Boosting the photoelectrochemical water oxidation performance of bismuth vanadate by ZnCo2O4 nanoparticles
Jingwei Hung,Yni Wng,Kiyi Chen,Tingting Liu,Qizho Wng,c,*
a School of Environment Science and Engineering,Chang’an University,Xi’an 710064,China
b College of Chemistry and Chemical Engineering,Northwest Normal University,Lanzhou 730070,China
c Tianjin Key Laboratory of Building Green Functional Materials,Tianjin Chengjian University,Tianjin 300384,China
ABSTRACT Due to the involvement of four-electron transfer process at photoanode,water oxidation is the ratelimiting step in water splitting reaction.To settle this dilemma,ZnCo2O4 nanoparticles are combined with BiVO4 to form a p-n ZnCo2O4/BiVO4 heterojunction photoanode,which is proved by an input voltage-output current test.The built-in electric field formed within the heterojunction structure promotes the effective separation of electrons and holes.ZnCo2O4 is also an effective water oxidation cocatalyst,since it could cause the holes entering the electrode/electrolyte interface rapidly for the subsequent water oxidation reaction.The photocurrent density of ZnCo2O4/BiVO4 composite photoanode reaches 3.0 mA/cm2 at 1.23 V vs. RHE in 0.5 mol/L sodium sulfate under AM 1.5G simulated sunlight,about 2.1 times greater than that of BiVO4(1.4 mA/cm2).These results suggest the potential of ZnCo2O4 nanoparticles for improving photoelectrochemical water splitting anode materials.
Keywords:Photoelectrochemical water oxidation BiVO4 ZnCo2O4 p-n junction
The conversion of solar energy into hydrogen by photoelectrochemical(PEC)water splitting is a promising way to produce hydrogen,which can simultaneously solve the current energy shortage and environmental problems caused by fossil fuel combustion[1–3].In the process of PEC water splitting,water oxidation(producing oxygen)and water reduction(producing hydrogen)reaction are involved.The water oxidation reaction involves a kinetic unfavorable four-electron transfer process,which determines the entire water splitting reaction rate[4,5].Researches are mainly focused on photoanodes where water oxidation reaction takes place.Metal oxides such as TiO2[6,7],Fe2O3[8,9],WO3[10],ZnO[11]and BiVO4[12-14]are often used as photoanode materials for PEC water oxidation.Among them,BiVO4is a very excellent photoanode material with beneficial energy band structure and a band gap of 2.4 eV,enabling it to absorb part of visible light[15-17].However,the slow migration rate of photogenerated electrons and holes in BiVO4and sluggish kinetics for water oxidation render BiVO4far below its theoretical photocurrent density[18,19].Strategies such as controlling morphology[20],constructing heterostructures[10]have been used to improve the performance of BiVO4.Besides,loading cocatalyst is an effective way to improve the surface reaction kinetics of BiVO4for PEC water splitting[21,22].
Cobalt-based cocatalysts,such as cobalt oxides[23–25],cobalt hydroxides[26,27],cobalt phosphide[28,29],Co-Pi[30,31],Cobased double hydroxide(LDH)[32],have been intensively studied as water oxidation catalysts due to their outstanding catalytic performance.Specially,ternary spinel MCo2O4(M = Ni,Mn,Zn,etc.)have improved bearing conductivity and possessing more active sites than Co3O4,resulting in a better water oxidation capability[33–35].Among them,ZnCo2O4has a definite structure in which Zn2+only replaces Co2+in the tetrahedral sites in Co3O4,rarely affecting the Co3+active site in the octahedral sites in Co3O4[36].Therefore,as compared to Co3O4,the use of ZnCo2O4could reduce the use of rare and relative expensive Co element without the compromise of catalytic activity.
