Visible-light-promoted radical alkylation/cyclization of allylic amide with N-hydroxyphthalimide ester:Synthesis of oxazolines
Zhiyng Yn,Bin Sun,*,Pnyi Hung,Hiyun Zho,Ho Ding,Weike Su,*,Cn Jin,,*
a Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals,Zhejiang University of Technology,Hangzhou 310014,China
b College of Pharmaceutical Sciences,Zhejiang University of Technology,Hangzhou 310014,China
ABSTRACT An efficient photocatalytic alkylation/cyclization of allylic amide with N-hydroxyphthalimide ester has been developed.The transformation is taken advantage of alkyl radicals to attack allylic amide with the assist of inexpensive rose bengal as photocatalyst to prepare a series of alkyl substituted oxazolines in moderate to excellent yields.High regioselectivity,operational safety,mild conditions and excellent substrate generality give this protocol broad application prospects.
Keywords:Photocatalysis Oxazolines Allylic amide N-Hydroxyphthalimide ester Alkylation/cyclization Decarboxylation
Nitrogen-containing heterocyclic compounds with unique biological and pharmacological activities are usually used as structural units of drugs and pesticides[1-9].As a kind ofN-heterocycle,oxazolines exhibit various notable bioactivities,such as antimicrobial,antimalarial,antiviral,antioxidant,antitumor(Fig.1)[10-13].The traditional methods for synthesis of oxazolines are realizedviathe condensation ofβ-aminoalcohols with carboxylic acids,aldehydes,esters or nitriles(Scheme 1a)[14].Additionally,[2+3]cyclization of olefins and amides(or benzoyl azides)is an efficient strategy for the synthesis of oxazolines(Scheme 1b)[15,16].Furthermore,allylic amide cyclization reaction provides a reliable approach to obtain oxazolines which focused on a halocyclization reaction with cationic halogen to form five-membered heterocycles(Scheme 1c)[17-20].Recently,the cyclization of allylic amide based on radical coupling has become a new strategy for preparation of oxazolines(Scheme 1d)[21-25].Although these processes are essential,some drawbacks such as hard conditions,the use of large amounts of metal complexes and oxidants,substrate limitations,still limit the further application.
