Adsorption of polyhaloalkane vapors by adaptive macrocycle crystals of WreathArene through C-halogen…π interactions
Shu Niu,Ling-Ling Mo,Hongyn Xio,Yongye Zho,Chen-Ho Tung,Li-Zhu Wu,Hun Cong,*
a Key Laboratory of Photochemical Conversion and Optoelectronic Materials,Key Laboratory of Bio-inspired Materials and Interfacial Science,Technical Institute of Physics and Chemistry,Chinese Academy of Sciences;School of Future Technology,University of Chinese Academy of Sciences,Beijing 100190,China
ABSTRACT Polyhaloalkanes are broadly useful yet environmentally harmful stock chemicals,therefore the development of adsorbent materials with capacity and selectivity for polyhaloalkane vapors is highly desirable.Here we report a novel macrocycle WreathArene,a fluorinated C3-symmetrical[16]-paracyclophane.In the crysalline state,WreathArene features guest-adaptive polymorphism for polyhaloalkanes including chloroform,tribromomethane,1,1,2-trichloroethane,and 1,2-dibromoethane.Non-covalent C-halogen…π interactions are observed in all of these host-guest structures.Based on these properties,the activated WreathArene crystals can be utilized as a selective and recyclable adsorbent for polyhaloalkane vapors with excellent capacity under user-friendly conditions.
Keywords:Macrocycle Paracyclophane Halogen…π Polyhaloalkane Adsorption
Polyhaloalkanes are important feedstock chemicals with versatile applications including solvents,refrigerants,synthetic reagents,and agrochemicals;however,they are often irritative volatile chemicals which are harmful to environment and health[1].In view of the utility and toxicity of polyhaloalkanes,the development of novel adsorbent materials for polyhaloalkane vapors with desirable selectivity and capacity should have profound potential in areas including chemical reagent purification and pollutant removal[2–11].Recently,nonporous adaptive crystals(NACs)of macrocycles have been a bourgeoning area in supramolecular chemistry for selective adsorption of organic vapors through noncovalent interactions[12–21].Successful NACs thus far are mostly electron-rich macrocyclic arenes which exhibit adsorptive affinity for hydrocarbons through C-H…πinteractions[22–24].Considering the opposite polarities between C-H and C-halogen bonds[25],we speculated that the use of electron-deficient macrocyclic arenes as adsorbents would show preference to polyhaloalkanesviaChalogen…πinteractions.
C-halogen…πinteractions are relatively weaker in energy compared to other non-covalent interactions,with halogen-centroid distances ranging from 3.3 ˚A to 3.8 ˚A[26].Although a number of literatures showed their important roles in supramolecular selfassembly and crystal engineering[27–29],C-halogen…πinteractions are less studied in the context of host-guest chemistry of macrocycles[30–33].For instance,halogenated solvent molecules are frequently present,but often neglected,in the crystal structures of macrocycles;and in-depth investigation of C-halogen…πinteractions involving polyhaloalkanes inside macrocyclic cavities would reveal supramolecular properties with potential applications.
Here we present a novel electron-deficient macrocyclic arene as adaptive crystals exhibiting affinity to polyhaloalkane vapors.This fluorinated neutral macrocycle carries alternatively located perfluorinated and unsubstituted paraphenylene subunits,and is named WreathArene(WA)because its C3-symmetrical shape resembles the popular holiday ornament(Fig.1).Crystal structures of WA with chloroform,tribromomethane(TBM),1,1,2-trichloroethane(TCE)and 1,2-dibromoethane(DBE),respectively,reveal universal C-halogen…πinteractions inside the guest-adaptive cavities of WA.Furthermore,the activated crystals of WA,as an adaptive adsorbent,show selective and recyclable vapor uptake for these polyhaloalkanes under user-friendly conditions.
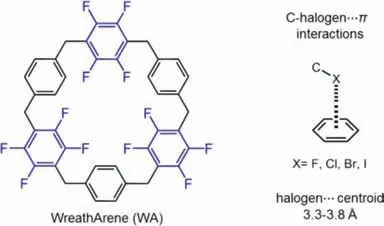
Fig 1.The macrocycle WreathArene(WA)and C-halogen…π interactions.

Fig 2.Synthesis of WreathArene.
[1n]-Paracyclophanes(PCP)are a class of neutral macrocycles consisting of methylene-bridged paraphenylenes[34].Owing to their structural symmetry and supramolecular properties,[1n]-PCP derivatives have been attractive synthetic targets for decades[35–40].While electron-rich[1n]-PCP are usually prepared using Friedel-Crafts-type reactions[41],new macrocyclization strategy is in need for the fluorinated target molecule.Instead of late-stage functional group manipulations on the macrocycle rims[42–45],our synthesis of WA entails a convergent route which directly constructs the macrocyclic scaffold employing sequential Pd-catalyzed C-H activation processes of fluorinated arenes[46].