In addition to loading cocatalyst,enhancing built-in electric field of a photoanode by constructing a heterojunction structure is also usually adopted.WO3@BiVO4heterojunction photoanode[37],Fe2O3/BiVO4heterojunction photoanode[38],g-C3N4@BiVO4heterojunction photoanode[39]etc.are recently reported to be favorable for the separation of photo-generated carriers due to the formation of built-in electric field between these different semiconductors in heterojunction.However,the water oxidation reaction on surface of these photoanodes is still sluggish.To address this problem,cocatalysts are still needed.For example,NiFe-LDH was used to load on Fe2O3/BiVO4heterojunction photoanode[40].WO3/BiVO4was modified by FeOOH to construct ternary WO3/BiVO4/FeOOH hierarchical photoanode[41].Considering the complicated fabrication process of above mentioned photoanodes,loading a semiconductor cocatalyst that could enhance the built-in electric field as well as act as cocatalyst is a new guiding ideology to construct a high performance photoanode for water oxidation.

Fig.1.SEM images of(a)BiVO4,(b)ZnCo2O4/BiVO4-35.TEM images of(c)BiVO4,(d)ZnCo2O4/BiVO4-35.(e,f)HR-TEM images of ZnCo2O4/BiVO4-35.
In this work,ZnCo2O4nanoparticles,acting as a p-type semiconductor and a cocatalyst simultaneously,were loaded on BiVO4to form ZnCo2O4/BiVO4composite photoanode by electrophoretic deposition.The formation of p-n junctions between ZnCo2O4and BiVO4is systematically confirmed,which introduces built-in electric field that is beneficial to the separation of photogenerated carriers.This composite photoanode shows high photocurrent for PEC water oxidation due to the improvement in charge separation effi-ciency and surface reaction efficiency.
As can be seen from Fig.1a,BiVO4presents a coral-like structure composed of porous nanoparticles.The nanoparticles are interconnected on the surface of the FTO to form pores and channels.These abundant channels facilitate the contact between the semiconductor and electrolyte.From Fig.1b,it can be clearly seen that ZnCo2O4nanoparticles are uniformly loaded onto the surface of BiVO4film by the electrophoretic deposition method.TEM image(Fig.1c)shows that BiVO4has a pore structure and smooth surface.However,a thin layer of nanoparticles appears on the surface of BiVO4after loading ZnCo2O4as shown in Fig.1d.Figs.1e and f show the HR-TEM images of ZnCo2O4/BiVO4photoanode.The spacing of 0.20 nm and 0.48 nm correspond to the(400)plane of ZnCo2O4(PDF card No.23-1390)and the(110)plane of BiVO4(PDF No.14-0688),respectively,demonstrating the successful combination of ZnCo2O4nanoparticles and BiVO4film to form ZnCo2O4/BiVO4photoanode.
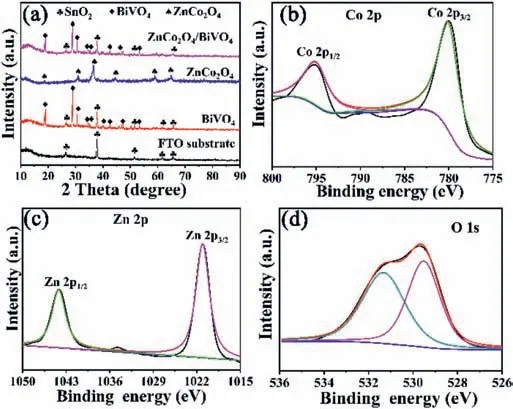
Fig.2.(a)XRD patterns of FTO substrate,BiVO4,ZnCo2O4 and ZnCo2O4/BiVO4 composites and XPS spectra of(b)Co 2p,(c)Zn 2p,(d)O 1s in ZnCo2O4/BiVO4.
