A bifunctional vinyl-sulfonium tethered peptide induced by thio-Michael-type addition reaction
Hongkun Xu,Xun Qin,Yping Zhng,Chun Wn,Rui Wng,Zhnfeng Hou,Xiofeng Ding,Hiling Chen,Ziyun Zhou,d,Yng Li,Chenshn Lin,Feng Yin,*,Zigng Li,,*
a State Key Laboratory of Chemical Oncogenomics,School of Chemical Biology and Biotechnology,Peking University Shenzhen Graduate School,Shenzhen 518055,China
b Pingshan translational medicine center,Shenzhen Bay Laboratory,Shenzhen 518055,China
c Anhui Medical University,Hefei 230032,China
d Cancer Hospital Chinese Academy of Medical Sciences,Shenzhen Center,Shenzhen 518000,China
1 These authors contributed equally to this work.
ABSTRACT The modification and functionalization of peptides is of great significance in modern biotechnology and drug development.Here we report a highly reactive Michael-type warhead for the covalently modification of cysteine on peptide and protein.By installing a vinyl group onto a methionine residue of peptide,the produced vinyl sulfonium can be efficiently nucleophilic added by appropriate cysteine residue of this peptide,and thus yield a cyclized peptide.This peptide cyclization strategy was proven to exhibit improved cell penetration and good stability.Moreover,a peptide ligand bearing vinyl sulfonium could covalently bind to the cysteine in the target protein,indicating the potential of vinyl sulfonium as a novel warhead for developing covalent peptide inhibitor.
Keywords:Vinyl sulfonium Michael-type addition Peptide cyclization Covalent peptide inhibitor Proximity-induced ligation
We constructed a novel vinyl-sulfonium tethered peptide with dual functional application.Cyclization with improved cellular uptake and good stability could be developed by thio-Michael-type addition reaction involving a cysteine residue in the peptide.Moreover,the vinyl-sulfonium in peptide could alkylate the thiol in cysteine residues of proteins of interestviathe Michael-type addition,which may be a potential strategy for covalent peptide inhibitor design.
Peptide drugs overcome the weak binding affinity of small molecule and low bioavailability of bio-molecule drugs,which have broad application prospects[1].However,linear peptides have a poor performance due to the variability of configuration and low activity[2-5].Therefore,peptide modification is important to enhance peptide activity.Currently,the general strategies for improving the activity of peptide contain cyclization,covalent inhibition and so on[6,7].Peptide cyclization strategies have been proven to improve their biophysical properties,including stability,cell permeability and target binding affinity[8,9].Peptide covalent strategies safeguard the reaction specificity and efficacy of the peptide inhibitor toward its target[6,10-13].Nevertheless,most of the cyclization and covalent methods are involved with non-natural amino acid,which may difficult to synthesize and may be biologically incompatible[14-24].Hence,developing a novel peptide modification with multiple potential based on natural amino acid is of great significance.
Sulfonium is a type of active functional groups that are commonly found in organisms,whereS-adenosylmethionine(SAM)is the most important methyl donor for biological methylation reactions(nucleotide,protein,lipid and other biological molecules,etc.),playing an important role in the post translational modification[25].Currently,there have developed several peptide modification methods based on sulfonium,which show a broad application prospect[26,27].Deming’s research took the lead in realizing the functionalization of methionine(Met)[28].Previously,we developed a general peptide macrocyclization strategy that involves selective methionine dialkylation/dealkylation process(Scheme 1a)[29,30].The methionine bis-alkylation of peptide showed time-dependent dealkylation under reductive conditions[29].The cysteine-methionine bis-alkylation could generate a cyclic peptide with an on-tether sulfonium warhead,which may further undergo proximity promoted Cys-substitution with the available Cys-in the vicinity of the binding pocket of proteins of interest(POI)[30].Moreover,Bernardes’s team reported an ultrafast Cysreactive reagent,vinyl/alkynyl pyridinium,as an excellent strategy for protein modification[31].Inspired by the unique reactivity of the sulfonium center and the combination of peptide cyclization and the installation of a selectively reacting moiety in one simple design,we attempted to introduce a vinyl sulfonium center to generate a novel warhead for peptide and protein modification.We found the formed vinyl sulfonium center could trigger a very facile thio-Michael-type addition under physiological conditions.When peptide contains a cysteine,cyclization with improved cellular uptake and good stability would occur by thio-Michael-type addition reaction involving a cysteine residue in the peptide.Whereas a free vinyl-sulfonium in peptide could alkylate the thiol in cysteine residues of POIviathe Michael-type addition,which may be a potential strategy for the design of covalent peptide inhibitor(Scheme 1b).
