Natural lotus root-based scaffolds for bone regeneration
Keqing Huang,Jun Huang,Jinmin Zhao,Zhipeng Gu,Jun Wu
a School of Biomedical Engineering,Sun Yat-sen University,Shenzhen 518107,China
b Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation,Sun Yat-sen Memorial Hospital,Sun Yat-sen University,Guangzhou 510120,China
c Guangxi Engineering Center in Biomedical Materials for Tissue and Organ Regeneration,the First Affiliated Hospital of Guangxi Medical University,Nanning 530021,China
d Research Institute of Sun Yat-sen University in Shenzhen,Shenzhen 518057,China
e College of Polymer Science and Engineering,State Key Laboratory of Polymer Materials Engineering,Sichuan University,Chengdu 610065,China
f Guangdong Academy of Sciences,Institute of Biological and Medical Engineering,Guangzhou 510316,China
1 These authors contributed equally to this work.
ABSTRACT A high incidence of bone defects and the limitation of autologous bone grafting require 3D scaffolds for bone repair.Compared with synthetic materials,natural edible materials possess outstanding advantages in terms of biocompatibility,bioactivities and low manufacturing cost for bone tissue engineering.In this work,attracted by the natural porous/fabric structure,good biocompatibility and bioactivities of the lotus root,the lotus root-based scaffolds were fabricated and investigated their potential to serve as natural porous bone tissue engineering scaffolds.The results indicated that the lotus root-based scaffolds possess suitable natural microstructure,excellent biocompatibility and promising functions,such as antioxidant capacity and angiogenesis promotion.Remarkably,lotus root scaffolds showed encouraging possibility of bone tissue engineering while the mineralized lotus root could further improve the bone regeneration in vivo.All the results demonstrated the bone regeneration potential of lotus root-based scaffolds equipped with suitable natural architecture,excellent biocompatibility,specific bioactivities and low manufacturing cost.
Keywords:Bone regeneration Lotus root Porous structure Mineralization
A high incidence of bone defects and the limitation of autologous bone grafting require the development of bone tissue engineering scaffolds with high efficacy[1,2].It has been well documented that the properties of ideal bone tissue engineering scaffolds are as follows:(1)high porosity microstructure with proper pore size(usually 100–200 μm),which are supposed to support cells attachment and migration with proper mechanical performance during controlled degradation;(2)biocompatibility,which could benefit the proliferation and differentiation of cells;(3)specific functions favorable for the enhanced tissue regeneration[3–8].Currently,many systems,including poly-L-lactic acid(PLLA),polyglycolic acid(PGA),poly(lactic-co-glycolic acid)(PLGA)and collagenbased derivatives,have been widely used to fabricate the bone tissue engineering scaffold,while various methods such as freezedrying,fiber bonding,foaming,and salt soaking have been utilized to create porous architecture[9–13].Compared with synthetic materials,natural materials,especially natural edible materials,might offer new options for the fabrication of scaffolds for improved bone tissue engineering[14–16].Gelatin,alginate or agarose have been investigated as bone tissue engineering scaffolds owing to their excellent biocompatibility and proper biodegradation[17,18].Remarkably,some proteins or carbohydrates derived from animal or plant based edible materials could exert an effect on shaping cell behaviorviachemical signals and biocompatibility[19–23].Nevertheless,only very limited edible materials have been studied for tissue engineering so far,and the previous studies tend to focus on one aspect of structure,composition or function of edible materials rather than the whole,leading to the delayed investigations of edible materials for tissue engineering applications.
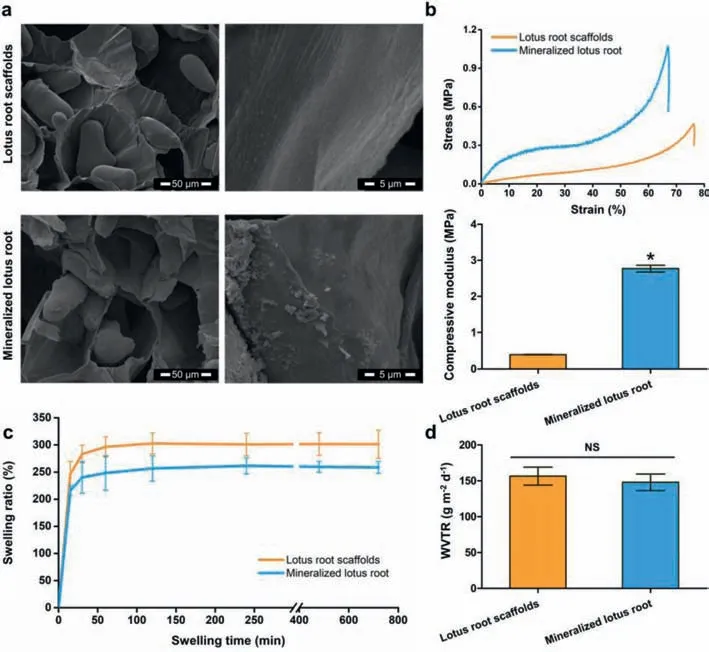
Fig.1.Characterization of lotus root-based scaffolds.(a)The interior morphology of lotus root-based scaffolds.(b)Mechanical properties of lotus root-based scaffolds.(c)Swelling kinetics of lotus root-based scaffolds.(d)The WVTR of lotus root-based scaffolds with thickness at 0.5 mm.*P <0.05 as compared to the lotus root scaffolds.NS:no significant difference.