In order to clearly grasp the crystal structure of the sample,we performed XRD testing on the photoanode material.As shown in Fig.2a,FTO substrate has the diffraction peaks located at 26.6°,37.8°,51.8°,61.7° and 65.7° corresponding to the(110),(200),(211),(310)and(301)lattice diffractions of SnO2(JCPDS No.46–1088),respectively.BiVO4shows diffraction peaks located at 18.7°,28.8°,30.5°,34.5°,35.2°,40.0°,42.5°,46.0°,47.3°,50.3°,53.3°,58.3° and 59.3°,indicating the formation of monoclinic phase BiVO4(JCPDS:14-0688).The synthesized ZnCo2O4powders have diffraction peaks mainly located at 19.0o,31.2o,36.8o,44.7o,59.3oand 65.1o.These diffraction peak positions correspond to the(111),(220),(311),(400),(511)and(440)crystal planes of the ZnCo2O4(JCPDS:23-1390),proving that the ZnCo2O4nanoparticles were successfully synthesized.The XRD pattern of ZnCo2O4/BiVO4composite electrode does not show obvious characteristic diffraction peaks of ZnCo2O4due to small loading amount of ZnCo2O4nanoparticles on BiVO4.X-ray photoelectron spectroscopy(XPS)test was used to determine the elements status of ZnCo2O4/BiVO4electrode.It can be seen from Fig.2b that two peaks of Co 2p in ZnCo2O4are located 795.0 and 780.0 eV,respectively belonging to the Co 2p1/2and Co 2p3/2peaks of Co3+in the octahedral site,and proving that the cobalt in the compound is+3 valence[36].The binding energies of the Zn 2p3/2and Zn 2p1/2peaks in the compounds are located at 1021.1 eV and 1044.2 eV,indicating that Zn in the ZnCo2O4is+2 valence in the compound(Fig.2c)[42].The O 1s spectrum in the ZnCo2O4/BiVO4photoanode can be fitted into two diffraction peaks(Fig.2d).The peak at 531.4 eV in the spectrum is derived from adsorbed OH-on the electrode,while the peak at 529.7 eV is assigned to oxide lattice[43].
The light absorption range of ZnCo2O4/BiVO4photoanode is broadened slightly by supporting ZnCo2O4cocatalyst(Fig.S1a in Supporting information).The light absorption intensity of the composite is also higher than that of BiVO4,indicating that ZnCo2O4improves the light absorption capacity of BiVO4.The band gap of BiVO4is about 2.46 eV,whilst ZnCo2O4/BiVO4electrode shows an apparent band gap of 2.43 eV,demonstrating ZnCo2O4can improve the light utilization efficiency of BiVO4photoanode(Fig.S1b in Supporting information).In order to investigate whether the catalytic active area of BiVO4film would be changed after loading ZnCo2O4,cyclic voltammetry(CV)tests of BiVO4and ZnCo2O4/BiVO4electrodes were carried out at different scan rates as shown in Figs.S1c and e(Supporting information).The catalytic active area of BiVO4and ZnCo2O4/BiVO4electrodes can be estimated from double layer capacitances that are linearly related to the slopes of difference values between anode current(Ja)and cathode current(Jc)in CVs against the scan rate as shown in Figs.S1d and f(Supporting information).Taking the current difference as y-axis and scanning rate as x-axis,the linear slope is twice over the double layer capacitances[44].It can be seen from Figs.S1d and f that the slope of ZnCo2O4/BiVO4is larger than that of BiVO4,demonstrating the catalytic active area of BiVO4photoanode increases after loading ZnCo2O4.
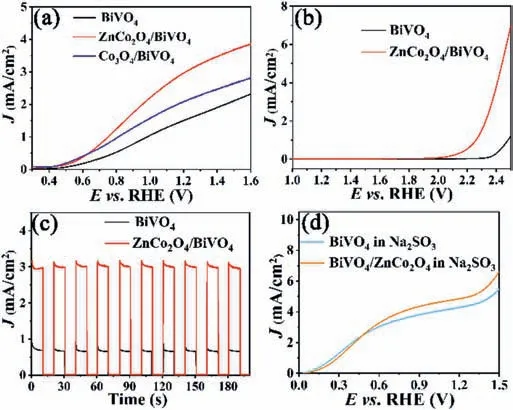
Fig.3.LSV curves of BiVO4,ZnCo2O4/BiVO4 photoelectrodes(a)with and(b)without light irradiation;(c)Chopped I-t curves of BiVO4,ZnCo2O4/BiVO4 photoelectrodes at 1.23 V vs. RHE in 0.5 mol/L Na2SO4.(d)LSV curves of photoanodes for sulfite oxidation(with 1 mol/L Na2SO3).
