Enhancing photocatalytic performance of metal-organic frameworks for CO2 reduction by a bimetallic strategy
Jihong Zhang,Yuchen Wang,Hongjuan Wang,Dichang Zhong,Tongbu Lu
MOE International Joint Laboratory of Materials Microstructure,Institute for New Energy Materials and Low Carbon Technologies,School of Materials Science and Engineering,Tianjin University of Technology,Tianjin 300384,China
1 These authors contributed equally to this work.
ABSTRACT Metal-organic frameworks(MOFs)as a type of crystalline heterogeneous catalysts have shown potential application in photocatalytic CO2 reduction.However,MOF catalysts with high efficiency and selectivity are still in pursuit.Herein,by a bimetallic strategy,the catalytic performance of a Co-MOF for photocatalytic CO2 reduction was enhanced.Specifically,the Co-MOF based on 4,5-dicarboxylic acid(H3IDC)and 4,4′-bipydine(4,4′-bpy)can catalyze CO2 reduction to CO,with high efficiency but relatively low selectivity.After replacement of 2/3 Co(II)with Ni(II)within Co-MOF,the resulted isostructural Co1Ni2-MOF not only retains high efficiency for photocatalytic CO2 reduction,but also shows enhanced CO selectivity.The CO evolution rate reaches 1160 μmol g-1 h-1 and the CO selectivity reaches as high as 94.6%.The enhanced photocatalytic CO2 reduction performance is supported by theoretical calculation results.This case demonstrates that bimetallic strategy is an effective mean to optimize the catalytic performance of MOF catalysts for photochemical CO2 reduction.
Keywords:Metal-organic framework Photocatalytic CO2 reduction Bimetallic strategy CO2–to–CO conversion Photocatalysis Dinuclear metal synergistic catalysis
Converting atmospheric excessive carbon dioxide(CO2)into fuel or useful chemicals driven by sunlight is a sustainable approach to relieve energy shortage and environment deterioration[1–9].The key is the development of efficient,selective and cheap catalysts.Metal-organic frameworks(MOFs)are a class of crystalline porous materials composed of metal ions(or metal clusters)and organic linkers[10–18].With structural diversity and ultrahigh surface area,MOFs can serve as promising photocatalysts for CO2conversion,especially for those constructed from transition metals[19–23].For instance,Wang group reported that Co-ZIF-9 can photocatalyze CO2reduction to CO,with the yield rate of 83.6 μmol/h and a selectivity of 58%[24].Lan and co-workers developed a stable Co(II)MOF for CO2reduction[25],which showed a CO evolution rate of 1140 μmol g-1h-1and a selectivity of 47.4%.Obviously,although great progress in developing MOFs-based photocatalysts for photocatalytic CO2reduction has been achieved,the activity and selectivity of these catalysts still need to be further enhanced[26–33].Very recently,we and others have demonstrated that the catalytic performance of metal complexes for photochemical CO2reduction can be improved by changing the metal centers and ligand modification[34–36].We have also found that the dinuclear metal synergistic catalysis(DMSC)within dinuclear metal complexes can greatly boost the photocatalytic CO2reduction activity[37–39].
To further improve the catalytic performance of molecule-based materials for photochemical CO2reduction,we tried to build heterogeneous catalysts with DMSC.Herein,we report a case well demonstrating a bimetallic strategy for greatly increase the photocatalytic CO2reduction performance.Specifically,a Co-MOF based on mixed ligands of 4,5-dicarboxylic acid(H3IDC)and 4,4′-bipydine(4,4′-bpy)[40]can efficiently catalyze CO2reduction to CO,while the selectivity to CO is relatively low.By gradually replacing the Co(II)with Ni(II)within Co-MOF,the CO selectivity of the resulted isostructural CoxNiy-MOFs(xandyrepresent the atomic ratio of Co and Ni,respectively)remarkably increases.The optimized Co1Ni2-MOF catalyst shows the best photocatalytic performance for CO2reduction,with a CO evolution rate of 1160 μmol g-1h-1and a selectivity of 94.6%.This work will pave a new way for developing high-performance MOFs-based catalysts for photocatalytic CO2reduction.