The visible-light mediated organic transformations which promoted chemical reactions by absorbing light as activation energy under very mild conditions have attracted widespread attention since the seminal work of MacMillan,Yoon,and Stephenson[26-45].Meanwhile,the alkylN-hydroxyphthalimide(NHP)esters which could be easily reduced by photocatalysts to generate extensive kinds of alkyl radicals with the eliminating of CO2and phthalimide anion are demonstrated to be effective alkyl precursors[46-55].Consistent with our ongoing research on photocatalytic radical-mediated C–H functionalization[56-60],herein,we uncover a metal and oxidant free approach to afford a variety of oxazolines through the radical alkylation/cyclization of allylic amide withN-hydroxyphthalimide(NHP)ester(Scheme 1e).This method features excellent regioselectivities,mild conditions,excellent substrate generality and good yields.
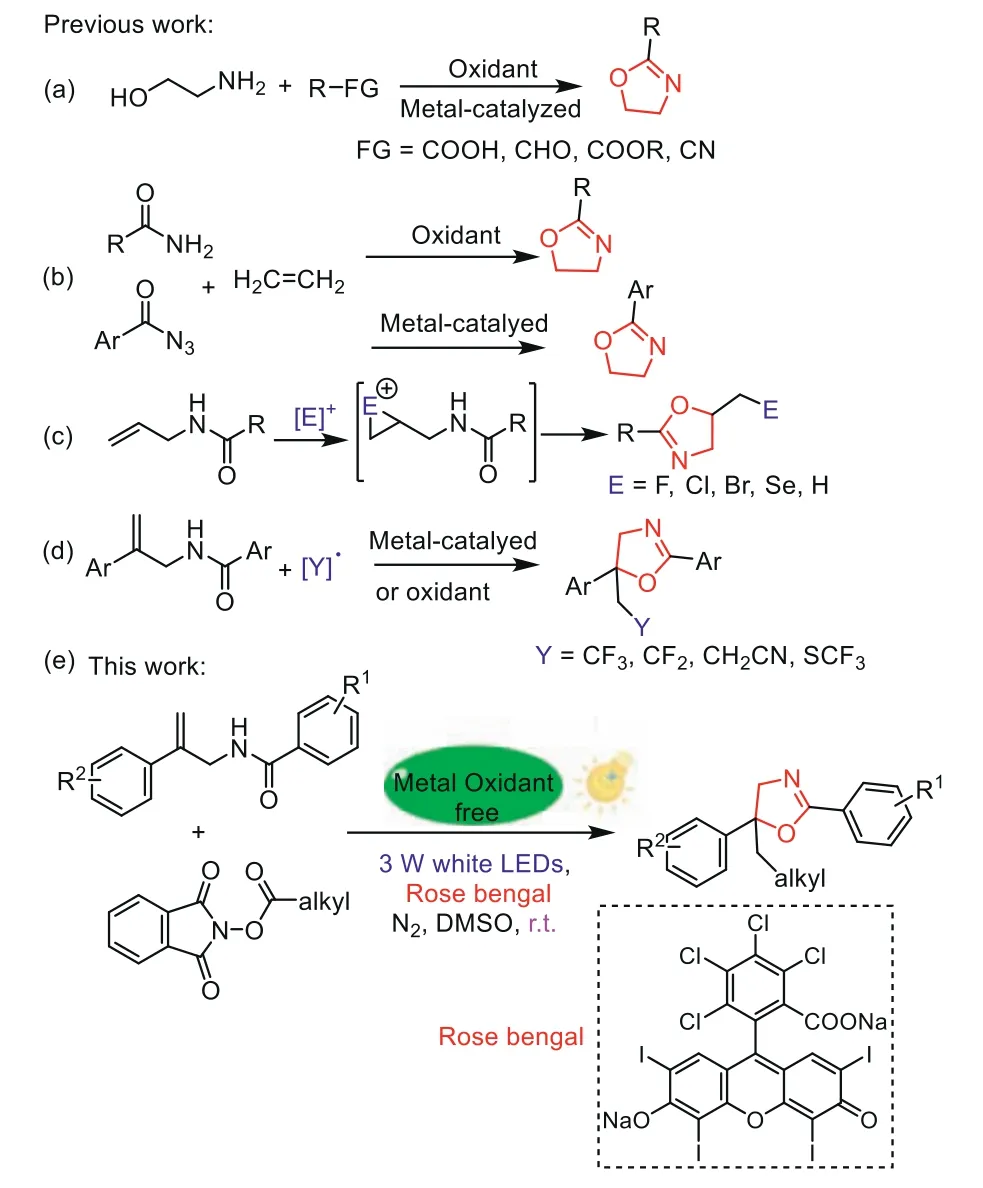
Scheme 1.Strategies for the synthesis of oxazolines.
To begin our study,N-(2-phenylallyl)benzamide 1a andt–butyl NHP esters 2a were utilized as model substrates to optimize the reaction conditions.Initially,the transformation was studied with Ir(ppy)3as a photocatalyst,irradiated with 3 W bule LEDs in DMSO under N2atmosphere for 36 h.Fortunately,the target 5-neopentyl-2,5-diphenyloxazoline 3aa was formed in 70% yield(Table 1,entry 1).In order to increase the yield of desired product,a variety of photocatalysts such as Rose bengal,Na2-Eosin Y,methylene blue,Ru(bpy)3Cl2were investigated(Table 1,entries 2–5).The experiment results revealed that only Rose bengal exhibited a better efficiency with a yield of 75%.Subsequently,a variety of commonly used solvents including DMF,MeCN,EtOAc,DCE,DCM were screened(Table 1,entries 6–10).Disappointingly,the yield was decreased when DMF,MeCN,EtOAc were employed,and no product was found with DCE or DCM as solvent.Thus,DMSO is still the most suitable solvent.To further improve the efficiency of this transformation,3 W blue LEDs were replaced by white or green LEDs(Table 1,entries 11 and 12).To our delight,the yield of 3aa was increased from 75% to 83% as 3 W white LEDs was applied.Contrast experiments indicated that either photocatalyst or visible light is indispensable for this transformation(Table 1,entries 13 and 14).Furthermore,only an extremely low yield was obtained when this reaction was executed under air(Table 1,entry 15).