The combination of Pd(OAc)2and PhJohnPhos(L)was optimized to facilitate the key C(sp3)-C(sp2)bond formation between tetrafluorobenzene 1 and benzyl bromide 2.One-pot demethylation/bromination of the coupling product 3 afforded bromide 4 smoothly when treated with BBr3.Various attempts to trimerize 4 directly to the target WA through coupling reactions were unsuccessful.Instead,under the aforementioned optimal coupling conditions,compounds 3 and 4 were converted to 5 and 6,respectively.Similarly,bis(benzyl bromide)7 was obtained from 5viaanother demethylation/bromination step.With both precursors in hand,cross coupling between 6 and 7 furnished the desired macrocyclization,affording WA within four longest linear steps(Fig.2).The concise and convergent synthetic route provides access to this electron-deficient neutral macrocycle in good amount for further study.
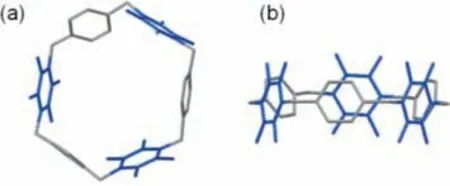
Fig 3.Crystal structure of WA.(a)Top and(b)side view of a single macrocycle.The tetrafluoroparaphenylene subunits are shown in blue.Hydrogen atoms and disordered solvents are omitted for clarity.
Next,single crystals were obtained by diffusion ofn-pentane into a CHCl3solution of WA.X-ray crystallographic analysis unambiguously confirmed the macrocyclic structure with a slightly distorted hexagonal skeleton(Fig.3).Inside the the crystals,π-πinteractions[47,48]and C-H…F hydrogen bonds[49–51]arrange the hexagonal WA molecules to form parallel honeycomb-like layers with one-dimensional channels(Fig.S18 in Supporting information).Each tetrafluoroparaphenylene subunit always faces an unsubstituted paraphenylene subunit of an adjacent molecule,glued by intra-layerπ-πinteractions.In addition,the parallel layers are neatly stacked in brick-wall-like pattern exhibiting long-range 1D channels which are stabilized by multiple inter-layer C-H…F hydrogen bonds.Such hydrogen bond networks in the solid state would rationalize the "upright" paraphenylene conformations of WA leading to its pillar-like cavity.
Because of the similar cavity geometries between WA and pillar[6]arenes[52],we reasoned that suitably sized molecules which are rich in high-electronegativity atoms should serve as possible guests for the electron-deficient WA.However,based on a survey of potential guest molecules,we were unsuccessful to identify evident guest inclusion of WA in solution by NMR analysis.This result would likely be linked to the poor solubility of WA:among common organic solvents,WA only dissolves well in dichloromethane and chloroform,both of which provide abundant chlorine atoms surrounding WA,thereby excluding other potential guests.
In view of the excellent crystallinity of WA,we next investigated its host-guest properties in the solid state.Notably,highly disordered chloroform molecules,which were used as solvent during crystallization,are present inside the macrocyclic cavities of the aforementioned structure of WA crystals(Fig.4a,cf.Fig.3),with C-halogen…πinteractions evidenced by an average chlorinecentroid distance of 3.6 ˚A.Encouraged by this observation,we then evaluated other polyhaloalkanes which are in general less investigated as guests for macrocycles.
Crystallization of WA in mixed solvents of CHCl3and TBM afforded single crystals TBM@WA.With each hexagonal WA occupied by a C3-symmetrical TBM guest at the cavity center,the C-Br bonds pointing to the centeroids of paraphenylenes show an average bromine-centroid distance of 3.5 ˚A(Fig.4b).The TBM@WA crystals are almost identical to WA crystallized from CHCl3solutions with regard to molecular packing.Honeycomb-like layers and brick-wall-like stacking were observed which are stabilized by intra-layerπ-πinteractions and inter-layer C-H…F hydrogen bonds,respectively(Fig.S19 in Supporting information).
