 0.16%Pt/Cu3.91%-ZnAl-LDO@Al2O3and 0.15%Pt/Ni-ZnAl-LDO@Al2O3,based on NH3-TPD profiles(Fig.S4 in Supporting information).So it can be deduced that the Cu sites promote isomerization selectivity by inhibiting hydrogenolysis reaction.The synergies of Ni and Cu sites on alloy phase is mainly responsible for the higher isomerization selectivity observed with NiCu1.19-ZnAl-LDO@Al2O3and NiCu2.11-ZnAl-LDO@Al2O3than with other catalysts.
In summary,NiCu bimetallic catalyst with uniform NiCu dispersion has been demonstrated to provide higher activity than Pt catalyst while with similar selectivity in the reforming reaction ofnheptane.The synergies between homogeneously dispersed Ni and Cu sites account for the enhancement of activity and selectivity.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
Financial supports from National Nature Science Foundation of China(NSFC,Nos.91634120 and 21521005),the National Key Research and Development Program of China(No.2017YFA0206804)and the Fundamental Research Funds for the Central Universities(No.XK1802-6)are gratefully acknowledged.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.120.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