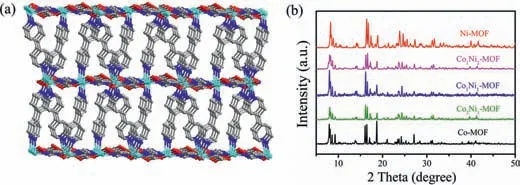
Fig.1.(a)Three-dimensional pillar-layered structure of CoxNiy-MOFs;(b)PXRD patterns of CoxNiy-MOFs.
The Co-MOF was synthesized according to the reported procedure by Cao group[40].The synthetic procedure of Ni-MOF was similar to that of Co-MOF(see Supporting information).Singlecrystal X-ray diffraction indicated that Ni-MOF is isostructural with Co-MOF(Tables S1–S3 in Supporting information),where the Ni(II)are linked with H3IDC to form a two-dimensional(2D)layers with hexagonal 24-membered rings,and the 4,4′-bpy ligands pillar these layers to yield a three-dimensional(3D)pillar-layered framework(Fig.1a).The powder X-ray diffraction(XRD)patterns of the assynthesized Co-MOF and Ni-MOF agree well with the simulated ones(Fig.S1 in Supporting information),demonstrating the successful syntheses of both MOFs.Simultaneously,a series of isomorphic bimetallic CoxNiy-MOFs were tried to be synthesized by adding a certain amount of Ni(II)to the Co-MOF synthetic system.A remarkable color change of the bimetallic CoxNiy-MOFs,from orange to yellow green were observed with the gradual replacement of the Co(II)with Ni(II)(Fig.S2 in Supporting information).The similar cuboids morphology and powder XRD patterns(Fig.1b and Fig.S3 in Supporting information)indicate that the synthesized bimetallic CoxNiy-MOFs maintained similar structures to that of monometallic MOF.The similar fourier transform infrared spectrometer(FT-IR)spectra and thermogravimetric(TG)curves further prove that these MOFs are isostructural(Figs.S4 and S5 in Supporting information).The CO2sorption experiments demonstrated that they also exhibit similar CO2uptake capacity(Fig.S6 in Supporting information).The element mappings of CoxNiy-MOFs show that C,N,O,Co and Ni are uniformly distributed(Fig.S7 in Supporting information),and the inductively coupled plasma mass spectrometry(ICP-MS)further demonstrate that the Co/Ni atomic ratio in CoxNiy-MOFs is consistent with the Co/Ni ratio used in synthesis(Table S4 and Fig.S8 in Supporting information).
Photocatalytic CO2reduction experiments were performed at room temperature with CoxNiy-MOFs as catalysts,[Ru(phen)3](PF6)2as a photosensitizer,and triethanolamine(TEOA)as an electron donor in a mixture of 5 mL CO2-saturated CH3CN/H2O(v/v = 4:1),irradiated by a 300 W Xe lamp with wavelengths over 420 nm(see Supporting information).The gaseous products were detected by gas chromatography,and the liquid products were examined by ion chromatography.As shown in Fig.2a and Tables S5 and S6(Supporting information),the monometallic Co-MOF exhibits good activity for CO2reduction,with a CO generation rate of 1247 μmol g-1h-1and a CO selectivity of 85.8%.Ni-MOF also displays catalytic activity for photochemical CO2reduction,with the CO generation rate of 597 μmol g-1h-1and almost 100% CO selectivity.Impressively,the bimetallic CoxNiy-MOFs show enhanced catalytic performance with the increase of the Ni content.Particularly for the CO selectivity,when the Co/Ni ratio is 1/2(Co1Ni2-MOF),a high CO selectivity of 94.6% is achieved,higher than pure Co-MOF(85.8%).The CO evolution rate reaches as high as 1160 μmol g-1h-1,twice than that of Ni-MOF(Fig.2b and Tables S5 and S6),and comparable to most reported MOFs-based catalysts(Table S7 in Supporting information).No liquid product was detected by ion chromatography(Fig.S9 in Supporting information).The results demonstrated herein clearly illustrate that bimetallic strategy is effective to build high-performance MOF catalysts for photochemical CO2reduction.
