Bioactive gelatin cryogels with BMP-2 biomimetic peptide and VEGF:A potential scaffold for synergistically induced osteogenesis
Lili Wng,Long Chen,Jiping Wng,Liying Wng,Chenyu Go,Bo Li,Yunzheng Wng,*,Jun Wu,*,Chngyun Qun,*
a Guangdong Provincial Key Laboratory of Sensor Technology and Biomedical Instruments,School of Biomedical Engineering,Sun Yat-sen University,Shenzhen 518107,China
b Department of Orthopedics,Guizhou Provincial People’s Hospital,Guiyang 550000,China
c School of Public Health,Yale University,New Haven,CT 06510,United States
d Department of Biomedical Engineering,Johns Hopkins University,Baltimore,MD 21218,United States
1 These authors contributed equally to this work.
ABSTRACT A poor biocompatibility and bioactivity of invasive materials remains major problems for biomaterialbased therapy.In this study,we introduced gelatin scaffolds carrying both bone morphogenetic protein-2(BMP-2)biomimetic peptide and vascular endothelial growth factor-165(VEGF)that achieved controlled release,cell attachment,proliferation and differentiation.To promote osteogenesis with VEGF,we designed the BMP-2 biomimetic peptide that comprised BMP-2 core sequence oligopeptide(SSVPT),phosphoserine,and synthetic cell adhesion factor(RGDS). In vitro cell experiments,the scaffold was conducive to the adhesion and proliferation of rat bone marrow mesenchymal stem cells(rBMSCs).The micro-CT 3D reconstruction of the rat cranial bone defect model showed that bone regeneration patterns occurred from one side edge towards the center area implanted with the prepared cryogel,and tissue section staining analysis demonstrated that the scaffold with double-growth factor can synergistically accelerate bone regeneration.These findings suggested that the obtained gelatin cryogel could serve as a cell-responsive platform for biomaterial-based nonbearing bone repair.
Keywords:Gelatin Cryogel Cytokine combination Osteogenesis Bioactive
In this study,we fabricated gelatin scaffolds carrying bone morphogenetic protein-2(BMP-2)biomimetic peptide and vascular endothelial growth factor-165(VEGF)to achieve controlled release,cell attachment,proliferation and differentiation.The scaffolds with dual growth factors demonstrated great potential in promoting bone regeneration.
With its three-dimensional interconnected porosity,cryogel has high similarity with extracellular matrix(ECM)and becomes an attractive candidate as base materials for bone tissue engineering[1].Cryogel has been widely used for its unique properties of heating and cooling,low cost,lack of immunogenicity,enzymatic degradability for matrix metalloproteinase(MMP)-degradable motifs,and presence of inherent peptide sequences such as RGD motifs that facilitate cell adhesion[2,3].Gelatin,a denatured product of collagen composed of 18 kinds of amino acids(7 of which are essential for the human body),is a proteinaceous material obtained by hydrolytic degradation of naturally occurring collagen[4,5].It contains no cholesterol that can cause human diseases[6],and thus mainly used as a medicinal capsule,wound dressing,and as a scaffold material in tissue engineering[7–15].In addition,only 5% of the amino acid residues in the molar ratio are potentially modifiable by methacrylic anhydride(MA)[16],implying that most of the bioactive amino acid motifs are maintained and functional(e.g.,RGD and MMP sequences).Moreover,high-density culture of bone marrow mesenchymal stem cells is conducive to the differentiation of cells into chondrocytes[17].When the degree of modification of GelMA is close to 80%,the wide cross-linking of macromolecules in the gel can impede cells proliferation,thereby promoting the differentiation of mesenchymal stem cells into chondrocytes.The degree of modification in 20%–80% GelMA can maintain stable physical and chemical properties[18].With the increase in the degree of modification,the hardness and durability of the cryogel increase while the pore size decreases.Therefore,modified gelatin could overcome the shortcomings of the low mechanical strength of cryogel,making it become a suitable scaffold material for bone tissue engineering.