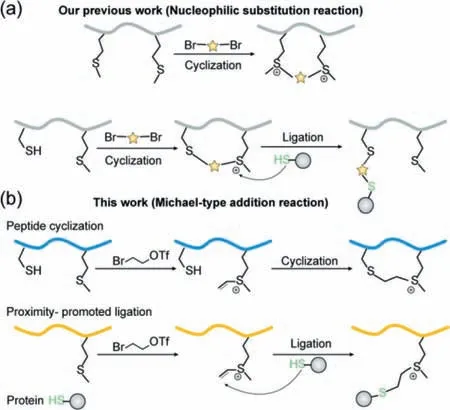
Scheme 1.(a)Our previous report of chemo-selective methionine bis-alkylation and cysteine-methionine bis-alkylation through Nucleophilic substitution reaction.(b)Schematic presentation of sulfonium tethered peptide macrocyclization and proximity-promoted protein ligation through Michael-type addition reaction..
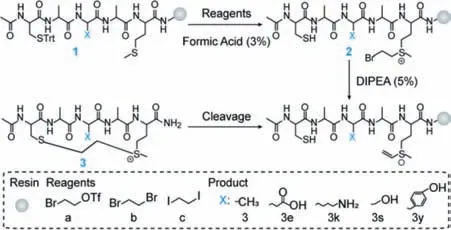
Scheme 2.Cyclization of peptides by vinyl sulfonium.
The synthesis of vinyl sulfonium requires dehydrohalogenation after alkylation with a haloethyl reagent(Scheme 2).Thus,we examined different haloethyl reagent,including 2-bromoethyltrifluoromethanesulphonate,1,2-dibromoethane and 1,2-diiodoethane,and peptide sequence in this section(Scheme 2).Obviously,peptide I-a showed the highest conversion of cyclization,suggesting 2-bromoethyl trifluoromethanesulphonate is the optimal substrate(Table 1).Besids,we analyzed the conversion of cyclized peptides with different ring size([i,i+1],[i,i+2],[i,i+3],[i,i+4],[i,i+7])and found thatonly the peptides constrained as[i,i+4]format has the higher conversion rate than others.Therefore,we chose the[i,i+4]ring size for later research.To verify the amino acid residues tolerance of this method,more sequence comprising glutamic acid(Glu),lysine(Lys),serine(Ser)and tyrosine(Tyr),were utilized as models for the study(Scheme 2).Most of the peptides were cyclized with moderate to high conversion(62%-78%)(Table 1).This result indicated that cyclization method of vinyl sulfonium would be suitable for the construction of complex cyclic peptides.Next,we applied the reaction between vinyl sulfonium of tetrahydrothiophene and ethyl mercaptan to optimize the condition,especially base.To validate the reactivity of vinyl sulfonium,a reaction between stoichiometric amounts of Ac-Cys-OH(10 mmol/L)and vinyl sulfonium 4(20 mmol/L)was conducted and monitored by1H NMR spectroscopy in Na3PO4buffer(20 mmol/L)in D2O(Fig.1a,Fig.S1 in Supporting information for additional detail).The reaction was complete within 10 min,and an ultrafast kinetic constant(kobs~116.5 L mol-1s-1)was observed,suggesting the high reactivity of vinyl sulfonium with Cys-side chain,and thus motivated its application in native protein modification.This result was been further validated by computational calculation of the model reaction between vinyl sulfonium 4 and ethanethiol,which was consistent with its high reactivity with Cys-side chain(Fig.1b and Fig.S2 in Supporting information).In addition,the circular dichroism(CD)spectroscopy showed that the chiral center in cyclic peptide had little effect on the peptide’s secondary structure(Fig.S3 in Supporting information).
