Oxalic acid cross-linked sodium alginate and carboxymethyl chitosan hydrogel membrane for separation of dye/NaCl at high NaCl concentration
Wenin Xie,Kongyin Zho,*,Lijing Xu,Ningning Go,Hui Zho,Zelong Gong,Linn Yu,Jun Jing
a State Key Laboratory of Separation Membranes and Membrane Processes/National Centre for International Joint Research on Separation Membranes,Tiangong University,Tianjin 300387,China
b SINOPEC Research Institute of Petroleum Processing,Beijing 100083,China
ABSTRACT Dye desalination is a challenge in the treatment of textile wastewater with high salt concentration.It is imperative to develop salt resistance membrane that is from sustainable materials to effectively treat dye/salt mixtures.And most polymer membrane materials are non-renewable petrochemical resources.In this paper,a green hydrogel membrane(CMCS-OA-NaAlg)was prepared by non-metallic ions of oxalic acid(OA)cross-linking of two natural macromolecules of sodium alginate(NaAlg)and carboxymethyl chitosan(CMCS).The membrane showed excellent anti-swelling at high salt concentration(swelling rate less than 8.0% in 10.0 wt% NaCl solution)and good anti-fouling performance.The membrane exhibited a rejection higher than 95.0% for dyes(bright blue,direct black,direct red,and Congo red)and lower than 7.0% for NaCl,which can achieve better dye/NaCl separation performance.This study provides a promising membrane material for high salt textile wastewater treatment only using water and carbohydrates as raw materials without any organic solvents.
Keywords:Hydrogel filtration membranes Oxalic acid Sodium alginate High salt wastewater Salt resistance
With the development of the spinning and dyeing industry,a large amount of printing and dyeing wastewater is produced every year[1,2].The printing and dyeing wastewater includes a large number of dyes and inorganic salts,which are extremely harmful to the environment if it is not treated properly and discharged[3].The dyes and inorganic salts in printing and dyeing wastewater are of good value for recycling[4].Therefore,the separation of dyes and salts from dyeing wastewater becomes critical.Traditional methods for treating printing and dyeing wastewater include biodegradation[5],physical adsorption[6]and catalytic degradation[7,8],but the traditional methods have many disadvantages such as complicated operation,low separation efficiency and high energy consumption[9].
Membrane separation is considered as an economical method for haemodialysis[10]and dyeing wastewater treatment.Dyeing wastewater does not have these disadvantages,but it has special requirements for membrane materials.High concentration salts dyeing wastewater requires membrane materials with good high salt resistance,but many commercial membranes have poor performance in terms of high salt resistance and general anti-fouling property[11,12].Therefore,it is necessary to develop a membrane material to solve these problems.
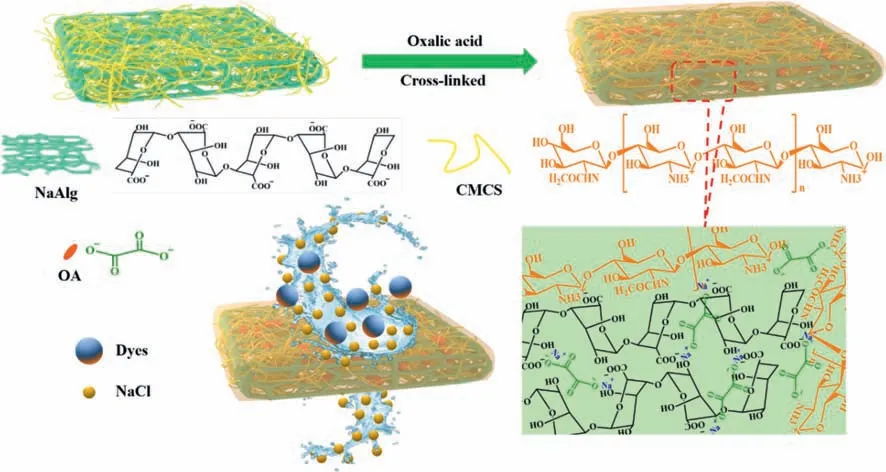
Fig.1.Illustration diagram of the fabrication processes and dyes/NaCl separation of CMCS-OA-NaAlg membrane.