As we know,bone morphogenetic protein-2(BMP-2)has been extensively used for tissue regeneration applications because it helps osteoblast progenitor cell recruitment,angiogenesis,and the stimulation of osteogenic differentiation[19,20].Through the downstream signaling of the Smad and MAPK pathways,BMP-2 can activate the transcription factors RUNX2 and Osx,and then initiate osteoblast-specific gene expression(e.g.,alkaline phosphatase(ALP),osteocalcin(OCN),and osteopontin(OPN))to accelerate ectopic and orthotopic bone formation[21–24].However,its instability limits clinical applications.Fortunately,BMP-2 biomimetic peptide with the BMP-2 core sequence(SSVPT)can be an alternative to natural BMP-2 protein[25,26].Not only can it maintain efficiency for significantly elevating osteogenic marker activity through interactions with type I and type II BMP receptors[20,27],it can also reduce costs and have higher stability[22,28].Furthermore,phosphorylation of serine promotes the deposition of endogenous calcium ions through chelation with calcium ions in the human environment[29].Although calcium phosphate minerals themselves do not have bone-inducing biological activity,they can dissolve in the body fluid environment and thereby increase the concentration of local calcium ions and phosphate ions,which plays an important role in differentiation of BMSCs or pre-osteoblasts[30,31].
Bone is a highly vascularized tissue,and the vascular system establishes a basis for its calcium-phosphorous and energy metabolism[32].Vascularization plays an important role in osteogenesis,including providing nutrition and oxygen,and transforming precursor cells and growth factors[33].Vascular endothelial growth factor 165(VEGF 165)can strengthen and accelerate both endochondral and intramembranous ossification with angiogenesis[34–38].When a fracture occurs,upregulated VEGF combines with the corresponding receptor,activates the downstream pathway,and promotes the migration and proliferation of vascular endothelial cells(VECs)[39].In particular,the VEGF 165 subtype,the most general secretion form,can be solublein vivoand has strong effect on inducing angiogenesis[40,41].Moreover,VEGF would activate the formation of nitric oxide in endothelial nitric oxide synthase(ENOS)to improve vascular permeability and deposition of plasma proteins in the ECM,thus providing a temporary matrix for VEC growth and migration[42].VEGF can also hydrolyze proteinases in the basement membrane and facilitate the migration of VECs to the broken end towards the concentration gradient of growth factor.
It has been reported that VEGF has already participated in vascularization of new bone before endochondral ossification[43].Synergistic effects of VEGF and BMP-2 can promote rBMSC proliferation and differentiation into osteoblasts[44–46].Angiogenesis occurs earlier than osteogenesis,and VEGF can upregulate the expression of BMP-2[47,48].More importantly,there is a relationship between the way of their interaction and the dosages[49].According to Peng[33]and Li’s research[50],the optimum concentration was the key factor for best efficiency when referring to the combined application of VEGF and BMP-2.For the specific concentration ratio,studies pointed out that excess VEGF would influence the mineralization of rBMSCs,thus reverse bone regeneration[51].In detail,the optimum concentration of VEGF/BMP-2 was about 0.4:1,but inhibiting effects gained the upper hand when the ratio was higher than 0.8:1[52].In Spector’s study,VEGF had an impact on their migration and differentiation,and the required concentration was 100 times lower than that of BMP-2[52,53].To achieve the desired biological response,incorporation of multiple bioactive components is required.Because of its small size and rapid diffusion rate,BMP-2 biomimetic peptide(BMPMP)can be immobilized on a scaffold to induce osteogenic differentiation and bone regenerationin vivo.For the same reason but for a larger size,it was also necessary to incorporate VEGF into carriers(i.e.,our prepared scaffolds)to reduce speed of release.
In this study,we fabricated and characterized gelatin cryogels formed by cryopolymerization,a method compatible with cytokines that achieves high interconnected porosity,a suitable hydrated pore size,and adequate mechanical properties.To achieve the desired bioactivity for osteogenesis,we first introduced both vascular endothelial growth factor 165(VEGF 165)and bone morphogenetic protein-2 biomimetic peptide(BMPBP)including cell adhesion factor(RGDS),phosphoserine,and BMPBP,onto gelatin cryogels to promote osteogenesis in different phases(Scheme 1).VEGF 165 can strengthen and accelerate both endochondral and intramembranous ossification with angiogenesis.BMPBP enhanced rBMSC adhesion and proliferation and intensified the deposition of endogenous calcium ionsviathe chelation reaction of calcium ionsin vitrocell experiments andin vivoanimal experiments.The cryogels have excellent biocompatibility and suitable physicochemical properties that stimulate bone regeneration under physiological conditions in clinical applications.