Lotus root,the rhizome of the lotus plant,a common vegetable in Asia,possesses a satisfactory natural porous structure with sufficient dietary fibers,vitamins and minerals,such as calcium,phosphorus and iron[24,25].Inspired by the microstructure of lotus root,preliminary experiments have studied the bionics of its unique structurevia3D printing strategies[26,27].The biomimetic materials with lotus root like structures showed improved cell attachment and enhancedin vivoosteogenesis,suggesting good potentiality for bone regeneration[26].Furthermore,it could be noticed that after incision the lotus root was darkened,which could be attributed to the phenolic compounds,the metabolites and natural antioxidants involved in plant growth,suggesting good antioxidant capacity of the lotus root[28].In traditional Chinese herb medicine,processed lotus root have been utilized for adjuvant therapy benefiting from the promising constituents[29].With porous microstructure,good biocompatibility and specific antioxidant bioactivities,the lotus root may do benefit to bone tissue engineering by serving as a natural scaffold with low manufacturing cost.Nevertheless,the potentials of lotus root as tissue engineering scaffolds have not been investigated yet.
Hence in this study,the lotus root scaffold with simple modification has been prepared for the first time to explore the potential as a natural scaffold for bone regeneration.Taking advantage of the microstructure of lotus root,a simple mineralization was further performed to improve the osteogenesis performance of lotus root.More details on fabrication of lotus root scaffolds could be found in Supporting information.
The intact lotus root usually possesses 3–5 tubers,and each segment could be about 10–20 cm in length and 5–10 cm in diameter.The internal morphologies of lotus root-based scaffolds have been investigated by scanning electron microscope(SEM).The SEM images revealed that the lotus root scaffolds possessed porous architecture with mean pore diameter at ~100 μm and starch grains at ellipsoidal shape(about 30 μm × 60 μm)(Fig.1a).And magnified 10,000 times by SEM,the surface of the hole wall in the lotus root revealed specific pattern of parallel strips,while the lotus root scaffold with mineralization showed irregular crystals on the surface of the hole wall.The rough texture of the hole wall may benefit the attachment of crystals,as well as cells,indicating promising potential of serving as bone tissue engineering scaffolds.
The typical stress-strain curve of each group showed that,compared with the lotus root scaffold,the mineralized lotus root scaffold could withstand greater loads with higher compressive modulus(Fig.1b).The mineralization of lotus root scaffold took the advantage of the specific patterns and internal morphologies of lotus root and introduced the bone-like surface apatite to benefit the mechanical behavior.Both the lotus root scaffold and mineralized lotus root scaffold could absorb moisture promptly and reached the swelling equilibrium in less than 2 h,suggesting suitable hydroscopicity for providing enough fluid with nutrition for cells during the bone regeneration(Fig.1c).Moreover,there was no significant difference in water vapor transmission rate(WVTR,g m-2d-1)between lotus root scaffolds and mineralized lotus root scaffolds,suggesting that the mineralization had no obvious effect on the permeability of the scaffolds(Fig.1d).All the characterization indicated that the porous lotus root-based scaffolds possessed unique internal morphologies with low density and high porosity,satisfactory mechanical properties,proper hygroscopicity and permeability,indicating the promising use in bone tissue engineering.

Fig.2.Cytocompatibility and antioxidant capacity of lotus root-based scaffolds.(a)The live/dead staining assay.Scale bar = 50 μm.(b)MTT assay.(c)DPPH radical scavenging capacity measurement.The percentage of radical scavenging efficiency was described as antioxidant activity(AA).(d)ROS levels of HUVECs cells induced by H2O2 with the treatment of lotus root-based scaffolds.Scale bar = 100 μm.(e)Quantitative analysis of the fluorescence intensity has been performed by imageJ.*P <0.05 as compared to the negative control group.NS:no significant difference.
