Facile construction of aggregation-induced emission molecular liquids via Piers-Rubinsztajn reaction for green fluorescent ink
Rong Fu,Longyue Yu,Junying Zhng,Huidong Yu,Shengyu Feng,Xing-Dong Xu,*
a Key Laboratory of Special Functional Aggregated Materials of Ministry of Education,Shandong Key Laboratory of Advanced Silicone Materials and Technology,School of Chemistry and Chemical Engineering,National Engineering Research Center for Colloidal Materials,Shandong University,Ji’nan 250100,China
b Shandong Qilu Zhonghe Technology Co.,Ltd.,Ji’nan 250100,China
ABSTRACT Solvent-free luminescent molecular liquids(LMLs),which exhibit nonvolatile fluidic nature and active optoelectronic properties,were widely used.For further development,we introduced siloxane units into AIE molecules,designed and synthesized TPE derivatives with siloxane side chains via facile Piers-Rubinsztajn reaction.The obtained AIE molecular liquids exhibit unique photophysical properties.Compared with the obtained alkyl TPE-solids,siloxane TPE show liquid state,which proves that the siloxane units have stronger liquefaction effect than alkyl.Viscosity test shows that siloxane TPE-liquids has far more lower viscosity and better fluidity than the long-chain alkyl molecular liquids in previous research.All those properties are attributed to the weak interaction between flexible molecular chains of siloxane.Besides,fluorescence test shows temperature responsiveness of siloxane TPE-liquids.We developed this lowviscosity nonvolatile AIE molecular liquid as green fluorescent ink.
Keywords:TPE derivatives Piers-rubinsztajn reaction Molecular liquids Aggregation-induced emission Green fluorescent ink
Soft matter such as hydrogels,ionic liquids,foams and liquid crystals is a class of innovative smart materials,which have had a significant impact in various fields such as chemistry,physics and material sciences in recent years[1–5].In the past decade,with the positive requirement for advanced soft chromophores,a new generation of soft matter termed as solvent-free luminous molecular liquids(LMLs)have caught the spotlight[6–9].LMLs exhibit nonvolatile,environmentally friendly,tunable optoelectronic,superior stability and excellent processability.In general,LMLs are synthesized by attaching aπ-conjugated chromophore with large,flexible,and low-melting side chains.The wrapping of the side chains appreciably suppresses the intermolecularπ-πinteractions and obtain free-flowing or amorphous states with strong emission,thus giving unparalleled photophysical properties in the solventfree state.Due to their unique characteristic,LMLs have been attracting growing research interest and giving impetus to many new applications of LMLs such as optoelectronic devices recently[10].
As a suitableπ-conjugated chromophore for solvent-free LMLs,tetraphenylethylene(TPE)has attracted considerable interest due to its typical aggregation induced emission(AIE)properties and its handy modification[11–18].However,there are only a few successful cases to liquefy AIE chromophore as LMLs.For example,some TPE-based materials with large and flexible side chains such as alkyl[19]and ethylene glycol[20]have been reported with extensive application prospects.In contrast,molecules with short alkyl or ethylene glycol chains show solid state[21–23].Therefore,in order to obtain the LMLs,rather large chains are required for modification[24–27],which can isolate the core unit and lead to the melting point(Tm)far below room temperature,and such LMLs exhibit high viscosity.
Inspired by the use of siloxane liquefied triarylamine for the optoelectronic devices[28],the performance of siloxane liquefied AIE molecules is worth exploring.Siloxane has a flexible molecular main chain composed of Si-O-Si bonds,and the interaction between chains is weak,therefore they exhibit low viscosity.Apart from its distinctive merits of inherent flexibility and thermal stability,siloxane is really convenient to modify,so they have shown great activity from material preparation to commercial-scale application[29–33].In the past few decades,the Piers-Rubinsztajn reaction has been proven to be an effective method in many different types of siloxane chemistries[34–41].The reaction is taken place under fairly mild and ambient conditions with low catalyst(tris(pentafluorophenyl)borane(BCF))loadings,and the substitution proceeds without metathesis or redistribution of the siloxane component,ending up with a high yield and the only byproduct is hydrogen or methane.In this study,the Piers-Rubinsztajn reaction was chosen as a powerful way to decorate chromophores with siloxane side chains,then obtain AIE molecular Liquids with unique properties.