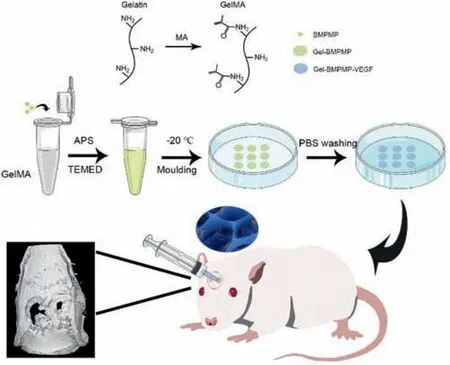
Scheme 1.Schematic illustration of bioactive gelatin cryogels with BMP-2 biomimetic peptide and VEGF for synergistically induced osteogenesis.
The repair of bone defects has always been a major problem in the field of clinical treatment.Bone tissue engineering is an effective way that combines advanced biomaterials to pursue the best bone repair effect.The idea of repairing biomaterials should involve a scaffold that can mimic natural bone tissue structure and possess appropriate mechanical properties and bioactive factors such as BMP or VEGF to enhance bone conductivity and to accelerate new bone formation.It is necessary to further optimize the performance of scaffold materials.In this study,VEGF 165 and BMPBP,consisting of cell adhesion factor(RGDS),phosphoserine,and BMPBP,were loaded onto gelatin cryogel and interact with host osteoblasts and vascular endothelial cells.The whole cryogel system synergistically promotes angiogenesis and osteogenesis to achieve a better bone defect repair effect.
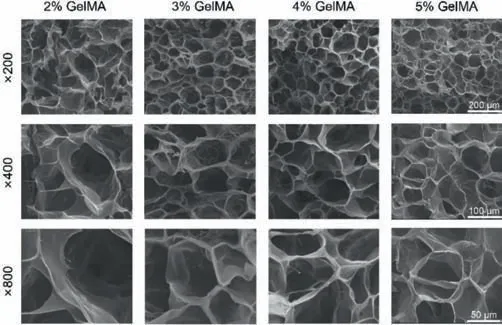
Fig.1.Scan electron micrograph images of GelMA cryogels with different concentrations.Scale bars(from up to down):200,100,and 50 μm.
The construction of the vascular network system is a prerequisite for bone tissue to survive and to integrate with host tissue,seed cells,cytokine slow-release systems and scaffold materials.High porosity and appropriately sized pores of the cryogel can ensure cell penetration,vascular growth,nutrient delivery and removal of metabolic waste.First,gelatin was modified by methacrylic anhydride,which could improve the mechanical properties and meet the requirements of bone tissue engineering applications.One of the double cytokines,BMP-2 biomimetic peptides,could promote mineral deposition on the cryogel scaffold and the adhesion of cells and promote the proliferation and differentiation of BMSCs.From the expression level of osteogenesisrelated genes,we further confirmed the results of cell adhesion,alkaline phosphatase activity and mineralization.As a result,combining the gelatin scaffolds with double cytokines together,adjusting the release rate of those factors,and considering the properties of BMPBP and VEGF,we first bonded BMPBP to GelMA by surface functionalization of Michael addition between-NH2and-C=C-COOH and then incorporated VEGF by physical entrapment.With the degradation of gelatin scaffolds,VEGF was rapidly initially released by diffusion and first boosted angiogenesis at the early bone healing stage.Subsequently,BMPBP was slowly exposed over a sustained period of time to provide sufficient bioactive units in the interior of gelatin scaffolds,induced osteogenic differentiation,and promoted bone tissue regeneration with VEGF synergistically.
The cryogel physicochemical properties included methacrylated grafting degree,bulk mechanical properties,and facilitation of mineralization.We found proton peaks of double bonds in methacrylamide at chemical shifts of 5.5 and 5.7 ppm(Fig.S1 in Supporting information)implied the successful synthesis of GelMA,and the resulting degree of substitution was 61.2% by comparing the proton peaks of methylene and residual freeε-amino groups.