During the process of bone regeneration,the implanted lotus root-based scaffolds could obviously affect the viability of cells.Therefore,the cytocompatibility of the lotus root-based scaffolds needs to be determined to ensure that the interaction between the cells and the lotus root-based scaffolds would not lead to the decreased cell viability.The human dental pulp stem cells(hDPSCs)have been employed as a kind of neural crests derived cell with MSC characteristics and the live/dead staining and MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)assay were utilized to explore the cytocompatibility of scaffolds.It can be observed that hDPSCs co-cultured with scaffolds exhibited elongated spindles,with green fluorescence,indicating good cell viability(Fig.2a).Moreover,the optical density(OD)values in the MTT analysis also demonstrated that the cells co-cultured with the lotus root scaffolds and mineralized lotus root scaffolds showed no significant difference in cell viability with good proliferation status compared with the control group without treatment during the culture period(Fig.2b).It was worth noting that the cells in the porous lotus root scaffold could grow in a three-dimensional(3D)environment with good morphology(Fig.S1 in Supporting information).
As a scaffold for bone tissue engineering,the immune responses should also be considered before implantation.The immune response of macrophagesin vitrounder the stimulation of lotus root was investigated(Figs.S2–S4 in Supporting information).The results suggested that lotus root could motivate slight immune response of macrophages at high concentrations.Moreover,the hemolysis of lotus root-based scaffolds could not be observed compared with the group treated with dd water and NaCl(0.9%),suggesting the good hemocompatibility of lotus root-based scaffolds(Fig.S5 in Supporting information).Meanwhile,whole blood clotting test was investigated to evaluate the hemostasis effect of lotus root-based scaffoldsin vitro.The results showed that the lotus root-based scaffolds possessed enhanced hemostasis capability compared with gelatin sponge,which may be conducive to the closure of the trauma site and subsequent tissue regeneration(Fig.S6 in Supporting information).All the results proved that the porous lotus root-based scaffolds possessed excellent biocompatibility to be utilized in bone regeneration.
The antioxidation of the lotus root-based scaffolds were detected by DPPH(1,1-diphenyl-2-picrylhydrazyl)radical scavenging capacity measurement and intracellular reactive oxygen species(ROS)evaluation.Compared with the lotus root group,the group with mineralized lotus root showed no obvious difference in radical scavenging efficiency(Fig.2c).Compared with the positive control group,the enhanced cell viability could be observed in the groups treated with lotus root-based scaffolds,indicating excellent antioxidation of lotus root-based scaffolds.The ROS levels of human umbilical vein endothelial cells(HUVECs)induced by H2O2were measured by using the probe 2′,7′-dichlorofluorescin diacetate(DCFH-DA)(Figs.2d and e).The positive control group treated with H2O2showed reduced cell number and increased green fluorescence compared to the other groups.Compared with the negative control group,no significant difference could be observed for cells in the groups treated with lotus root-based scaffolds,which could further indicate the good antioxidation of lotus root-based scaffolds.
To verify the effects of lotus root-based scaffolds on cells migration,in vitroexperiments on the migration of HUVECs were performed by the scratch test assay.As shown in Figs.S7a and b(Supporting information),migration of HUVECs was enhanced under the stimulation of lotus root-based scaffolds compared to the control group in 12 h and no significant difference could be observed among the various treatments.The lotus root-based scaffolds were efficient in inducing the migration of HUVECs,and the coverage of the scratch area by HUVECs could be completed in no more than 24 h.Moreover,the secretion of VEGF showed significant increase with the treatment of lotus root-based scaffold compared with control group(Fig.S7c in Supporting information).Meanwhile,the lotus root-based scaffolds significantly stimulated tube formation of HUVECs compared with control group(Figs.S8 and S5 in Supporting information).Collectively,the results indicated good angiogenesis capacity of lotus root-based scaffolds,suggesting good potentiality in bone tissue regeneration.