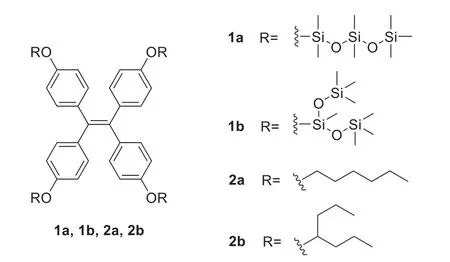
Fig.1.Structures of AIE-active TPE derivatives.
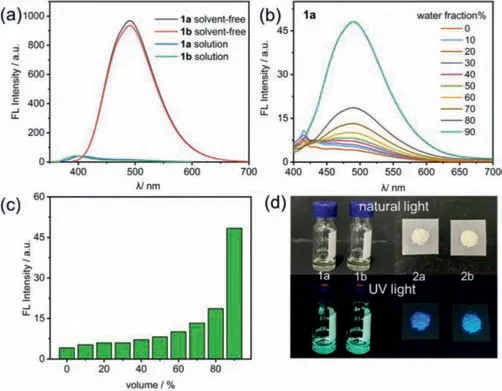
Fig.2.Fluorescence spectra of(a)solvent-free 1a,1b supported on quartz plate and 1a,1b solution in THF(c = 2.0 × 10-5 mol/L),(b)1a in mixed solvents of THF/H2O(c = 2.0 × 10-5 mol/L,λex = 370 nm),and(c)a plot of fluorescence intensity of 1a at 490 nm in THF/H2O mixtures with different H2O fractions(c = 2.0 × 10-5 mol/L,λex = 370 nm).(d)Picture of 1a,1b,2a and 2b in natural light and UV light.(All the ex slit:5 nm,em slit:5 nm).
Herein,based on our previous work[42–45],we designed siloxane TPE(1a and 1b)to obtain AIE molecular liquids,as shown in Fig.1.As a contrast,we synthesized alkyl TPE(2a and 2b)with similar side chain lengths.Furthermore,straight and branched chains are also set as an affect of the different properties.The synthesis of 1a,1b,2a and 2b are carried out according to Scheme 1.The detailed synthetic procedures and characterization data of1H NMR and13C NMR,or MS are shown in the Supporting information(Figs.S1-S14 in Supporting information).In the synthesis process,through mild and efficient Piers-Rubinsztajn reaction,the molecules 1a and 1b with siloxane chains were found to be lowviscous liquids,while 2a and 2b with alkyl chains are solids at room temperature(Fig.2d).Combined with Takashi’s previous research[19],this interesting phenomenon indicates the awesome liquefaction ability of the siloxane chains.
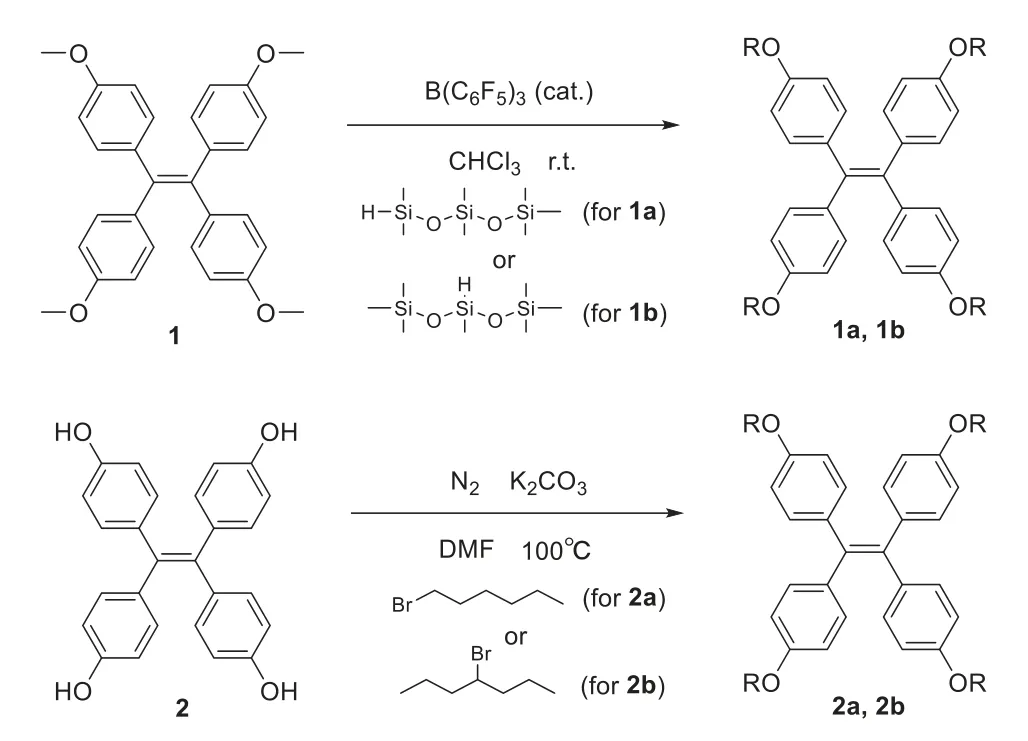
Scheme 1.Synthesis of 1a,1b,2a and 2b.