The LSV curves of BiVO4and ZnCo2O4/BiVO4photoanodes were tested under AM 1.5G simulated sunlight(100 mW/cm2)from back side of the sample.As shown in Fig.3a,the photocurrent density of BiVO4is 1.4 mA/cm2at 1.23 Vvs.RHE,while the photocurrent density of 3.0 mA/cm2is obtained for the ZnCo2O4/BiVO4photoanode.Besides,the onset potential of BiVO4photoanode shiftsca.0.1 V after loading ZnCo2O4.Co3O4/BiVO4photoanode was also fabricated for comparison using the same electrophoretic deposition method as shown in Fig.3a,giving a photocurrent of 2.2 mA/cm2at 1.23 Vvs.RHE at same reaction condition.Obviously,ZnCo2O4shows more higher catalytic activity than that of Co3O4.The photocurrent density of ZnCo2O4/BiVO4is 2.1 times that of BiVO4.This indicates that the performance of BiVO4for PEC water splitting can be enhanced by ZnCo2O4.The comparison of ZnCo2O4/BiVO4photoanode with other BiVO4and Co based photoanodes is shown in Table S1(Supporting information).In order to investigate the effect of deposition time on the catalytic performance of ZnCo2O4/BiVO4,the electrophoretic deposition time of ZnCo2O4was set be to 15,20,25,30,35 and 40 s for comparison.These composite photoanodes are named as ZnCo2O4/BiVO4-15,ZnCo2O4/BiVO4-20,ZnCo2O4/BiVO4-25,ZnCo2O4/BiVO4-30,ZnCo2O4/BiVO4-35 and ZnCo2O4/BiVO4-40 accordingly.As demonstrated in Fig.S2(Supporting information),the photocurrent density of ZnCo2O4/BiVO4-35 has the highest photocurrent at 1.23 Vvs.RHE,indicating that ZnCo2O4/BiVO4-35 has the best PEC water splitting performance.When the deposition time is less than 35 s,small photocurrents were obtained probably due the lower content of ZnCo2O4.However,when the amount of the supported ZnCo2O4is excessive,the transport of holes to the electrode surface is hindered,leading to low photocurrents.ZnCo2O4/BiVO4-35 was used in all the experiments without further description.Fig.3b is LSV curves of the electrodes in the absence of light.The initial potential of BiVO4is about 2.4 V,while the initial potential of the ZnCo2O4/BiVO4composite photoanode is about 2.1 V.The initial potential of ZnCo2O4/BiVO4negatively shifts 300 mV compared to that of BiVO4.This indicates that ZnCo2O4can reduce the overpotential of PEC water splitting.The choppedI-tcurves of the photoanodes were tested at 1.23 Vvs.RHE.It can be clearly seen from theI-tcurves in Fig.3c that the photocurrent density of the composite ZnCo2O4/BiVO4photoanode is stable and higher than that of BiVO4at constant voltage.Fig.3d shows the LSV curves of BiVO4and ZnCo2O4/BiVO4photoanodes tested in 0.5 mol/L Na2SO4solution with 1 mol/L Na2SO3.As a hole trapping agent,Na2SO3is easily oxidized by holes.When Na2SO3is present,the photogenerated holes reaching the surface of BiVO4and ZnCo2O4/BiVO4are all involved in the oxidation of Na2SO3.Therefore,the photocurrent density for Na2SO3oxidation represents the amount of photogenerated holes reaching on the surface of BiVO4and ZnCo2O4/BiVO4.In the presence of Na2SO3,the photocurrent density of ZnCo2O4/BiVO4is higher than that of BiVO4,indicating that the amount of photogenerated holes reaching the surface of ZnCo2O4/BiVO4is higher than that of BiVO4.This means that the ZnCo2O4/BiVO4heterojunction structure promotes the separation of photogenerated charges.Fig.S3(Supporting information)shows the chopped LSV curves of BiVO4and ZnCo2O4/BiVO4with chopped light illumination.When the simulated sunlight was used to illuminate the photoanode,the photocurrent density of the BiVO4and ZnCo2O4/BiVO4electrodes immediately increased.When the light is blocked,the photocurrent density of the both photoanodes immediately reduced to almost 0 mA/cm2,suggesting that the photoanodes is very sensitive to light.The photocurrent densities of the electrodes are disparate at different voltages.At the same potential,the photocurrent density of the ZnCo2O4/BiVO4photoanode is distinctly higher than that of the BiVO4electrode,implying ZnCo2O4nanoparticles can significantly improve the PEC water oxidation performance of BiVO4photoanode.