Fig.S2A(Supporting information)showed that the compressive modulus of the cryogels increased from 2.5 kPa to 32 kPa,while the concentration of GelMA increased from 2.0% to 5.0%.This increase can be explained by the decreasing interconnected porosity of cryogels as shown in Fig.S2B(Supporting information).It was clear that the interconnected porosity of the cryogel decreased rapidly from 65% to 18%.The interconnected porosity of the cryogel can also be seen from the SEM results(Fig.1).GelMA cryogels(2%–5%)all showed a through-connected porous scaffold structure with abundant interconnected holes.As the concentration of GelMA increased,interconnected holes decreased in size and became denser.When 2% GelMA was added,we observed interconnected holes with a diameter of approximately 100 μm,which is suitable for cell growth and transportation of nutrients and metabolic wastes.The microstructure results observed by SEM were aligned with the interconnected porosity we calculated before.Considering the above experimental results,for small nonbearing bone defects,the 2% GelMA cryogel has the most suitable pore structure for cell growth while simultaneously satisfying the basic mechanical properties of bone tissue engineering scaffolds.Therefore,in this study,a 2% GelMA cryogel was chosen as an ideal scaffold for further cell and animal experiments.
We studied surface topographies of mineralized cryogels with different concentrations of BMPMP(Fig.S3 in Supporting information).Three groups all had mineral deposition on their surface,and cryogels with higher BMPBP concentrations showed more visible mineral deposition.The designed and synthesized BMP-2 biomimetic peptide,which contains phosphorylated serine,chelated calcium ions in simulated body fluids(SBFs)and generated more calcium and phosphorus deposition.The Ca-P ratio on the surface of mineralized colloids was obtained by energy dispersive spectrometry.The elements C,N and O were taken as the basis,and the surface of the nonmineralized colloid scaffold did not contain Ca and P.In contrast,the surface Ca-P ratios of the mineralized cryogels,the Gel-LBMP and Gel-HBMP groups,were(P 1.02%,Ca 0.18%),(P 1.27%,Ca 0.22%),and(P 1.40%,Ca 0.38%),respectively(Table S1 in Supporting information).This result was consistent with the results of the thermal field SEM,and can be explained by the phosphorylated serine in BMPMP.However,the difference in Ca-P ratios among groups was not obvious,which may cause by the low concentration of polypeptide.At the same time,this result may be due to good biocompatibility of gelatin itself.Gelatin has a certain role in inducing mineral deposition in simulated body fluid.
To closely monitor cell morphology and adhesion,after incorporating cytokines for 24 h,we observed rBMSCs on the cryogels by confocal laser scanning microscopy.As shown in Fig.S4A(Supporting information),cells survived and grew well in the scaffold,and cells were more aggregated in cryogels containing BMPMP than in pure cryogels.However,cells in the Gel-BMPMP-VEGF group gathered more tightly and spread well,showing contact pseudopodia and a larger spreading area.The cell adhesion factor RGDS,which consists of BMPMP,can specifically bind to adhesion proteins on the cell surface to promote cell attachment in cryogels.In addition,cells in the cryogel containing BMPMP and VEGF presented different adhesion morphologies as shown in Fig.S4B(Supporting information).Because of the narrow or wide network structure inside the cryogel bulk,cells changed their shapes into corresponding elongated,shuttled and spheroidal shapes,indicating the excellent biocompatibility of cryogels composed of BMPMP and VEGF.
The proliferation of BMSCs on the scaffold was calculated by the CCK-8 assay.As shown in Fig.S5(Supporting information),the cell number of BMSCs on all the scaffold groups increased well with culturing time.Groups with high BMPMP concentration have obvious higher cell numbers than groups with low BMPMP.Based on the result,the scaffolds showed no apparent cytotoxicity or adverse effects on cell growth,and cell proliferation was concentration-dependent on the BMP-2 biomimetic peptide.In the initial period of time,the Gel-HBMP-VEGF group gathered significantly more cells than the Gel,Gel-LBMP,and Gel-LBMP-VEGF groups.Over time,both the Gel-HBMP-VEGF groups and Gel-HBMP groups had dramatically significant differences in Gel groups and had significant differences in Gel with low BMPMP scaffolds on days 3 and 7.It has been reported that BMP-2 has the ability to induce the proliferation and differentiation of bone marrow mesenchymal stem cells into chondrocytes and osteoblasts,promote the maturation of osteoblasts,help angiogenesis,participate in bone and cartilage growth and tissue reconstruction,and accelerate the process of bone defect repair[19,20].Therefore,the cell proliferation was dependent on BMP content in the early stage of cell growth.It should be noted that the cell number of the Gel-HBMP groups was slightly higher than that of the Gel-LBMP-VEGF groups,but there was no significant difference between them.This result was consistent with the point of view that the effect of BMP-2 on BMSCs might be inhibited when VEGF content is high[34].It is possible that the amount of BMPMP released in the early stage was lower,but the accumulated amount of BMPMP on day 7 was higher than the optimal amount of BMPMP balanced with VEGF,and there was slight inhibition of cell growth.