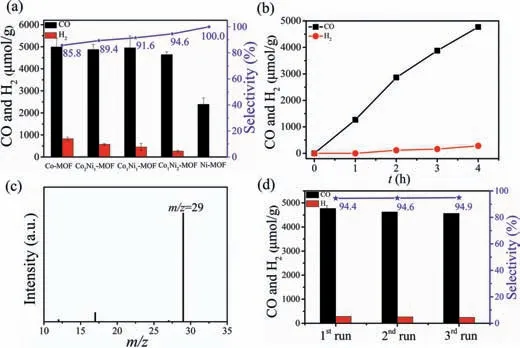
Fig.2.(a)Photocatalytic CO2 reduction with CoxNiy-MOFs;(b)Time profile of CO and H2 evolution rate,(c)Mass spectrum of the gas generated from photocatalytic CO2 reduction by using 13CO2,(d)Cycle experiments of Co1Ni2-MOF for photocatalytic CO2 reduction.Reaction conditions:catalyst(2 mg),[Ru(phen)3](PF6)2(0.43 mmol/L),TEOA(0.3 mol/L),CH3CN/H2O(v/v = 4:1;5 mL),300 W Xe lamp(λ >420 nm),4 h,25 °C.
A series of control experiments with Co1Ni2-MOF were performed to identify the key factors for photochemical CO2–to–CO conversion.As shown in Table S5,no product was detected in the absence of photosensitizer,TEOA or illumination,suggesting that the photosensitizer,sacrificial reductant,and light irradiation are all indispensable to CO2–to–CO conversion(Table S5,entries 6–8 in Supporting information).In the absence of Co1Ni2-MOF,negligible CO was detected under the same condition(Table S5,Entry 9 in Supporting information).In addition,no CO was detected when Ar instead of CO2(Table S5,Entry 10 in Supporting information),illustrating that the CO was originated from the CO2reduction.Furthermore,in order to provide direct evidence for the carbon source of evolved CO,we performed an isotopic tracing experiment by replacing CO2with13CO2.As shown in Fig.2c,the peak ofm/z= 29 assigned to13CO was observed,solidly confirm that the produced CO originates from photocatalytic CO2reduction.To get the possibly higher catalytic activity of Co1Ni2-MOF,12 mg of Co1Ni2-MOF was used for photocatalytic CO2reduction.The results show that the selectivity to CO also remains a high level of 94.7%,and 41.7 μmol CO was generated,much higher than that generated by 2 mg of Co1Ni2-MOF(9.3 μmol)under similar conditions.However,the CO yield rate did not increase accordingly(Fig.S10 in Supporting information).This may be ascribed to the small catalytic reactor,where many Co1Ni2-MOF could not fully contribute to the photocatalytic CO2reduction activity.
The photocatalytic stability is also crucial for catalysts.The cyclic catalytic experiments of Co1Ni2-MOF for photochemical CO2reduction show no noticeable activity decrease after three runs(Fig.2d).The powder X-ray diffraction(PXRD)pattern,morphology and IR spectra of Co1Ni2-MOF after photocatalytic reaction are also similar to those freshly prepared.The above results illuminate that Co1Ni2-MOF is robust during photocatalytic CO2reduction process(Figs.S11–S13 in Supporting information).To reveal the catalytically active sites within CoxNiy-MOF,the photochemical CO2reduction experiments by Co1Ni2-MOF were carried out in the absence and presence of KSCN.The consistent PXRD pattern affirmed that the Co1Ni2-MOF maintain original crystal structure after treatment with KSCN(Fig.S14 in Supporting information).As Co1Ni2-MOF show dramatic activity decrease in the presence of KSCN,the Co/Ni in Co1Ni2-MOF can be assigned the catalytic centers for photocatalytic CO2reduction(Fig.S15 in Supporting information).
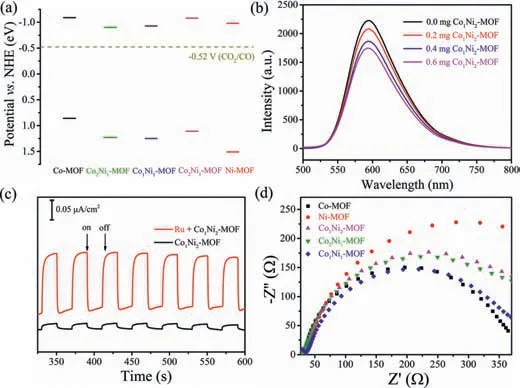
Fig.3.(a)The band structures of CoxNiy-MOFs;(b)Steady state fluorescence spectra of[Ru(phen)3]PF6 upon the addition of Co1Ni2-MOF;(c)Photocurrent response of Co1Ni2-MOF and Co1Ni2-MOF containing[Ru(phen)3]PF6;(d)Nyquist plots of CoxNiy-MOFs.