To assess the optical affect of different kinds of side chains on the TPE derivatives,the synthesized molecules were characterized by fluorescence emission spectra(Fig.2).According to Fig.2a,the fluorescence of siloxane TPE-liquids 1a and 1b were highly emissive in solvent-free state,although they are liquids,and on the contrary,nearly quenched in good solvents such as THF.Further analysis of the absorption and emission spectra are shown in Fig.S15(Supporting informaiton).As shown in Fig.S15b,different from the alkyl TPE-solids 2a and 2b,of whichλemappeared at around 440 nm,those of siloxane TPE-liquids 1a and 1b appeared at around 490 nm.Theλemof siloxane TPE-liquids 1a and 1b redshifted to longer wavelength was owing to the extended effective conjugated length,roughly due to the better coplanarity of the liquid molecules.In aggregated state,compared with 2a and 2b,1a and 1b have lower emission intensity due to the low-viscosity siloxane side chains,according to Figs.S15b and c.And in good solvent,they show similar low emission intensity(Fig.S15f).In addition,the difference in fluorescence performance between straight and branched chains under such short chain length is not obvious.
Furthermore,the fluorescence emission spectra were carried out in a THF/H2O solution mixture with various ratios at a fixed concentration(2.0 × 10-5mol/L),as shown in Fig.2b and Fig.S16(Supporting information).With the addition of the poor solvent H2O,the fluorescence intensity slightly increased at first and then showed a sharp increase upon the water fraction(fw)increasing to beyond around 80%.Uponfwincreasing up to 90%,the intensity reached a maximum.This is due to the effect of the restricted intramolecular rotation(RIR)effects,exhibiting typical AIE behavior.It can be clearly observed from Fig.2c that the fluorescence intensity at 490 nm showed a trend of increase.Moreover,the molecules 1b,2a and 2b showed similar emission spectrum(Fig.S16),which exhibits typical AIE behavior as well.
The photophysical properties for all compounds are included in Table 1.The siloxane TPE-liquids with straight chains 1a and branched chains 1b exhibited the quantum yield(ΦF)values of 0.11 and 0.21,respectively.The alkyl TPE-solids 2a and 2b showed theΦFvalues of both 0.47.The time constant(τ)was larger for the alkyl TPE-solids(2–3 ns)than that for the siloxane TPE-liquids(<1 ns).According to the calculation results of the rate constant of the radiative(kr)and nonradiative processes(knr),krwere roughly identical in both liquid and solid molecules(0.1–0.3 ns-1),while solid molecules showed smallerknr(<0.3 ns-1)compared to that of liquid molecules(>0.9 ns-1).In the case where thekrof liquid and solid molecules are similar,the higherknrof the liquid molecules is the main reason for the lowerΦFandτ.Furthermore,viscosity measurements for siloxane TPE-liquids 1a and 1b revealed the intrinsic viscosity to be merely 302.5 cP and 104.4 cP,respectively.As a contrast,according to Machida’s previous report[19],their long-chain alkyl TPE-liquids show relatively highΦF(>0.65)and lowknr(<0.091)due to the high viscosity(ca.6000 cP).Therefore,for siloxane TPE-liquids,the intramolecular motion is not so restricted due to low viscosity,causing higherknrand weak-AIE behavior.