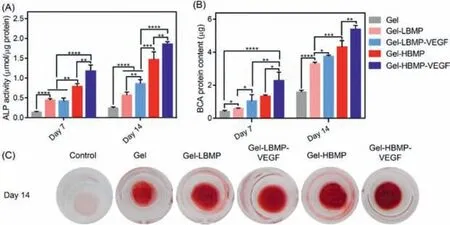
Fig.2.(A)ALP activity and(B)BCA protein content of rBMSCs after incubated with Gel,Gel-LBMP,Gel-LBMP-VEGF,Gel-HBMP,and Gel-HBMP-VEGF scaffolds for 7 and 14 days,respectively.(C)Optical macro images of alizarin red staining of rBMSCs cultured on Gel,Gel-LBMP,Gel-LBMP-VEGF,Gel-HBMP,and Gel-HBMP-VEGF scaffolds for 14 days.
To assess the effect of cryogels on the osteogenic differentiation of BMSCs,the activities of ALP were evaluated.As shown in Fig.2A,during the initial period of time,the ALP activity of the Gel-LBMP group increased significantly compared with that of the Gel group.When the BMPMP concentration was low,regardless of whether VEGF was added,it had no significant effect on ALP activity on the 7thday.However,the ALP activity of the Gel-LBMP-VEGF group was significantly higher than that of the Gel-LBMP group on the 14thday,which indicated that BMPMP cooperated with VEGF better and promoted BMSCs to differentiate into osteoblasts when it reached a certain level.In addition,there was a significant difference between the Gel-HBMP group and the Gel-LBMP group,and the difference was more obvious as the culturing time was prolonged.Furthermore,the ALP activity of Gel-HBMP-VEGF showed the most significant growth difference among all the groups,and the increase in Gel-HBMP-VEGF was more significant than that of the Gel-HBMP group.A similar trend was observed in terms of the BCA protein content among groups(Fig.2B).The difference was that the increase in BCA protein in the low BMPMP group resulted in a higher content than that of ALP activity.We also examined the efficiency of osteogenic differentiation in the mineralization stage by using Alizarin Red S staining as a marker of inorganic calcium.The results showed a more intuitive mineralized formation in osteogenic medium under macroscopic view(Fig.2C).BMSCs cultured in Gel-HBMP-VEGF scaffolds showed the most apparent mineralized formation compared with Gel-LBMP and Gel scaffolds on the 14thday,which confirmed the above results.
To clarify the effect of scaffolds on the expression of osteogenic differentiation-related genes in BMSCs,we studied several cell differentiation-related marker genes,including RUNX2,ALP,OCN,OPN and Col I,which are essential during osteogenesis after 7 and 14 days of culture in osteogenic medium.We focused on groups including GEL,GEL-BMPMP and GEL-BMPMP-VEGF to study the role of BMPMP and VEGF in gelatin cryogels.The results of real-time polymerase chain reaction(RT-PCR)analysis were shown in Fig.S6(Supporting information).On day 7,ALP expression was relatively low in the absence of BMP-2.In contrast,the ALP gene expression of BMSCs in cryogels containing BMPMP and VEGF showed a significant difference from that of the gel group in the early stage,and the difference was more significant on the 14thday.These results suggested that BMP-2 cooperating with VEGF has a strong potential to induce early osteogenic differentiation of hMSCs in a short time.ALP is an early osteogenic marker that is mainly distributed on nuclear transport proteins,promoting cell maturation and bone tissue calcification.The expression of ALP could reflect the differentiation level of osteoblasts.The higher the activity of ALP is,the more obvious the differentiation of BMSCs into mature osteoblasts is.Moreover,the Gel-BMPMP-VEGF group was also significantly different from the Gel-BMPMP group,indicating that the addition of VEGF enhanced the osteogenesis induced by BMPMP.On the 7thday,the expression of Runx-2 showed a significant difference among the groups,but it was relatively weak on the 14thday.There was still a significant difference between the group with BMPMP and the pure Gel group,but there was no significant difference between the Gel-BMPMP-VEGF group and the Gel-BMPMP group.Runx-2 was expressed in the early stage of BMSC differentiation,and the expression level gradually decreased in the later stage.For BMP-2-induced osteogenesis,Runx-2 is the main transcription factor that stimulates osteoblast marker genes in the early stage of differentiation.The same expression of Col I was observed on the 14thday,but the difference was that there was no significant difference in the expression of Col I among the groups on the 7thday.OCN reflects the differentiation and maturation of osteoblasts and the expression of OCN increases during the late differentiation and mineralization stage of osteoblasts.It was obvious that OCN showed almost no expression in each group on the 7thday.On both the 7thand 14thdays,the expression level of OPN followed the preceding trend of the Gel-BMPMP-VEGF group>Gel-BMPMP group>Gel group.OPN and OCN are osteogenic markers of BMSCs in the early and late stages of osteogenic differentiation,and OPN also has rich expression in the late stage.