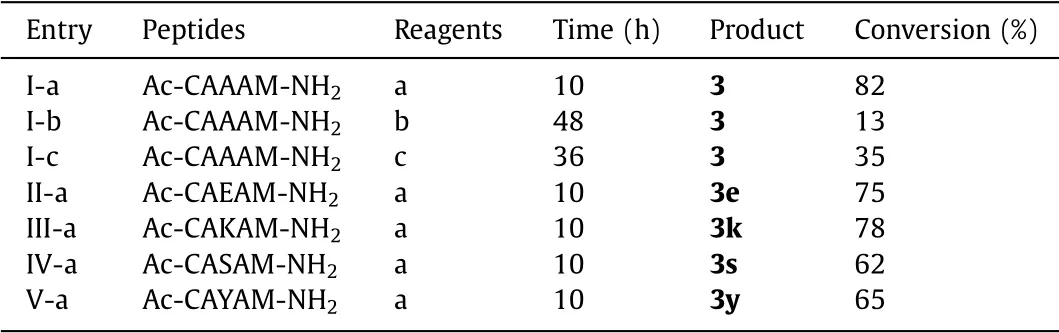
Table 1 Seven different peptides were tested for functional residue tolerance.
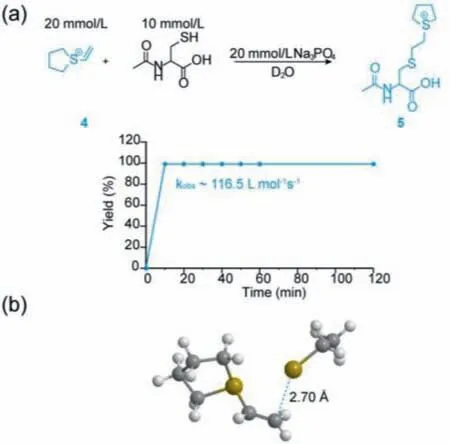
Fig.1.(a)The kinetic study between vinyl sulfonium 4 and Ac-Cys-OH.The observed second-order rate constants kobs were derived from the slope of a linearlyfitted plot of the inverse of the Cys-concentration(1/[E]) versus time.(b)Structure of the transition state of the model reaction between vinyl sulfonium 4 and ethanethiol by computer-assisted calculation.
Next,we investigated the changes in the biophysical properties of cyclic peptides constructed by this method.Previous studies have demonstrated that the methionine alkylation was easily reversible using appropriate reductants under mild conditions(PBS,pH 7.4,37 °C)[28-30].Here we studied the reduction of vinyl sulfonium cyclic peptides by 2-mercaptopyridine.The LC analysis clearly revealed that the relatively little reduction of cyclic sulfonium tethered peptides(1 mmol/L)with 2-mercaptopyridine,indicating its relatively reductive stability under strong reducing environment(Fig.S4 in Supporting information).PDZΔRGS3 is a commonly used model protein for sulfonium tethered peptide(PDZΔRGS3 denotes the PDZ domain of PDZ-RGS3)[30].To further investigate the stability of cyclic peptide,we constructed two cyclic peptide ligands with different cyclization sites for PDZΔRGS3 as shown in Fig.S5a(Supporting information).In our previous work,when the cysteine-methionine bis-alkylation cyclized peptide ligand selectively recognizes its protein target with a proximate cysteine,a rapid nucleophilic substitution could occur between the protein Cys-and the sulfonium center on the peptide to form a conjugate.Whereas in this work,when vinyl sulfonium was introduced into peptide and cyclized through addition reaction,the thiol of cysteine in proteins hardly open the sulfonium ring and form a covalent conjugation,despite the vicinity induced by ligand(Fig.S5b in Supporting information).Moreover,we used more proteins containing free Cys-residues(BCL2,BCL-XL,MgrA)to confirm the results(Fig.S5c in Supporting informaiton).The cyclized peptide hardly covalently conjugated to the cysteine in the proteins.The activity of alkyl linker generated in the peptides cyclized by vinyl sulfonium was milder than the activity of benzyl linker generated in peptides cyclized by bis-alkylation nucleophilic substitution,leading to the relative stability of vinyl sulfonium cyclized peptides.