The efficiency of PEC water splitting is determined by light capture efficiency,charge separation efficiency in the bulk(ηbulk)and surface reaction efficiency(ηsurface)of photoelectrode.To analyze these efficiencies,the maximum theoretical photocurrentsJabsunder AM 1.5G simulated sunlight was calculated from UV diffuse reflection data of BiVO4and ZnCo2O4/BiVO4photoanodes(Fig.S4 in Supporting information).ηbulkwas calculated through dividing the photocurrent for Na2SO3oxidation(JNa2SO3,Data in Fig.3d)byJabs[44].ηsurfacerepresents the ratio of surface holes participated in the reaction,which is obtained through dividing photocurrent for water oxidation(data in Fig.3a)byJNa2SO3.Fig.4a shows thatηbulkof ZnCo2O4/BiVO4reaches 70% at 1.23 Vvs.RHE,while theηbulkof pure BiVO4is 50%,indicating the loading of ZnCo2O4can enhance charge separation efficiency of BiVO4.Fig.4b represents theηsurfaceof the ZnCo2O4/BiVO4photoanode reaches 50% at 1.23 Vvs.RHE,while theηsurfaceof the BiVO4photoanode is merely 25%.As a water oxidation cocatalyst,ZnCo2O4improves the kinetics of water splitting reaction.Based on LSV curve shown in Fig.3a,applied bias photon-to-current efficiency(ABPE)values can be obtained[45].In Fig.4c,the maximum efficiency of the BiVO4film is 0.1% at 1.1 Vvs.RHE,while the ZnCo2O4/BiVO4photoanode attains a maximum ABPE value of 0.6% at 0.9 Vvs.RHE.The maximum efficiency of ZnCo2O4/BiVO4composite photoanode is 6 times that of BiVO4.This indicates that ZnCo2O4improves the photoelectrochemical performance of BiVO4.The IPCE values of BiVO4and ZnCo2O4/BiVO4photoanodes were tested at 1.23 Vvs.RHE.As can be seen from Fig.4d,the IPCE value of ZnCo2O4/BiVO4electrode is higher than that of BiVO4in the wavelength range of 320~510 nm.The IPCE value of ZnCo2O4/BiVO4electrode can reach 40% at 380 nm.Absorbed photon-to-current conversion effi-ciency(APCE)of BiVO4and ZnCo2O4/BiVO4electrodes were calculated through dividing IPCE values by light absorption values and the resulting APCE values are given in Fig.S5(Supporting information).Almost half absorbed photons can be converted to current by ZnCo2O4/BiVO4electrode,about 20% higher than that of BiVO4,indicating that the light utilization rate of the electrode is improved after loading ZnCo2O4.To confirm the reliability of our tests,the integrations of IPCE of BiVO4and ZnCo2O4/BiVO4photoanodes with the AM 1.5G solar current were carried out.As shown in Figs.4e and f,the photocurrents of BiVO4and ZnCo2O4/BiVO4photoanodes obtained by integrating AM 1.5G solar currents are 1.8 and 3.2 mA/cm2,which are approximate equivalent to the photocurrents in LSV measurement,indicating the tests are reliable.