In summary,PCR analysis confirmed the results of cell adhesion,alkaline phosphatase activity and mineralization deposition at the gene level.After culturing in osteogenic medium for 2 weeks,the results showed that the expression levels of osteogenic differentiation-related genes in double-cytokine-coated cryogels were generally significantly upregulated compared with those observed in pure cryogels.The expression levels of these five genes in the Gel-BMPMP-VEGF group were always the highest among the three groups.In fact,it has been proven that BMP-2 guides the expression of osteogenic marker genes through the Smad and MAPK signaling pathways.BMP-2 was also reported to promote cell homing.Extracellular BMP-2 is absorbed by cells through reticulin-mediated endocytosis.It has a specific binding affinity with VEGF.The isoelectric point of BMP-2 is 8.5,and BMP-2 has a positive surface at pH 7.4.It is known that positively charged materials can facilitate cell adhesion,which is consistent with the expression data in Fig.S5.The overall results here indicated that BMP-2 had great potential to induce osteogenic differentiation of hMSCs in 3D culture,and VEGF was superior for assisting the BMP-2 peptide.
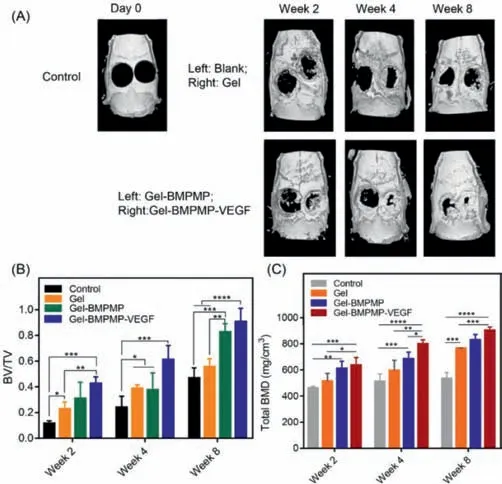
Fig.3.(A)Micro-CT 3D reconstruction images of newly bone tissues at 2,4 and 8 weeks after implantation of Gel,Gel-BMPMP,and Gel-BMPMP-VEGF scaffolds into rat cranial defects.(B)Proportion of new bone formation(BV/TV)and(C)total bone mineral density(total BMD)were calculated to analyze the effect of Gel,Gel-BMPMP,and Gel-BMPMP-VEGF scaffolds on repairing bone defects.
Micro-CT images presented accurate information on new bone regeneration after scaffold implantation(Fig.3A).New bone growth showed that the GEL-BMPMP-VEGF group had the best radiologic performance compared with all other groups.For the scaffold group,the new bone grew from the surrounding edge to the center of the defect.With increasing healing time,different degrees of repair were observed in each scaffold group at different time periods compared with those immediately after the operation.In particular,the GEL-BMPMP-VEGF group showed an obviously better bone repair effect than the other groups at 2 weeks postoperatively and achieved almost complete regeneration at 8 weeks postoperatively.To analyze quantitatively the amount and quality of new bone formation,bone volume fraction(BV/TV)and total BMD were determined as shown in Figs.3B and C.The bone tissue volume/total tissue volume(BV/TV)and total bone mineral density(BMD)of the scaffold group were all significantly higher than those of the control group at all time points.However,the GEL-BMPMP-VEGF group presented the highest BV/TV and total BMD,which implied quicker and more fruitful bone regeneration.