To reveal the reason of increased catalytic performance of Co1Ni2-MOF,the optical properties and band gap energies of these materials were investigated by UV-vis diffuse reflectance spectroscopy(DRS,Fig.S16 in Supporting information).According to the Tauc plot,the corresponding band gaps were calculated to be 1.95(Co-MOF),2.13(Co2Ni1-MOF),2.18(Co1Ni1-MOF),2.19(Co1Ni2-MOF)and 2.49(Ni-MOF)eVvs.NHE(Figs.S17–S21 in Supporting information).By Mott Schottky plots(Figs.S22–S26 in Supporting information),the flat band position(Vfb)were determined to be-0.99(Co-MOF),-0.8(Co2Ni1-MOF),-0.83(Co1Ni1-MOF),-0.98(Co1Ni2-MOF)and-0.88(Ni-MOF)eVvs.NHE,respectively.Since it is generally believed that the bottom of the conduction band(CB)in many n-type semiconductors is more negative by about 0.10 V than theVfb,[41–45]the CB of Co-MOF,Co2Ni1-MOF,Co1Ni1-MOF,Co1Ni2-MOF and Ni-MOF was estimated to be-1.09,-0.9,-0.93,-1.08 and-0.98 eV,respectively.Combined with the results of band gaps,the valence band(VB)of CoxNiy-MOFs were estimated to be 0.86(Co-MOF),1.23(Co2Ni1-MOF),1.25(Co1Ni1-MOF),1.11(Co1Ni2-MOF)and 1.51(Ni-MOF)eVvs.NHE.Based on the above results,the energy-band alignment of Co-MOF,CoxNiy-MOFs and Ni-MOF can be drawn in Fig.3a.Obviously,the CB potentials of CoxNiy-MOFs are more negative than the redox potential of photocatalytic CO2reduction to CO(-0.52 Vvs.NHE),indicating that CoxNiy-MOFs satisfy the thermodynamic requirement of CO2–CO conversion.In addition,the electrochemical experiments of CoxNiy-MOFs measured in 0.5 mol/L Na2SO4under Ar/CO2showed larger current in CO2than in Ar,further indicating that CoxNiy-MOFs are potential catalysts capable of catalyzing CO2reduction(Figs.S27–S31 in Supporting information).The enhanced photocatalytic CO2reduction performance of CoxNiy-MOFs over Co-MOF/Ni-MOF also highlights the advantage of dimetallic catalysts.Compared with monometallic catalysts,the introduction of the second metal will change the interficial electronic structure and charge dispersion,which thus affects the photocatalytic performance.
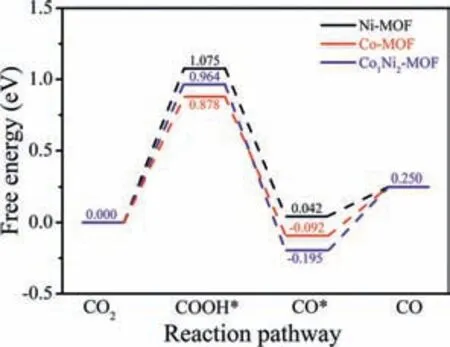
Fig.4.Calculated free-energy diagrams for the conversion of CO2 to CO.