To investigate the influence of the branched or straight chain on the solvent-free molecules,compounds 2a and 2b were employed to carry out single crystal X-ray analysis(Fig.3 and Table S1 in Supporting information).Single crystals of 2a and 2b were obtainedviaa common recrystallization process,which can be seen in Figs.3a and b.It is shown in Figs.3c and d that themolecular packing natures of 2a and 2b were extremely different.For 2a,theπ–πinteractions on the phenyl group withca.2.8 ˚A were observed,in addition,there are C···H–H···C interactions between the hydrogens on the terminal alkyl groups withca.2.2–2.9 ˚A.And for 2b,C···H-πinteractions between the alkyl group and phenyl group withca.2.9 ˚A and C···H–O interactions between the Oxygen atom and phenyl group with 2.5 ˚A were observed.However,the different molecular packing properties of 2a and 2b did not show obvious difference in macroscopic properties.
Each solvent-free state compound was characterized by differential scanning calorimetry(DSC)to evaluate the effect of molecular chains branching and silicone substitution on the glass transition temperatures(Tg)and melting point(Tm)of the materials.Upon heating,solvent-free liquid compounds 1a and 1b illustratedTgat-81.5 °C and-73.8 °C,respectively(Table 1 and Fig.S17b in Supporting information),and there is no obviousTm.These lowTgsuggested that the siloxane TPE 1a and 1b are amorphous molecules with a temperature-stable liquid state.On the other hand,the alkyl TPE 2a and 2b showedTmat 86 °C and 92.8 °C,respectively(Table 1 and Fig.S17a in Supporting information)upon the heating process.At the same time,by comparing the branched and straight chains molecules(Tgof 1a<1b,Tmof 2a<2b),it is found that the movement of branched chains molecules requires more energy,it probably because the viscosity of 1b(302.5 cp)is greater than that of 1a(104.4 cp).Besides,although supercooling liquid behavior is often observed in alkyl-substituted liquid[8],siloxane TPE-liquids shows to be completely liquids in spite of short side chains,which is obvious in DSC thermogram.This is come from the fluidic nature of siloxane chain.
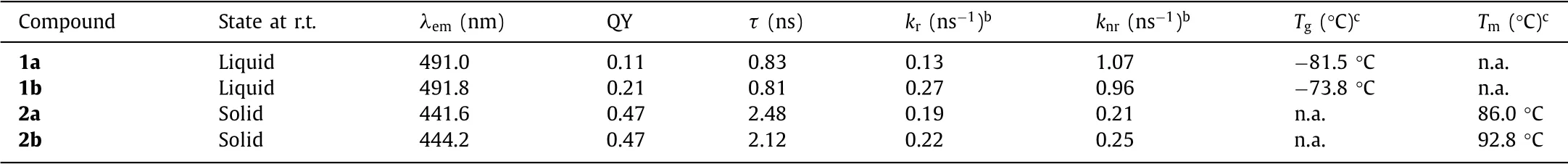
Table 1 Summary of photophysical and thermal properties of TPE derivatives in the solvent-free statea.
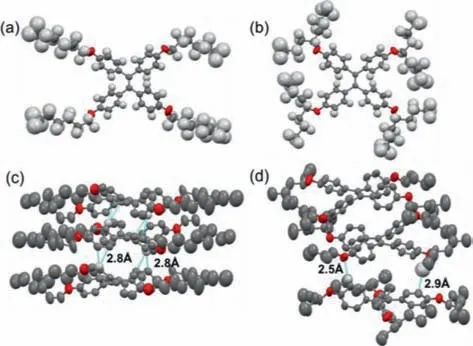
Fig.3.X-ray single crystal structures of 2a(a),2b(b),and crystal packing of 2a(c)and 2b(d).Solvent molecules and most hydrogen atoms are omitted for clarity.