Histological and immunohistochemical analyses were performed to verify the osteogenic ability of this double-cytokinecombined cryogel systemin vivo(Fig.S7 in Supporting information).The H&E staining results showed that the defect of bone tissue in the control group(without any implantation,the wound healed naturally)was still prominent and the drilling site was obvious,which means the bone regeneration tissue was less,and the boundary between the new tissue and the surrounding normal tissue was incoherent at week 2 post-implantation.Meanwhile,in the group implanted with cryogels,cartilage fibers were found to varying degrees,and the newly formed cartilage structure was not similar to the natural bone tissue.In addition,there was more connective tissue and new cartilage fibers in the groups containing BMPMP than in the pure Gel group,while a small amount of bone trabeculae,which was similar to the natural bone structure,appeared in the Gel-BMPMP-VEGF group.Over time,new bone trabeculae gradually appeared in the control group at 4 weeks after surgery.Moreover,round and oval hypertrophic chondrocytes were found in the Gel-BMPMP group and Gel-BMPMP-VEGF group,which is a medium-term phenomenon that is difficult to capture.These chondrocytes buried in the cartilage stroma and formed cartilage lacuna.At 8 weeks after surgery,the new connective tissue in the control group was still not fused with the surrounding bone tissue while the bone connective tissue in the Gel group was well connected with the surrounding bone tissue,which showed that the cryogel scaffold played a good bridging role in the process of bone tissue repair.A similar phenomenon could be seen in the Gel-BMPMP group and Gel-BMPMP-VEGF group,but the connective tissue was replaced by more bone trabeculae.The gap between bone trabeculae was the smallest,and the connective tissue in the middle grew tightly together in the Gel-BMPMP-VEGF group.Subsequent Masson trichrome staining was used to show collagen in the connective tissue,which was labeled blue while other tissues were stained red.The results showed that there was no obvious connective tissue and almost no stained collagen in the control group in the early postoperative period,but collagen fibers appeared in the other three groups,and the Gel-BMPMP-VEGF group appeared to have the most collagen fibers among them.In the early bone repair stage,there was less red-stained area but more blue-stained area around the bone nucleus and osteoblasts.As the repair time increased,collagen fibers appeared in the control group.In the Gel-BMPMP-VEGF group,the red-stained area increased gradually,and the blue-stained area correspondingly reduced the color depth.Newly mature bone tissue increased gradually in the middle and later periods of bone repair.At 8 weeks after surgery,there was a large area of red-stained area and a small amount of blue-stained area,which indicated that collagen fibers were gradually replaced by mature bone tissue.In summary,the implantation of cryogels made the progress of bone tissue repair enter the middle and late stages in advance,and the double cytokines further promoted the repair task.
In addition,we further labeled several exceptional biochemical markers of bone formation,including CD31,Col I and OCN,to indicate the osteoinductivity of the double-cytokine combined cryogel systemin vivo(Fig.4).CD31 is a specific marker molecule along vascular endothelial cells,and its immunohistochemical staining represented the vascularization of each group in the bone defect site.The results showed that there was new angiogenesis with different diameters in the group implanted with cryogels at 2 weeks after surgery.There was only a few CD31-positive staining at 2 and 4 weeks after surgery and slightly more vascular endothelial cells at 8 weeks after surgery in the control group.There was more CD31-positive staining in the Gel group,Gel-BMPMP group and Gel-BMPMP-VEGF group,but the Gel-BMPMP-VEGF group exhibited the most vascularization.The above phenomena indicated that VEGF in the cryogel system could promote the formation of vascular endothelial cells and bone tissue vascularization to a large extent,and the synergistic effect of VEGF and BMPMP could produce a better bone repair effect.The immunohistochemical staining results of Col I indicated the formation of fibrocollagen in different stages of bone repair.In the Gel-BMPMP-VEGF group,a large amount of fibrocollagen appeared and was densely distributed at 2 weeks after surgery,followed by the Gel-BMPMP group.At 8 weeks after surgery,the fibrocollagen in the Gel-BMPMP-VEGF group was relatively reduced and replaced by mature bone tissue(red).At this time,there was more fibrocollagen in the Gel-BMPMP group than in the Gel group at the previous stage.The immunohistochemical staining results of Col I were almost consistent with the Masson trichrome staining results.BMPMP promoted osteogenic differentiation and secreted more collagen,and the synergistic effect of VEGF and BMPMP promoted the process of osteogenic differentiation.OCN,as an osteogenic marker in the late stage of osteogenic differentiation of BMSCs,showed a small amount in the Gel-BMPMP group at 2 weeks after surgery and was more abundant in the Gel-BMPMP-VEGF group.Sparsely distributed positive staining of OCN was found at 8 weeks after surgery in the control group.Based on the above immunohistochemical staining results,VEGF embedded in the cryogel system began to play an important role in promoting the generation of vascular endothelial cells in the early stage,and the synergistic effect of VEGF and BMP-2 biomimetic peptide promoted the process of osteogenic differentiation and bone tissue repair.