Peptide drugs with excellent cell-penetration ability are a key prerequisite for their efficacy.To test whether our cyclization strategy could improve cell penetration of peptide,we explored the changes in cell penetration between vinyl sulfonium cyclic peptides and linear peptides using TAT peptide as a model(Fig.2a).HeLa cells were treated with 10 μmol/L FAM-labeled peptide,cultured at 37 °C for 8 h,and then incubated with 0.05% trypan blue before flow cytometric analysis.Compared with linear peptides,vinyl sulfonium cyclic peptides showed a significant increase in cell-penetration(Fig.2b).Confocal microscopy images are consistent with those measured by flow cytometry(Fig.2c),the results confirm that the significantly improved penetrating ability of the cyclic peptides.Besides,the results of a cyclic peptide with only three arginine residues were also elucidated the similar cell permeability(Fig.S6 in Supporting information),indicating a significant improvement of cell permeability by sulfonium tethering.Taken together,these data demonstrate that peptides constructed with vinyl sulfonium method showed good stability and enhanced cellular uptake.
To further explore the potential of this methodology,we attempted to investigate whether the vinyl sulfonium in peptide could be used as a novel warhead for alkylating the thiol in cysteine residues of POIviathe Michael-type addition.Using PDZΔRGS3 peptide ligands as a model,we synthesized three peptides with different methionine substitution sites and used 2-bromoethyl trifluoromethanesulphonate to generate vinyl sulfonium warhead(Fig.3a).We explored the conjugation between peptides and PDZΔRGS3 protein.Peptides PDM1 to PDM3 were reacted with PDZΔRGS3 in PBS(protein/peptide 20/100 μmol/L,37 °C)for 12 h and then analyzed by SDS-PAGE.All the three peptides showed covalent conjugation whereas the different sites of vinyl sulfonium salt had a limited effect for the conjugation(Fig.3b).We then chose PDM2 peptide as a model to examine the reaction kinetics and stoichiometric study between peptides and PDZΔRGS3.The results showed PDM2 peptide moderately conjugated PDZΔRGS3 in a dose-and time-dependent manner(Figs.3c and d),which indicated the potential of vinyl sulfonium in peptide covalent inhibitors development.
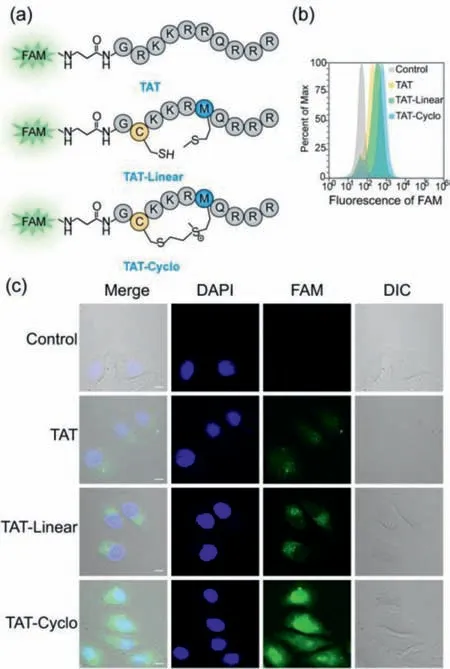
Fig.2.(a)The sequence of FAM-labeled TAT peptide,FAM-labeled TAT Linear peptide and FAM-labeled TAT-Cyclo peptide.(b)The histogram of cellular uptakes in HeLa cells treated with 10 μmol/L FAM-labeled peptides for 8 h using flow cytometry.All cells were incubated with 0.05% trypan blue for 3 min before further analysis.(c)Confocal microscopy analysis of cellular uptakes of the cyclic and linear peptides in HeLa cells.The cells were imaged using laser scanning confocal microscope with a 60×oil objective.Scar bar:10 μm.