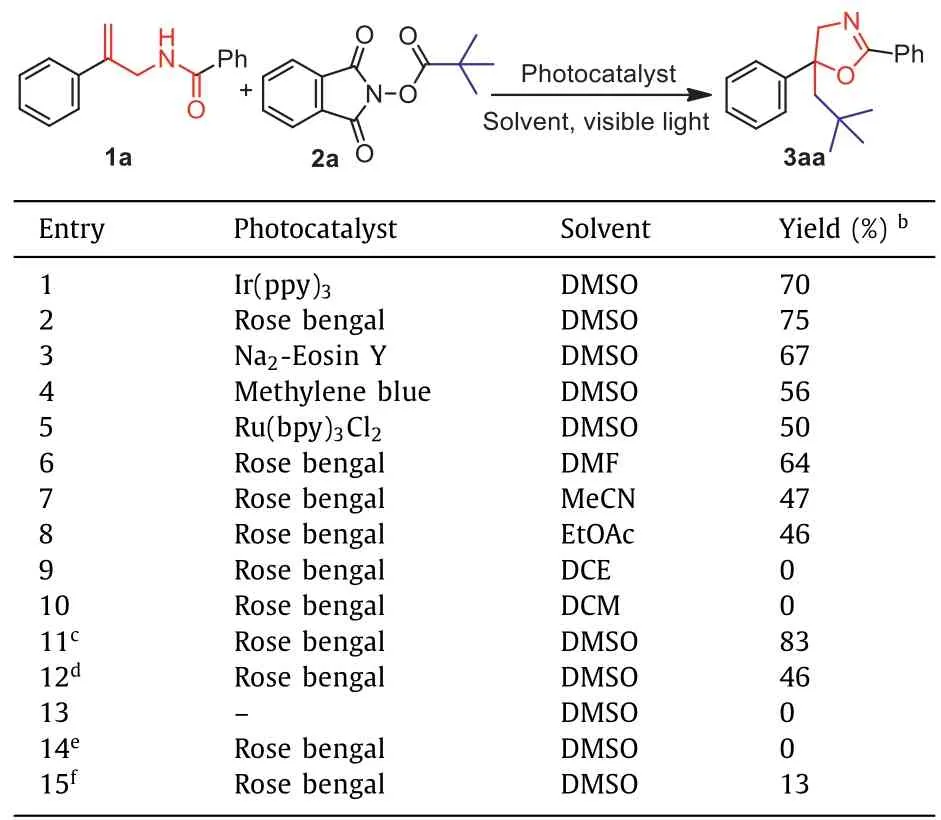
Table 1 Optimization of the reaction conditions.a
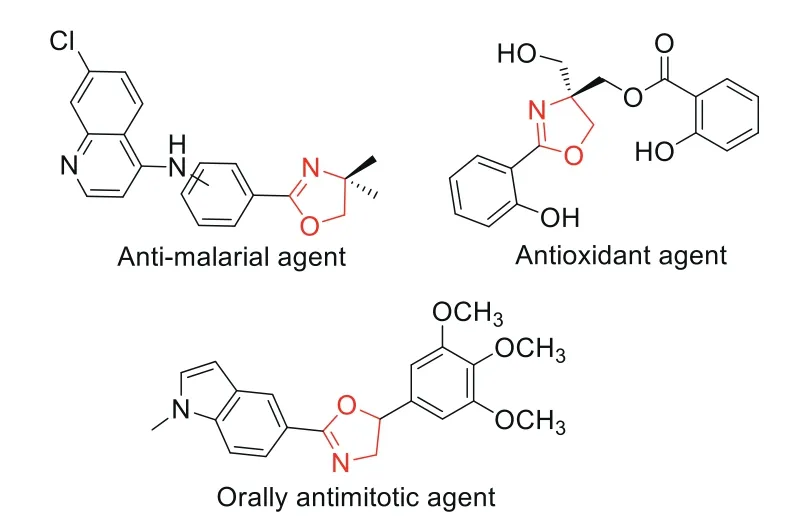
Fig.1.Examples of oxazoline-typle pharmaceutical.