Most of the current membrane materials such as poly(vinylidene fluoride)(PVDF),polyacrylonitrile(PAN)and polyurethane(PU),whose raw materials are non-renewable petrochemical resources and it is not conducive to sustainable development.Sodium alginate(NaAlg)is regarded as a green hydrogel membrane material[13].Zhaoet al.prepared the calcium alginate(CaAlg)hydrogel membrane with excellent anti-fouling property and controlled separation performance by cross-linking with Ca2+and NaAlg[14].Baiet al.prepared the Cu2+/CaAlg hydrogel membrane by Cu2+secondary cross-linking of CaAlg.With three alternating filtration cycles,the flux recovery rate(FRR)of the membrane can still reach 85% without cleaning,and the rejection of Coomassie Brilliant Blue G250 reaches 99.8%,whereas the rejection of Na2SO4is less than 10.0%[15].Zhanget al.prepared the BaAlg-PET membrane using polyester(PET)non-woven fabric as the support layer and by Ba2+cross-linking.The membrane has high dye rejection(methyl blue(MB)>99.7%),low salt rejection(NaCl less than 8.1%)[16].However,common alginate hydrogels are prepared by metal-ion cross-linking,which have poor salt resistance because the monovalent salt displaces the metal ion and thus decreases the hydrogels cross-link density[17].Pérez-Madrigalet al.used small organic molecules to replace metal ions(M2+)to quickly prepare calcium-free hydrogels[18].However,the strength of hydrogel obtained by cross-linking with sodium alginate is very low and the hydrogel cannot be used as filtration membrane.Carboxymethyl chitosan(CMCS)has good biocompatibility and hydrophilicity[19].Chenet al.used carboxymethyl chitosan(CMCS)and NaAlg to prepare a pH-sensitive hydrogel[20].Pérez-Calderónet al.prepared oxalic acid/chitosan hydrogels using oxalic acid cross-linked chitosan,and the hydrogels exhibited good anti-swelling properties and chemical structural stability[21].CMCS plays an important role in improving the mechanical properties of the acid crosslinked hydrogels.Therefore,CMCS and NaAlg are promising to prepare a non-metal ion cross-linked hydrogel membrane.To our knowledge,no study of filtration membrane about M2+-free alginate hydrogel has been conducted so far.
In this paper,oxalic acid cross-linked carboxymethyl chitosan and sodium alginate(CMCS-OA-NaAlg)hydrogel filtration membrane was prepared.The CMCS-OA-NaAlg membrane was characterized by scanning electron microscope(SEM),atomic force microscopy(AFM).Fourier transforms infrared spectra(FT-IR),water contact angle and thermal stability.The swelling properties and anti-fouling properties of the membranes were investigated in high concentration NaCl solution.A series of NaCl(100 g/L)and dye(Coomassie brilliant blue G250(CBB),direct black 38(DB),direct red 23(DR)and,Congo red(CR)0.1 g/L)aqueous solutions were used for filtration tests to evaluate the membrane’s permeability.This membrane has potential application prospects in the separation of dyes,high salt concentration wastewater and purification of active ingredients of Chinese herbal medicine.
Fig.1 shows the schematic diagram of the preparation of CMCSOA-NaAlg hydrogel filtration membrane and the separation of dyes and salts.Firstly,CMCS(2.5 wt%)was dissolved in deionized water,followed by constant stirring and the casting membrane solution was prepared by adding NaAlg with a mass fraction of 3.5 wt%.After vacuum defoaming,the membrane was evenly scraped onto a clean glass plate with a glass rod(with 0.5 mm diameter brass wire wrapped around both ends).The CMCS content of 0.0 wt% and 2.5 wt% of the membrane was named as OA-NaAlg and CMCS-OA-NaAlg.The membranes were obtained after 8 h immersions in oxalic acid(OA)solution with a concentration of 0.5 mol/L.The obtained CMCS-OA-NaAlg membranes were stored in 0.5 mol/L OA solution before any treatment or experiment.
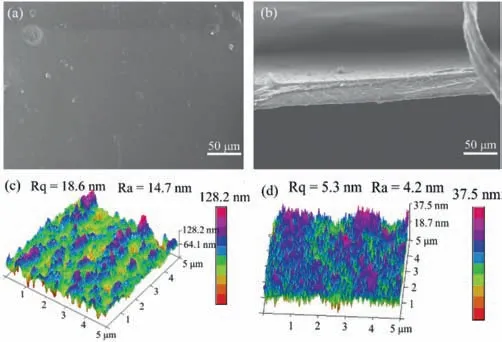
Fig.2.Surface(a)and cross-section(b)SEM image of CMCS-OA-NaAlg membranes(scale bar:50 μm).AFM image of OA-NaAlg membrane(c)and CMCS-OA-NaAlg membrane(d).