Single crystals of TCE@WA and DBE@WA were further obtained respectively by crystallization using the corresponding polyhaloalkane as solvent.Crystallographic analysis revealed the intriguing guest-adaptive features of WA crystals[53],in which the macrocycles adopt relatively more procumbent conformations so that the adaptive cavities could better match the less-symmetrical polyhaloalkane guests.In TCE@WA,a single conformation was observed for the macrocyclic host,the cavity of which is occupied by one TCE molecule of two conformations(Fig.4c).Both conformations maintain staggered with different dihedral angles,and are stabilized by non-covalent C-halogen…πand C-H…πinteractions with WA.With regard to DBE@WA,two conformations for WA were identified with a ratio of 50:50,and each macrocycle could host one DBE molecule within its adaptive cavity assisted by C-halogen…πand C-H…πinteractions(Fig.4d).One of the two macrocycle conformers exclusively pairs DBE guests ofanticonformation.The cavities of the other macrocycle conformer are occupied by two differentgauche-conformations of DBE.
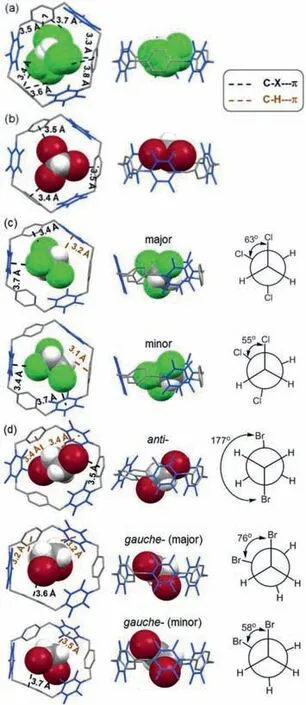
Fig 4.Crystal structures of polyhaloalkane@WA.(a)CHCl3@WA,(b)TBM@WA,(c)TCE@WA,(d)DBE@WA.The tetrafluoroparaphenylene subunits are shown in blue.Hydrogen atoms of WA are omitted for clarity.
Similar to TBM@WA,the WA in TCE@WA and DBE@WA pack into honeycomb-like layers,but their more procumbent conformations found in the latter two crystals cause less intra-layerπ-πinteractions(Figs.S20 and S21 in Supporting information).Whereas the presence of multiple inter-layer C-H…F hydrogen bonds can assist to pack the staggered layers into brick-wall-like patterns,longrange channels no longer exist in TCE@WA or DBE@WA.
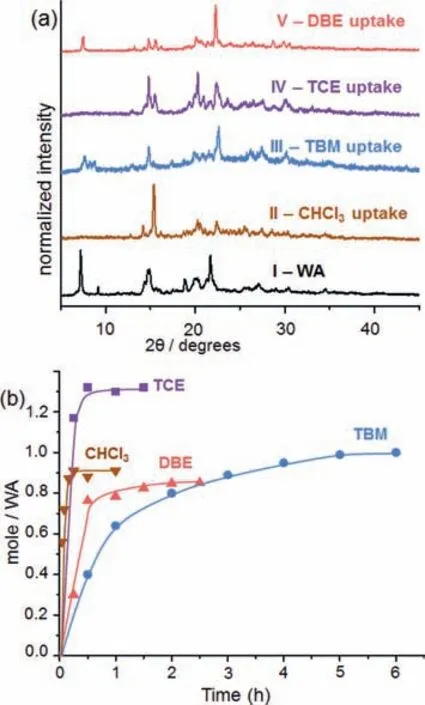
Fig.5.(a)PXRD patterns of WA samples:(I)activated WA crystals,(II)after CHCl3 uptake(2 h),(III)after TBM uptake(6 h),(IV)after TCE uptake(2 h),(V)after DBE uptake(3 h).(b)Time-dependent vapor uptake plots.All vapor adsorptions were conducted at 30 °C.
Based on the non-covalent interactions in the polyhaloalkane@WA crystals,the vapor adsorption capability of WA was explored.WA crystallized from CHCl3/n-pentane was activated by heating under vacuum to remove volatile components.The resulting white powder of activated WA was found crystalline by PXRD,(Fig.5a,sample I),and its patterns match the simulated patterns of the guest-free WA crystals(Fig.S25 in Supporting information).Subsequently,polyhaloalkane vapor uptake experiments confirmed excellent adsorptive capability of WA,which is evidenced by the distinct PXRD pattern changes for all four polyhaloalkanes under evaluation(Fig.5a,samples II-V).After adsorption,the PXRD patterns of WA are consistent with the simulated patterns of the corresponding polyhaloalkane@WA crystal structures(Fig.S25).Such similarities between the experimental and simulated PXRD patterns provide informative insight for solid-vapor adsorptions based on crystal structures.