After determining the optimal reaction conditions,including Rose bengal as a photosensitizer at room temperature in DMSO by irradiation of 3 W white LEDs under N2atmosphere for 36 h,the scope of NHP esters was examined through the radical alkylation/cyclization of 1a.It can be concluded from Scheme 2 that this protocol is applicable to different types of NHP esters,affording the desired products in moderate to excellent yields.Initially,the tertiary alkyl NHP esters containingt–butyl,methylcyclohexyl and adamantyl showed excellent tolerance for this transformation,and gave the desired products 3aa-3ac in excellent yields.Subsequently,secondary alkyl NHP esters were experimented under optimal conditions,and the results exhibited that alkyl NHP esters including isopropyl,cyclohexyl,3-heptyl were also well adapted to this protocol and the corresponding products 3ad-3af were obtained in 72% to 79% yields.It is worth mentioning that primaryalkyl NHP esters which could generate primary alkyl radicals were also suitable well for this protocol.For example,63%-67% yields can be obtained by takingn-pentyl,n-octane and 3-phenylpropyl as the reactive radicals to participate in the reaction(3ag-3ai).Additionally,the primary alkyl NHP esters containing functional groups involving carbonyl,ester and alkenyl on aliphatic chain are apposite to this transformation and provided the desired product in yields of 66%–71%(3aj-3al).

Scheme 2.Scope of alkyl NHP esters.Reaction conditions:1a(0.6 mmol),2(0.9 mmol),Rose bengal(0.012 mmol)in DMSO(3.0 mL)at room temperature with irradiation by 3 W white LEDs under N2 atmosphere for 36 h.
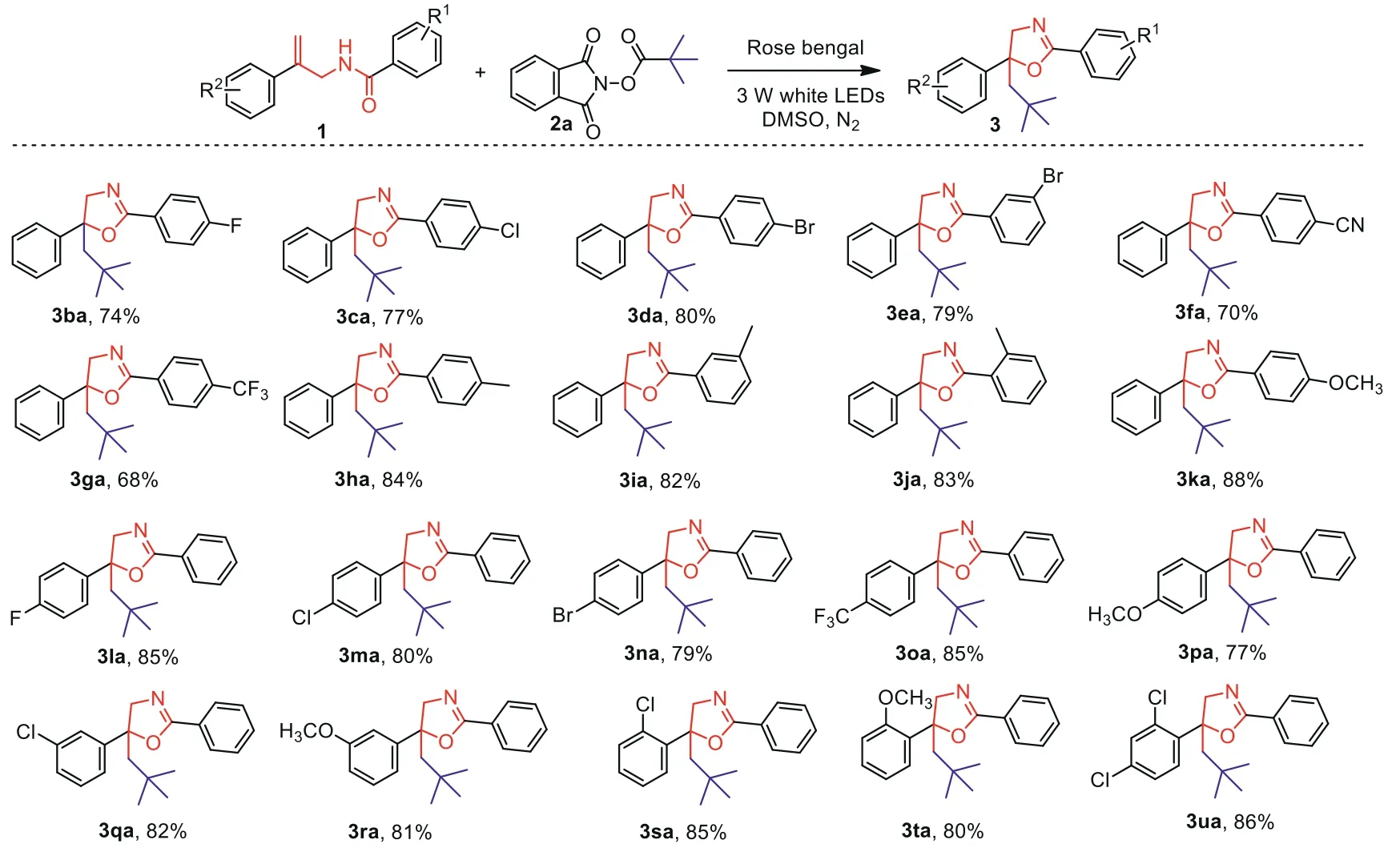
Scheme 3.Scope of allylic amides.Reaction conditions:1(0.6 mmol),2a(0.9 mmol),Rose bengal(0.012 mmol)in DMSO(3.0 mL)at room temperature with irradiation by 3 W white LEDs under N2 atmosphere for 36 h.