Figs.2a and b show the surface and cross-section SEM of the CMCS-OA-NaAlg hydrogel membrane,respectively.It is found in Fig.2a that there are some crystal particles distributed on the surface of the CMCS-OA-NaAlg membrane.These crystal particles are oxalic acid crystals.At the same time,a small number of convex structures can be seen on the surface of the membrane.This may be due to the presence of oxalic acid crystals in the interior of the membrane causing the membrane surface to bulge.It is found in Fig.2b that the membrane is a homogeneous membrane without finger-like pore structure,and some crystals can also be seen inside the membrane.
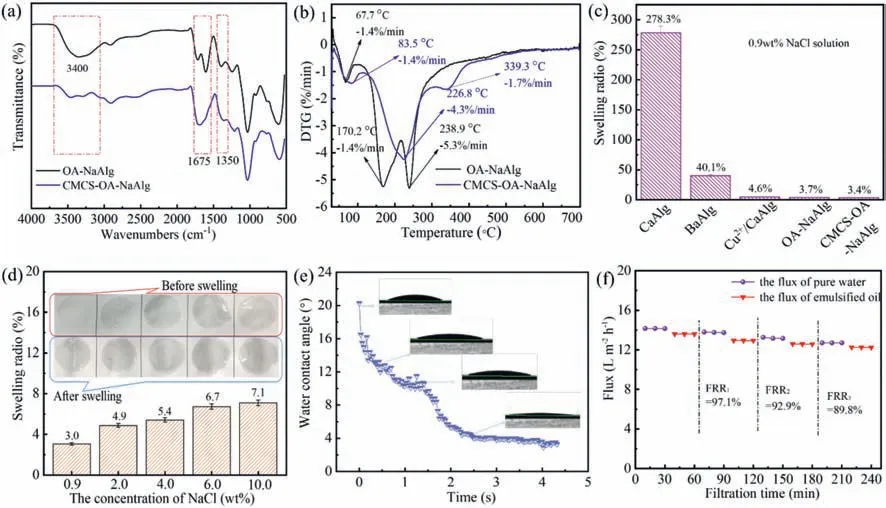
Fig.3.FI-IR spectrum(a)and DTG thermograms(b)of OA-NaAlg and CMCS-OA-NaAlg membrane.(c)Comparation of the swelling performance of CMCS-OA-NaAlg membrane and other alginate hydrogel membrane in 0.9 wt% NaCl solution.(d)Swelling of CMCS-OA-NaAlg membrane with different NaCl concentrations.(e)Water contact angle of CMCS-OA-NaAlg membrane with different time.(F)The FRR of CMCS-OA-NaAlg membrane to emulsified oil.
The surface roughness of the CMCS-OA-NaAlg membrane is further characterized with AFM as shown in Figs.2c and d.The roughness of the surface of the hydrogel membrane decreased more significantly with the addition of CMCS.For the OA-NaAlg hydrogel membrane,Rq=18.6 nm,Ra=14.7 nm(Fig.2c).However,for the CMCS-OA-NaAlg hydrogel membrane,Rq=5.3 nm,Ra=4.2 nm(Fig.2d).The increased interaction between CMCS,NaAlg and OA resulted in a denser three-dimensional interpenetrating network structure of the hydrogel.The addition of CMCS makes the cross-linked binding between C2O42-and NaAlg tighter,which results in a smoother membrane surface.In addition,the low roughness value is helpful to improve anti-fouling performance because it difficult for contaminants to accumulate on the membrane surface through mechanical interlocking[22].
Fig.3a shows the FT-IR plots of OA-NaAlg and CMCS-OA-NaAlg hydrogel membrane.Compared with OA-NaAlg membrane,the peaks of CMCS-OA-NaAlg membrane at 3400 cm-1,1675 cm-1and 1350 cm-1changed significantly.The changed peak at 3400 cm-1indicates that-NH2in CMCS interacts strongly with OA[23].In addition,-NH2also interacts with-OH of NaAlg due to the hydrogen protonation of OA[24,25].The peak at 1675 cm-1is formed by the two shifted peaks to form a peak where the formamide in CMCS forms a strong interaction with the free OA and-COOH in NaAlg,and the hydrogen bonding between the-COO-of NaAlg and CMCS is enhanced at 1350 cm-1.It is indicated that the CMCS makes the interaction between OA and NaAlg more complex,and increases the mutual binding,which forms a denser three-dimensional interpenetrating network.
Fig.3b shows DTG thermograms of OA-NaAlg and CMCS-OANaAlg hydrogel membranes.Both OA-NaAlg and CMCS-OA-NaAlg hydrogel membranes exhibit three different regions of thermal weight loss.For the OA-NaAlg membrane,the first stage around 67.7°C is the decomposition of water absorbed by the material,the second stage at 170.2°C is the loss of bound water in the alginate hydrogel and the third stage at 238.9°C is the decomposition of the NaAlg macromolecule chain cleavage.The decomposition temperature of the three stages of CMCS-OA-NaAlg membrane was improved after the addition of CMCS because CMCS is beneficial to increase the inter-cross-linked entanglement nodes of the membrane,so CMCS can improve the thermal stability of the CMCSOA-NaAlg membrane.