By monitoring the vapor uptake kinetics at 30 °C,we established that the adsorption capacities of WA are 0.9,1.0,1.3 and 0.85 in mole ratios(14 wt%,33 wt%,23 wt%,and 21 wt% relative to WA)for chloroform,TBM,TCE,and DBE,respectively.The adsorption rates show positive relationship with the boiling points of the polyhaloalkane guests(Fig.5b).At 30 °C,CHCl3(b.p.61 °C)rapidly reaches saturated adsorption within 20 min,and in parallel experiments,30 min for TCE(b.p.114 °C),2 h for DBE(b.p.131 °C),and 5 h for TBM(b.p.150 °C).Furthermore,time-dependent PXRD monitoring the adsorptive progress of TBM revealed that rapid crystalto-crystal phase transition of WA starts within 5 min of vapor contact(Fig.S26 in Supporting information).
With single-component adsorption confirmed,we wondered whether WA can serve as a selective adsorbent with preferential affinity to polyhaloalkanes in the presence of other organic vapors.Considering the different volatility of these organic liquids,we defined a preference factor to quantitatively evaluate the selectivity of the competitive mixed-vapor adsorptions.The preference factor of a two-component vapor adsorption equals to the molar ratio of adsorbed vapors divided by the ratio of vapor pressures under the adsorption temperature(Fig.6a).
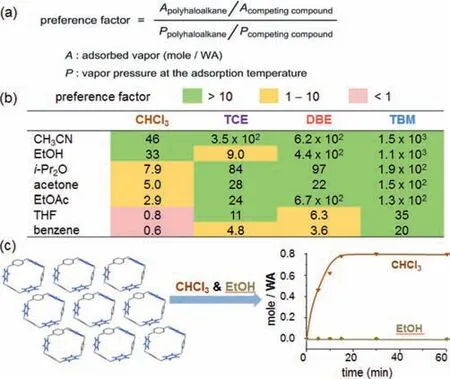
Fig 6.Adsorptive preference of WA at 30 °C.(a)The definition of preference factor.(b)Preference factors for competitive adsorptions(2 h)of two-component mixed vapors containing a polyhaloalkane and an organic solvent.(c)Time-dependent vapor uptake of mixed chloroform/ethanol vapors(3:1,v/v).All vapor pressure ratios were calculated using Antoine Equation[54,55].
The activated WA crystals were subjected to an array of two-component mixed vapors containing a polyhaloalkane and a common organic solvent(Fig.6b).The resulting preference factors exhibit excellent differentiation of polyhaloalkane over nonhalogenated vapors in general.Among the 28 evaluated combinations of two-component mixed vapors,19 combinations show preference factors above 10.The polyhaloalkanes’preference factors follow the trend of TBM>DBE>TCE>CHCl3,indicating the highest affinity for WA toward TBM.The general trend of preference factors for non-halogenated vapors are in the order of CH3CN>EtOH>i-Pr2O>acetone>EtOAc>THF>benzene.
The commercial chloroform is usually stabilized by doping ethanol(b.p.78 °C)for extended storage to avoid phosgene formation.Single-component evaluations revealed that activated WA crystals can rapidly adsorb chloroform vapor at 30 °C(Fig.5b),whereas no adsorption was observed for ethanol under the same condition(Fig.S33 in Supporting information).Next,adsorptive separation of mixed vapors of CHCl3and ethanol(v/v,3:1 at 30°C)[54,55]showed exclusive adsorption of CHCl3by WA(Fig.6c).Time-dependent vapor uptake indicated that CHCl3reaches saturation within 20 min,similar to the corresponding single-component sorption.
The recyclability of activated WA crystals was tested by five adsorption-activation cycles of mixed CHCl3and ethanol,and each cycle maintained excellent adsorptive capacity and selectivity(Fig.S35 in Supporting information).According to the PXRD patterns recorded before and after recycling,the crystalline structure of WA could be well preserved(Fig.S36 in Supporting information).
In summary,we have developed WA,a novel macrocycle featuring C-halogen…πinteractions to host polyhaloalkanes in the crystalline state.Activated guest-free crystals of WA can function as an adaptive and selective adsorbent for polyhaloalkane vapors,exhibiting excellent capability and recyclability.Given that polyhaloalkanes are widely useful yet harmful stock chemicals,the development of macrocycle WA represents a new adaptive adsorbent with future potentials for pollutant removal and energysaving separation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support from the National Natural Science Foundation of China(Nos.21672227,21922113,21988102,22071257),Chinese Academy of Sciences(Nos.XDB17000000),the National Key Research and Development Program(No.2017YFA0206903),K.C.Wong Education Foundation,and the TIPC Director’s Fund is gratefully acknowledged.We thank Prof.Congyang Wang(ICCAS),Drs.Xiaodi Yang(Shanghai University of Traditional Chinese Medicine),Jie Su and Wen Zhou(PKU)for the help with compound characterizations.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.090.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