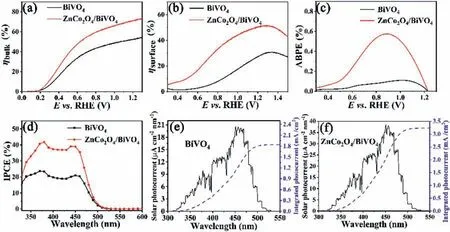
Fig.4.(a)Charge separation efficiency in the bulk(ηbulk)and(b)surface reaction efficiency(ηsurface)of BiVO4 and ZnCo2O4/BiVO4 photoanodes.(c)ABPE curves of pure BiVO4 and ZnCo2O4/BiVO4 photoelectrodes.(d)IPCE values of BiVO4 and ZnCo2O4/BiVO4 photoanodes;Solar photocurrents of(e)BiVO4 and(f)ZnCo2O4/BiVO4 anodes(left ordinate)and integrated photocurrent at 1.23 V vs. RHE(right ordinate).
The relative magnitude of the charge transfer rate of BiVO4and ZnCo2O4/BiVO4photoanodes was investigated by electrochemical impedance spectroscopy(EIS).The Nyquist plots of EIS spectra of BiVO4and ZnCo2O4/BiVO4photoanodes are a semi-circular arc whatever in dark conditions or under AM 1.5G simulated sunlight(Figs.S6a and b in Supporting information).The impedance of the circuit can be explained by the simplified Randles circuit model,in which RΩrepresents the solution resistance.Rctrepresents charge-transfer resistance at the interface of semiconductor and electrolyte andCctrepresents the capacitance of the bulk BiVO4[46].The smaller the diameter of the arc,the stronger the charge transfer capability is.ZnCo2O4/BiVO4exhibits a smaller arc diameter than BiVO4,indicating that the charge transfer rate in the photoanode increases after the ZnCo2O4cocatalyst is supported.It is worth noting that the arc diameter is smaller under light irradiation,indicating charge transfer become easy with light irradiation.The PEC water splitting reaction of ZnCo2O4/BiVO4photoanode was carried out in a closed reactor.Prior to the water splitting reaction,the reactor was purged with N2for 1 hour to remove air from the reactor.The PEC water splitting reaction was carried out for 3 h at 1.23 Vvs.RHE.1 mL of gas was withdrawn from the reactor every half hour,and the gas was quickly pumped into the gas chromatograph to detect the amount of hydrogen and oxygen produced.After 3 h of reaction,153.7 μmol of H2and 79.8 μmol of O2were produced with the mole ratio of H2to O2of 1.9:1(Fig.S6c in Supporting information).The average Faradaic efficiency of PEC cell for H2and O2production(Fig.S6d in Supporting information)is about 90% and 86%,respectively,which indicates that the photoanode ZnCo2O4/BiVO4has good water splitting efficiency.
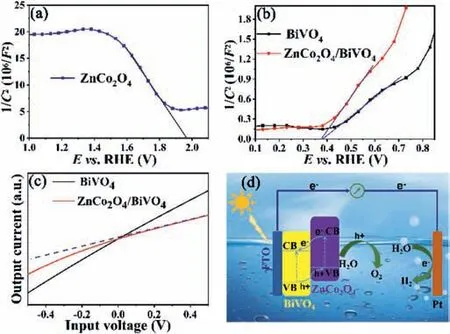
Fig.5.Mott-Schottky plots of(a)ZnCo2O4 and(b)BiVO4,ZnCo2O4/BiVO4;(c)Input voltage-output current characteristic curves of BiVO4 and ZnCo2O4/BiVO4 anodes.The dotted line is used as a linear reference;(d)Heterojunction illustration and water splitting mechanism of ZnCo2O4/BiVO4 photoanode.