To further explore the affect of temperature on the fluorescence of the solvent-free TPE derivatives,the synthesized molecules were characterized by emission spectra(Fig.4).According to Figs.4a and e,siloxane TPE-liquids 1a and 1b both shows much higher fluorescence intensity at-196 °C(both>4000 a.u.)than either at room temperature or at 100 °C(both<500 a.u.),and theλemblueshifted to lower wavelength at-196 °C.At-196 °C,far below theTgof 1a(-81.5 °C)and 1b(-73.8 °C),the freezing of TPE siloxane side chains leads to the RIR effects,which causes the enhancement of fluorescence emission.Futhermore,freezing will deteriorate the coplanarity of the molecules[15],leading to the blue-shift of the emission wavelength.Besides,as shown in Fig.S18(Supporting information),alkyl TPE-solids 2a and 2b both shows much lower fluorescence intensity at 100 °C(both<500 a.u.)than either at-196°C or room temperature(both>2500 a.u.).When heated to 100°C,the temperature is above theTmof 2a and 2b(86.0 °C and 92.8°C),then 2a and 2b change to liquid state,causing weaker RIR effects.Meanwhile,the better coplanarity of the molecules cause the red-shift of the emission wavelength.
Inspired by the unique fluorescence performance and lowviscosity of solvent-free siloxane TPE-liquids,we tried to pack compound 1a and 1b as green fluorescent ink in a ballpoint pen refill or use them as inkpads to make the fluorescence pattern on paper substrate that can only be seen under the UV light(Figs.4bd and Figs.4f-h).As shown in Figs.4b-d,a black panda and fluorescent bamboos are painted with normal ink and 1a fluorescent ink,respectively,and a stamp is covered with 1a fluorescent ink.Under natural light,only a panda can be seen(Fig.4b).While,a bright fluorescent stamp and bamboos can be observed under UV light.During the process of returning to room temperature after freezing in liquid nitrogen(Figs.4c and d),the stamp and bamboos gradually changed from blue(λem= 449.6 nm)to green(λem= 492.8 nm)under UV light(λex= 365 nm).The result is consistent with the fluorescence spectrum in Fig.4a.As shown in Figs.4e-h,using 1b as the fluorescent ink,the painting showed similar results to 1a.Therefore,we successfully developed a facile green fluorescent ink,which is a solvent-free siloxane TPE-liquids.Different from traditional ink,the new fluorescent ink use UV light to identify patterns on the paper substrates,and due to its solventfree characteristics,it is stable and environmentally friendly.
In summary,we reported two kinds of siloxane TPE-liquids(1a with straight side chains and 1b with branched side chains)as members of AIE molecular liquids,which were synthesized using short siloxane chainsviafacile Piers-Rubinsztajn reaction,and they exhibited room-temperature liquid state.With similar chain lengths,alkyl TPE-solids(2a with straight chains,2b with branched chains)were synthesized as a comparison,which exhibited roomtemperature solid state.This indicates that the siloxane chain has a stronger liquefaction effect to construct LMLs than the alkyl chain.Besides,viscosity test shows that siloxane TPE-liquids has much lower viscosity(302.5 cp and 104.4 cp)than the long-chain alkyl molecular liquids(ca.6000 cp)in previous research.That is due to the flexible molecular chains of siloxane and weak interaction between chains.Furthermore,siloxane TPE-liquids also shows completely liquids,longer emission wavelength and weaker-AIE behavior than alkyl TPE.Due to the low viscosity,which makes them easy to be packed in the refill,and other characteristic such as fluorescence temperature responsiveness,nonvolatile,environmental friendly,stable and room-temperature liquids,siloxane TPE-liquids 1a and 1b can be developed as environmentally friendly fluorescent inks.Therefore,the current platform broadens the avenue for the preparation and application of AIE molecular liquids.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.21602124),Natural Science Foundation of Shandong Province(No.ZR2016BQ11),and the Young Scholars Program of Shandong University(No.2018WLJH40)

Fig.4.Fluorescence spectra of solvent-free 1a(a)and 1b(e)in the bulk state at different temperatures(λex = 370 nm),ex slit:2.5 nm,em slit:2.5 nm.(b-d)Schematic diagram of painting and stamp with 1a as ink from-196 °C to room temperature under natural light or UV light.(f-h)Schematic diagram of painting with 1b as ink from-196 °C to room temperature under natural light or UV light.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.018.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