The anti-swelling performance of hydrogel filtration membranes is a relatively important factor in the treatment of high-salt wastewater.Fig.3c shows the swelling rates of CMCS-OA-NaAlg membrane and other alginate-based hydrogels in the literatures.The swelling rate of the alginate-based hydrogel membrane crosslinked by Ca2+,Ba2+,Ca2+/Cu2+(Ca2+:Cu2+=5:1)and OA-NaAlg was 278.3%,40.1%,and 4.6%,respectively in 0.9 wt% NaCl solution[15,16].However,the swelling rates of OA-NaAlg and CMCSOA-NaAlg hydrogel membranes cross-linked by oxalic acid are 3.7% and 3.1%,respectively.Compared with CaAlg and BaAlg,the swelling rates of the CMCS-OA-NaAlg membrane were decreased by 81.9 times and 11.8 times,respectively.Due to the-COOH residing on theβ-D-guluronic acid(G)segment of NaAlg is selectively connected to divalent metal ions.When there are a large number of Na+in the system,the divalent cations will be exchanged with the monovalent cations,which reduces the degree of crosslinking[26].OA can form a complex and tight electrostatic interaction with CMCS as well as NaAlg with an increased cross-link density[27],which makes the whole hydrogel membrane maintains good stability in high concentration salt wastewater.
Fig.3d shows the swelling rates of CMCS-OA-NaAlg in different concentrations of NaCl.As the concentration of NaCl increasing,the swelling rate of the membrane increases gradually,but even in 10.0 wt% NaCl solution,the swelling rate is still less than 8.0%.The density of the solution penetrating the interior of the hydrogel increases with the increasing of the NaCl concentration.So the swelling rates of the CMCS-OA-NaAlg membrane increase gradually.There was no change in the membrane before and after swelling in NaCl solution.It fully demonstrates that the CMCS-OA-NaAlg membrane has good anti-swelling property,and the membrane has potential significance in the treatment of highsalt wastewater.
The anti-fouling performance of membrane is an important factor to ensure the long-term stable operation and is closely related to the hydrophobicity of the membrane surface.Fig.3e shows the dynamic water contact angle of the CMCS-OA-NaAlg hydrogel membrane.The water contact angle of the CMCS-OA-NaAlg hydrogel membrane gradually decreased from 20.3° to 3.5° in 2.5 s,indicating that the membrane has good hydrophilicity.Because the increase of the hydrogen bond between the-NH2of CMCS and the-COOH of NaAlg makes the entire membrane structure denser and also makes more OA attached to the surface of the membrane.So the membrane surface is super hydrophilic.
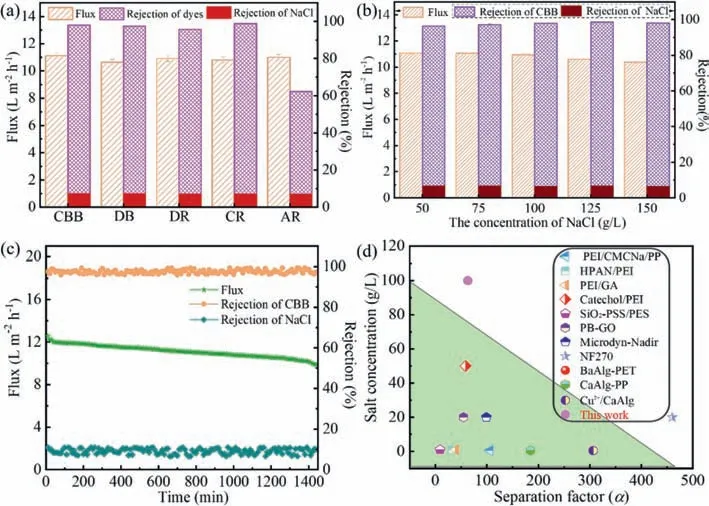
Fig.4.Flux and rejection of CMCS-OA-NaAlg membranes:Different dyes(a),the concentration of NaCl(b),long-term stability(c).(d)Comparison of the performance of the CMCS-OA-NaAlg membrane with that of commercial membranes and other membranes reported previously.