Mott-Schottky tests were performed to study the semiconductor type of ZnCo2O4,BiVO4and ZnCo2O4/BiVO4photoanodes.As demonstrated in Fig.5a,ZnCo2O4shows a negative slope,indicating that ZnCo2O4is p-type semiconductor.While BiVO4photoanode shows positive slope,meaning the n-type property of BiVO4(Fig.5b).The intersection of Mott-Schottky curves and x-axis are close to 0.38 Vvs.RHE with/without loading ZnCo2O4,demonstrating the flat band potentials(Vfb)of BiVO4photoanodes are 0.38V.
TheVfbvalue of BiVO4is 0.38V.Considering that theVfbof ntype semiconductor is slightly positive(~0.1-0.3 V)than the CB potential,the conduction band(CB)potential of BiVO4is about 0.18 V[47].The band gap of BiVO4is 2.46 eV(Fig.S1b).According to the relationship between CB and valence band(VB),the VB of BiVO4can be calculated to be 2.64 V.The p-type semiconductor ZnCo2O4has aVfbvalue of 1.98 V(Fig.5a).Because VB position of a p-type semiconductor is approximately 0.2 V higher than itsVfbvalue,the VB position of ZnCo2O4is 2.18 V.ZnCo2O4has a band gap width of 2.10 eV,so the CB position of ZnCo2O4is calculated to be 0.08 V.The staggered energy levels of BiVO4and ZnCo2O4allows them to form p-n junction.
The formation of p-n junctions between BiVO4and ZnCo2O4was proved by input voltage-output current test as shown in Fig.5c.BiVO4photoanode shows a linear relationship between input voltage and output current due to the ohmic contact between BiVO4and FTO substrate[48].On the contrary,ZnCo2O4/BiVO4anode represents a nonlinear relation between output current and input voltage,indicating the interface contact type between ZnCo2O4and BiVO4is p-n junction[49].Built-in electric field in the p-n junction favors the transfer of holes from BiVO4to ZnCo2O4for water oxidation.
The PEC water splitting mechanism of ZnCo2O4/BiVO4is proposed as shown in Fig.5d.BiVO4and ZnCo2O4are excited to generate electrons and holes by illuminating from the back of the photoanode.The electrons in conduction band of ZnCo2O4migrate to the conduction band of BiVO4,and then transfer to the counter electrode under external circuit for H2evolution reaction.The holes in valence band of BiVO4transfer to the valence band position of ZnCo2O4with the assistance of built-in electric field for water oxidation reaction(O2evolution reaction).After a long-time reaction,the photocurrent of ZnCo2O4/BiVO4anode presents only a small decrease(Fig.S7 in Supporting information).
In summary,we have constructed a p-n heterojunction ZnCo2O4/BiVO4photoanode by a simple electrophoretic deposition method.The formation of p-n junction between BiVO4and ZnCo2O4is proved by input voltage-output current test.Compared with BiVO4,this composite photoanode ZnCo2O4/BiVO4has better photoelectrochemical water splitting performance.The photocurrent density of ZnCo2O4/BiVO4reaches 3.0 mA/cm2at 1.23 Vvs.RHE,about 2.1 times greater than that of BiVO4.The formation of a p-n heterojunction between the BiVO4and ZnCo2O4improves the separation efficiency of carriers while the cocatalyst ZnCo2O4accelerates the surface reaction kinetics,leading to enhanced charge separation efficiency and surface reaction efficiency.After 3 h reaction,the produced H2and O2achieve 153.7 and 79.8 μmol,respectively.All results demonstrate that ZnCo2O4can boost the photoelectrochemical water oxidation performance of BiVO4.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China(Nos.21808189 and 21663027),Natural Science Basic Research Fund of Shaanxi Province(No.2020JZ20),and Fundamental Research Funds for the Central Universities of Chang’an University(No.300102299304).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.08.082.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