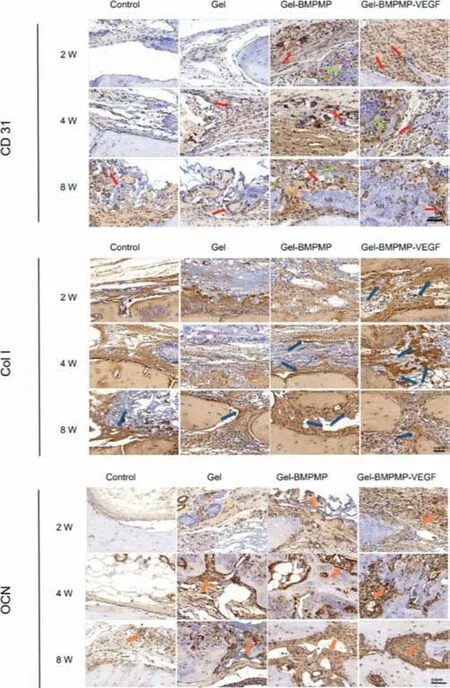
Fig.4.The immunohstochemical staining specific for anti-CD 31,Col I and OCN images of the bone regeneration tissues in different groups at 2,4 and 8 weeks after surgery.
Overall,we designed a novel nonload-bearing bone repair system that aimed at promoting bone generation and will have promising prospects in clinical application.This bone repair system consisted of BMP-2 biomimetic peptide(BMPMP),VEGF cytokines,and modified gelatin cryogels.We chose 2% GelMA as a cytokine-coated architecture,which had a hundred-micron aperture structure for cell growth and migration.When the concentration ratio of BMP-2 biomimetic peptide to VEGF was 3:2(0.06 mg/mL:0.04 mg/mL),the prepared double-cytokine-coated cryogel system most effectively promoted the proliferation and differentiation of bone marrow mesenchymal stem cells.In addition,micro-CT analysis ofin vivoanimal experiments showed that the Gel-BMPMP-VEGF group had an obvious advantage in bone repair at 2 weeks after surgery and was almost completely repaired at 8 weeks after surgery.Compared with the other groups,the bone tissue regeneration rate of the Gel-BMPMP-VEGF group was the fastest,and the bone repair degree was the highest.The results of immunohistochemical analysis also showed that the cryogel scaffolds could play an excellent bridging role in the process of bone tissue repair,and the coating of the double cytokines further promoted the process to obtain a better repair effect.In detail,VEGF physically embedded in the cryogel played an important role in promoting vascularization in the early stage;subsequently,the synergistic effect of VEGF and BMP-2 biomimetic peptide promoted the process of osteogenic differentiation and bone tissue repair.
In conclusion,we explored the optimal concentration ratio of BMP-2 biomimetic peptide and VEGFin vitroand demonstrated its effect of promoting bone formationin vivo.The BMP-2 biomimetic peptide and VEGF from the cryogel system could develop synergistic effects on bone defect repair to a certain extent.This paper demonstrated the potential of this double-cytokine-containing cryogel as a bone tissue engineering scaffold and is instructive in promoting research in the field of bone defect repair.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from Science and Technology Program of Guangzhou,China(No.201804010146),Natural Science Foundation of Guangdong Province,China(No.2016A030313341),Science and Technology Planning Project of Guangdong Province,China(No.2014B020215001),National Natural Science Foundation of China(No.51973243),Science and Technology Planning Project of Shenzhen(No.JCYJ20190807155801657).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.070.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