After the scope of alkyl NHP esters was screened,a variety of allylic amides were then experimented in alkylation/cyclization reaction witht–butyl NHP ester 2a under the standard conditions.As given in Scheme 3,this protocol was found to be applicable to various allylic amides containing different functional groups and gave the corresponding products in moderate to excellent yields.Initially,allylic amides with different substituents of R1were investigated.The experimental results indicated that the allylic amides possessing virous halogens(1b-1e),such as F,Cl and Br at different position of acylbenzene,all proceeded well and provided the desired products in yields of 74%-80%.Remarkably,even cyano and trifluoromethyl groups which are considered as strong electron withdrawing group were also well suitable for this protocol,and moderate yields have been obtained(3fa,3ga).Additionally,when the substituents R1were replaced by electron-donating groups including methyl and methoxy,the desired products were obtained in 82%-88% yields(3ha-3ka).Then,the electronic effect of R2group was screened.Substrates with electron-withdrawing or electron-donating groups at theparaposition of the benzene ring exhibited good reactivity for this reaction(3la-3pa).The transformation also survived well and provided the products in 80%-85%yields when substrates with substituents at different position of the benzene ring(3qa-3ta)were used.Satisfactorily,this protocol was also suitable for disubstituted substrate,afforded the desired product in 86% yield(3ua).
In order to explore the reaction mechanism,several control experiments were carried out as shown in Scheme 4.A radical scavenger TEMPO(2,2,6,6-tetramethyl-1-piperidinyloxy)(3.0 equiv.)was applied to the model reaction,and this alkylation/cyclization reaction was totally suppressed(Scheme 4a).The same result was obtained when using BHT as another radical scavenger in the same system(Scheme 4b).These results revealed that the transformation might be carried on with a radical mechanism.To confirm the existence of alkyl radical,1,1-diphenylethylene was added into the transformation between NHP ester and allylic amide under the standard conditions,the adduct 4 was obtained as the only product in a yield of 33%(Scheme 4c).Furthermore,EPR experiment was performed and strong signal peaks of alkyl radical adduct with DMPO were detected(for details,see Supporting information).