In order to reveal the catalytic CO2reduction mechanism,room temperature photoluminescent(PL)quenching experiments of[Ru(phen)3](PF6)2were performed by addition of Co1Ni2-MOF or TEOA in a CO2degassed CH3CN/H2O solution(v/v = 4:1).As shown in Fig.3b,the PL intensity of excited[Ru(phen)3]2+*gradually decreases with the increase of the amount of Co1Ni2-MOF,which should be attributed to the electron transfer from the excited[Ru(phen)3]2+*to Co1Ni2-MOF.By contrast,no obvious fluorescence decrease was observed with the addition of TEOA(Fig.S32 in Supporting information).Therefore,the quenched mode of the excited[Ru(phen)3]2+*can be assigned to an oxidatively quenched pathway[46].Photocurrent response measurements performed in several on–off cycles at a bias of-0.2 VversusAg/AgCl showed that the Co1Ni2-MOF exhibits a small photocurrent response,while the photocurrent was greatly enhanced after addition of[Ru(phen)3](PF6)2(Fig.3c).This observation indicates the rapid migration of photogenerated electrons of excited[Ru(phen)3]2+*to Co1Ni2-MOF[41],which is consistent with the results of steady-state PL.In addition,electrochemical impedance spectroscopy(EIS)measurements showed that Co-MOF possesses a smallest capacitive loop diameter,Ni-MOF has a biggest one,and CoxNiy-MOFs are between those of Co-MOF and Ni-MOF(Fig.3d).These results indicate that Ni-MOF has the largest charge-transfer resistance,which is consistent with the relatively lower catalytic activity of Ni-MOF.
Based on the above discussion,the photocatalytic mechanism of CoxNiy-MOFs for CO2reduction was proposed.Upon visible-light irradiation,the photosensitizer[Ru(phen)3]2+is excited to form[Ru(phen)3]2+*,which is immediately quenched by Co1Ni2-MOF to generate the oxidized[Ru(phen)3]3+.Meanwhile,the excited electrons are transferred to CO2molecules adsorbed in Co1Ni2-MOF,leading to the activation and reduction of CO2.The oxidized[Ru(phen)3]3+is reduced to[Ru(phen)3]2+by TEOA,to complete the entire catalytic cycle.
DFT calculations were further performed to understand the catalytic performance of Ni-MOF,Co1Ni2-MOF and Co-MOF for the conversion of CO2to CO(Fig.S33 in Supporting Information).The proposed reaction pathways and the corresponding free-energy diagrams are shown in Fig.4.First,the CO2molecule undergoes a proton coupled electron transfer(PCET)process to form the COOH*intermediate(asterisk denotes an adsorption status),which is generally regarded as the rate-limiting step.The free energy change(ΔG)for this step over Ni-MOF,Co1Ni2-MOF and Co-MOF is 1.075,0.964 and 0.878 eV,respectively.The results demonstrate that Co-MOF and Co1Ni2-MOF have appropriate ΔGvalues in the ratelimiting step,while Ni-MOF exhibits a relatively higher ΔGvalue.Then,the COOH*intermediate undergoes the second PCET process,forming*CO and a H2O molecule.The ΔGvalues for this step over the three catalysts are negative(–1.033,–1.159 and–0.970 eV,respectively),indicating this step is spontaneous.Finally,the CO desorbs from the catalyst surface.For Ni-MOF,Co1Ni2-MOF,and Co-MOF,the CO desorption is slightly endothermic(0.208,0.445 and 0.342 eV,respectively).Overall,the DFT calculations show that Co-MOF and Co1Ni2-MOF have better catalytic capacity for CO2to CO conversion than Ni-MOF,which well supports the experimental results.
In summary,a series of isostructural CoxNiy-MOFs based on H3IDC and 4,4′-bpy were synthesized for photocatalytic CO2reduction.The experimental and DFT calculation results reveal that bimetallic strategy is an effective way to optimize the photocatalytic performance of Co-MOF.The optimized bimetallic Co1Ni2-MOF exhibits remarkably enhanced photocatalytic performance for CO2–to–CO conversion,with an evolution rate of 1160 μmol g-1h-1and a high selectivity of 94.6%,superior to those of monometallic Ni-MOF and Co-MOF.This work paves a new way for developing high-performance MOFs-based catalysts for photochemical CO2reduction.
Declaration of competing interest
We declare that we have no competing interests for the manuscript entitled“Enhancing photocatalytic performance of metal-organic frameworks for CO2reduction by bimetallic strategy”.
Acknowledgments
This work was financially supported by the National Key R&D Program of China(No.2017YFA0700104),the National Natural Science Foundation of China(Nos.22071182,21861001,21931007 and 21790052),the 111 Project of China(No.D17003)and the Science&Technology Development Fund of Tianjin Education Commission for Higher Education(No.2018KJ129).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.035.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