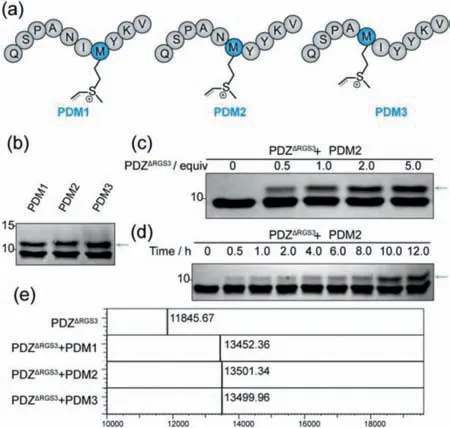
Fig.3.(a)The sequences of peptide PDM1,PDM2,and PDM3.(b)The covalent conjugation between peptides and PDZΔRGS3(protein/peptide 20/100 μmol/L,PBS,pH 7.4,12 h).(c)Stoichiometric study of peptide PDM2 from 0.5 equivalent to 5.0 equivalent incubating with PDZΔRGS3 for 12 h.The samples were analyzed by SDSPAGE gels and stained with Coomassie(CBB).(d)Reaction kinetic study of peptide PDM2 with PDZΔRGS3(protein/peptide 20/100 μmol/L,pH 7.4)for 0,0.5,1.0,2.0,4.0,6.0,8.0,10.0,12.0.The covalent ligation reacted in a time-dependent manner.The covalent conjugates were indicated with green arrowheads.(e)The mass analysis of peptide-PDZΔRGS3 conjugation,original MS data shown in Supporting information.
In summary,we report a highly reactive vinyl sulfonium warhead with dual functional application through the thio-Michaeltype addition reaction(intramolecularly or intermolecularly)under physiological conditions using natural amino acid.Cyclization with improved cellular uptake and good stability could be triggered by addition between intramolecular Cys-and vinyl sulfonium.Furthermore,the vinyl-sulfonium installed peptide could intermolecularly alkylate the thiol in cysteine residues of POIviathe Michael-type addition,showing the possibilities for the design of covalent peptide drug.This facile,efficient and biocompatible reaction could substantially expand the chemical toolbox of peptide modification and design of covalent inhibitor.With further undergoing studies,we believed that this new vinyl sulfonium warhead could play more roles in biological applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge financial support from the National Key Research and Development Program "Synthetic Biology" Key Special Project of China(No.2018YFA0902504);the China Postdoctoral Science Foundation(No.2021M690220),the National Natural Science Foundation of China(Nos.21778009 and 21977010);the Natural Science Foundation of Guangdong Province(Nos.2019A1515110487,2020A1515010522 and 2019A1515111184);the Shenzhen Science and Technology Innovation Committee(Nos.JCYJ20180507181527112,JCYJ20180508152213145,and JCYJ20170817172023838);the Foundation for Basic and Applied Research of Guangdong Province(No.2019A1515110489);Guangdong Medical Science Foundation(No.A2021413).We also acknowledge the financial support from Beijing National Laboratory of Molecular Science Open Grant(No.BNLMS20160112)and Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions(No.2019SHIBS0004).This work was supported by the high-performance computing platform of Peking University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.071.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