In order to further test the anti-fouling performance of the membrane,emulsified oil was used as a pollutant,and the flux recovery rate(FRR)was evaluated by alternately filtering pure water and emulsified oil.Fig.3f shows that the FRR was greater than 97.1% after the first cycle,92.9% after the second cycle,and 89.8%after the third cycle.The CMCS-OA-NaAlg hydrogel membrane still shows excellent anti-fouling performance even the membrane has not undergone any cleaning process due to the super hydrophilic and low roughness values of CMCS-OA-NaAlg hydrogel membrane surface[28,29].Superhydrophilicity facilitates the formation of a hydration layer on the membrane surface,thus reducing the interaction forces between hydrophobic organic contaminants and the membrane surface[28],and ultimately the organic contaminants attached and deposited on the membrane surface are reduced.
Fig.4 shows the dye/NaCl separation performance of CMCS-OANaAlg hydrogel membrane under different conditions.It is found in Fig.4a that there is no obvious change for the flux and rejection with decreasing molecular weight of dyes.The rejection of NaCl is less than 7.0%,and the rejection of dyes with molecular weights greater than 696 Da(CBB,DB,DR and CR)are greater than 95%,but the rejection of AR with molecular weight 604 Da is only 62%.This membrane can well achieve the separation of dyes with molecular weight greater than 696 Da and the rejection molecular weight of the membrane is 696 Da.
During membrane treatment of dye wastewater and dye separation,NaCl concentration has a significant effect on the separation performance.The CMCS-OA-NaAlg membrane was evaluated to explore the influence of different NaCl addition concentrations(50–150 g/L)and kept the CBB concentration at 0.1 g/L.It is found in Fig.4b that the flux of the mixed solution decreased slightly with the increase of NaCl concentration from 50 g/L to 150 g/L.However,the CMCS-OA-NaAlg membrane remains stable for the rejection of dye and NaCl,indicating its enormous potential for treating highsalinity textile wastewaters.
From the perspective of practical applications,the separation stability of the membrane is an important consideration for long-term filtration.The CBB/NaCl mixture solution(0.1 g/L CBB and 100 g/L NaCl)was used for 24 h continuous filtration tests at 0.1 MPa to investigate the membrane separation stability,as shown in Fig 4c.During the 24 h continuous filtration process,the permeation flux of CMCS-OA-NaAlg membrane dropped from 12.6 L m-2h-1to 9.9 L m-2h-1.In addition,the rejection of CBB and NaCl remained stable during the entire operation.After 24 h of filtration,the rejection of CBB and NaCl were 98.5% and 7.0%,respectively.The membrane has good long-term stability.
Fig.4d and Table S2(Supporting information)summarize the separation factor(α)and salt concentration of some filtration membranes for dye/NaCl separation,compared with CMCS-OANaAlg membrane.The separation factor of CMCS-OA-NaAlg membrane is 63.0,which is similar to most membranes.But the salt concentration over 100 g/L used in this paper is far greater than other membranes.Especially,comparing with similar hydrogel membranes Cu2+/CaAlg,CaAlg-PP,and BaAlg-PET[15,16],the salt concentration increased from 0.5 g/L to 100 g/L,which is a good solution to the problem that metal cross-linked alginate hydrogel cannot be applied to treat high salinity dye wastewater.Therefore,this membrane has important research significance for the treatment of high salinity printing and dyeing wastewater.In future work,we will continue to investigate the improvement of the separation coefficient of CMCS-OA-NaAlg membrane.
In this study,highly salt-resistant alginate-based hydrogel membranes were prepared using non-metallic ions(oxalic acid)as cross-linking agents.The swelling rate of the CMCS-OA-NaAlg hydrogel membrane was less than 3.4% in 0.9 wt% NaCl solution.Furthermore,the CMCS-OA-NaAlg membrane demonstrated excellent anti-fouling performance by maintaining a flux recovery rate of 89.8% after three cycles of emulsified oil contaminants.Besides,the rejection of NaCl was less than 7.0% in the mixed solution of dye/NaCl with high-salt(100 g/L),and the membrane also showed more than 95.0% rejection of typical dyes(CBB,DB,DR,CR),which was of great significance for achieving dye/NaCl separation of high salinity textile wastewater.In addition,the membrane still maintained a good separation of dye/NaCl during long-term filtration.The use of OA instead of traditional metal ions for the preparation of NaAlg hydrogel membranes can effectively improve the salt resistance of hydrogel membranes,which was expected to be extended to more organic small molecules for the preparation of hydrogel membranes.It is worth noting that the membrane has a much-improved salt resistance compared to commercial membranes and also has excellent anti-fouling performance.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The research is supported by the National Natural Science Foundation of China(Nos.51708407 and 51803150),and the Science and Technology Plans of Tianjin(Nos.19JCQNJC02900,18ZXJMTG00120,20JCYBJC00120).
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