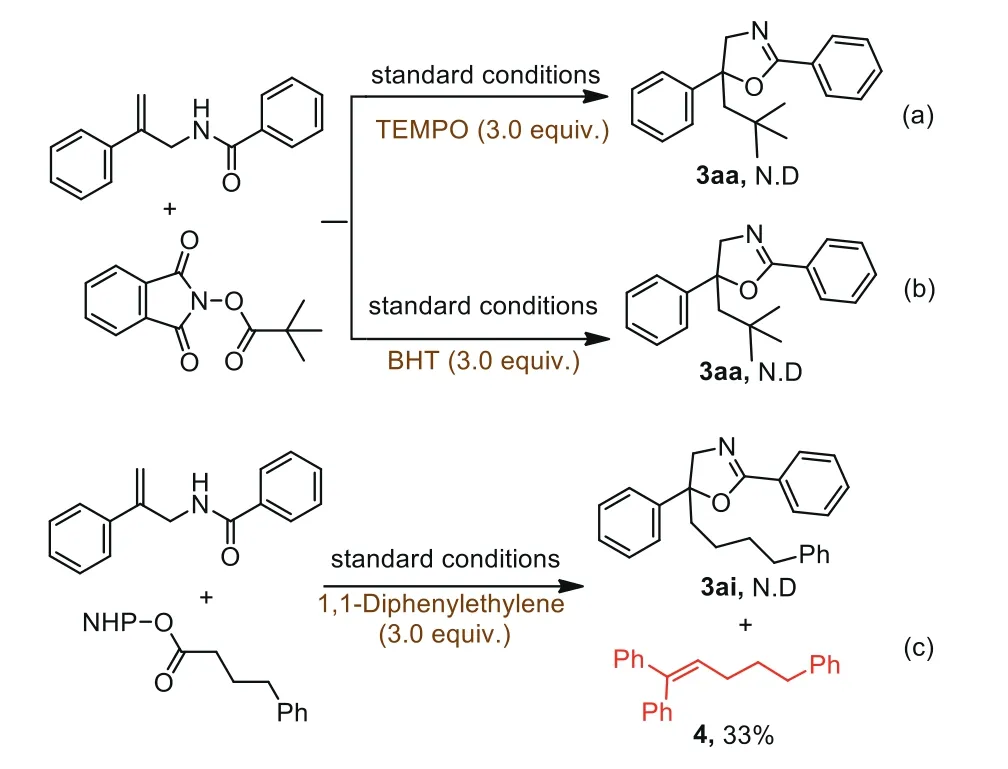
Scheme 4.Control experiment.
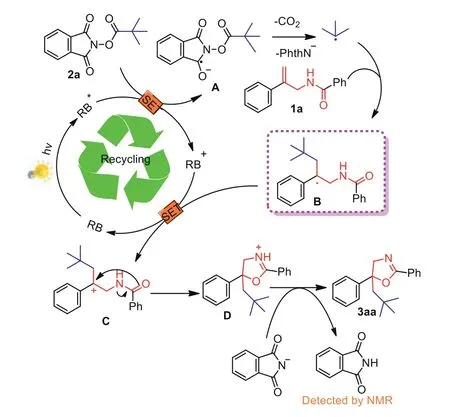
Scheme 5.Proposed reaction mechanism.
In addition,the Stern-Volmer experiment was carried out and the results indicated that Rose bengal*was quenched by alkyl NHP esters,suggesting that the reaction might proceed through an oxidative quenching process(Fig.2).
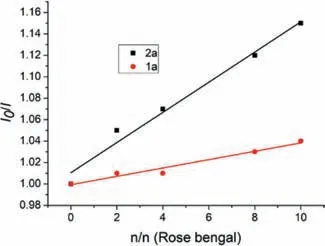
Fig.2.Stern–Volmer plot:fluorescence quenching of Rose bengal using N-(2-phenylallyl)benzamide 1a and t–butyl NHP ester 2a.
According to the above-mentioned observations and previous literature reports[61],a plausible mechanism for this transformation was proposed in Scheme 5.Initially,the photosensitizer Rose bengal(RB)was irradiated by 3 W white LEDs to form its activated species,which can transfer a single electron to NHP ester 2a to generate the radical A,accompanied by the oxidation of RB*to its oxidative state RB+(E1/2RB+/RB*=-1.32 Vvs.SCE,E1/2(NHP esters)≈-1.26 Vvs.SCE)[62,63].Then thet–butyl radical was formedviarelease of carbon dioxide and phthalimide anion from radical A,which was subsequently captured by the double bond in allylic amide to give the radical B.Through a single electron transfer process between RB+and radical B,the carbocation intermediate C was generated.The desired product 3aa was finally obtained after undergoing an intramolecular cyclization of C between carbocation and carbonyl,followed by a deprotonation process with phthalimide anion.
In summary,we successfully synthesized a series of oxazoline derivatives through the alkylation/cyclization reaction of allylic amide with NHP ester by employing a metal-free photocatalytic system under white LEDs irradiation.A wide range of alkyl NHP esters and allylic amides bearing different types of substituents were demonstrated to survive for this protocol,and provided a variety of oxazolines in moderate to excellent yields.Furthermore,this method has the characteristics of high regioselectivity,operational simplicity,mild conditions and excellent substrate generality.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.22078299)and Zhejiang Provincial Natural Science Foundation of China(Nos.LY21B060005,LQ20B060007).We are also grateful to the College of Pharmaceutical Sciences,Zhejiang University of Technology and Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals for the financial help.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.067.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