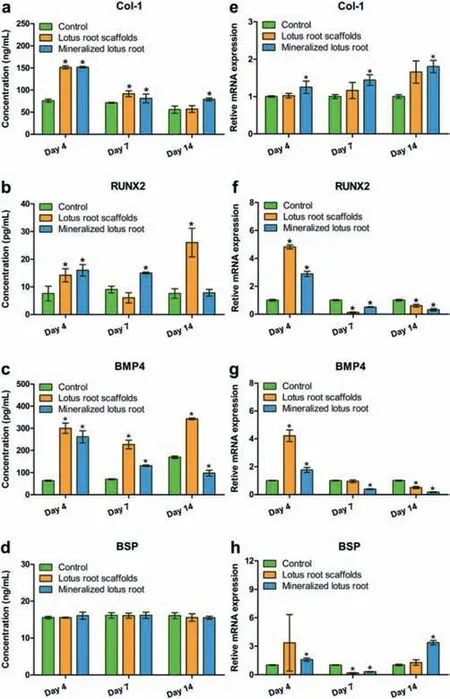
Fig.3. In vitro osteogenic differentiation of hDPSCs co-cultured with lotus rootbased scaffolds.(a–d)The secretion of osteogenesis-related proteins in the culture medium measured by ELISAs.(e–h)Expression of osteogenesis-related genes in hDPSCs evaluated with RT-qPCR and the data was normalized to GAPDH expression.*P <0.05 as compared to the control group.
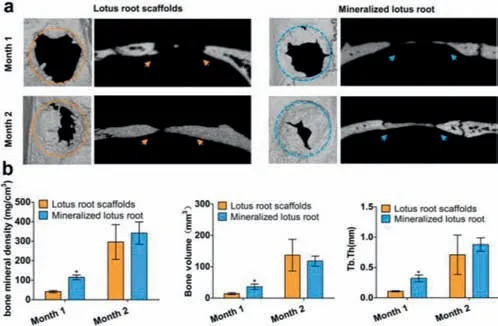
Fig.4.Bone formation in vivo.(a)Representative micro-CT images of bone defects.(b)Quantitative analysis of bone mineral density,bone volume and Tb.Th.*P <0.05 as compared to the group treated with lotus root scaffolds.
As bone regeneration implants,lotus root-based scaffolds are required the appropriate ability to promote osteogenic differentiation of cells.Here,enzyme-linked immunosorbent assay kits(ELISA)and real-time quantitative polymerase chain reaction(RTqPCR)were deployed to confirm the osteogenic related proteins and genes expression in hDPSCs under the stimulation with lotus root-based scaffold.The ELISA results affirmed that the concentrations of type I pro-collagen(Col-1),runt-related transcription factor 2(RUNX2)and bone morphogenetic protein 4(BMP4)in hDPSCs treated with lotus root-based scaffolds significantly increased compared to the control group,especially on day 4(Figs.3a–d).The concentrations of RUNX2 and BMP4 in hDPSCs co-cultured with the lotus root scaffolds increased on day 14,while the concentrations of RUNX2 and BMP4 in hDPSCs under the stimulation of mineralized lotus root scaffolds significantly increased on day 7,indicating that the mineralized lotus root scaffolds could promote the osteogenic differentiation during bone formation.Meanwhile,the RT-qPCR results further showed the expression of corresponding osteogenic differentiation-related genes(Figs.3e–h).The expression of the Col-1 showed an upward trend during the 14-day incubation,while the mineralized lotus root scaffold group possessed a significantly different from the control group.The gene expressions of RUNX2,BMP4,and bone sialoprotein(BSP)were all up-regulated in both the lotus root scaffold group and mineralized lotus root scaffold group on day 4,and the down-regulation trend appeared after 7-day incubation,which may because the stimulation of lotus root-base scaffolds accelerate the process of osteogenic differentiation regulation.Allin vitroresults show that porous lotus root-based scaffolds can promote osteogenic differentiation with promising bone regeneration potential.
Encouraged by the resultsin vitro,the ability of lotus root-based scaffolds to promote bone regeneration was continuously investigatedin vivo.After 1-or 2-month implantation of lotus rootbased scaffolds in the bone defects(diameter,5 mm)of SD rats,the bone tissue corresponding to the defect areas in each group was collected for further investigation.Since the untreated control group did not survive more than one month after the experiment,no bone tissue has been collected from the control group.New bone formation could be observed both in the lotus root scaffolds groups and mineralized lotus root scaffolds groups,while the significantly increased new bone formation could be found in the mineralized lotus root scaffolds group compared with the group without mineralization(Fig.4a).In addition,statistical analysis including bone mineral density,bone volume,and trabecular thickness(Tb.Th)proved that the mineralized lotus root scaffold could accelerate new bone formation with more mature bone tissue after 1-month implantation(Fig.4b).The specific states of bone regeneration treated with lotus root-based scaffolds were further exploredviahistological and immunohistochemical examinations(Fig.S9 in Supporting information).All the results demonstrated that the natural porous lotus root-based scaffold with satisfactory cytocompatibility,especially the mineralized lotus root scaffold,possessed promising ability to promote osteogenic differentiation and accelerate new bone formation.
In this work,inspired by the natural porous and fabric structure of the lotus root,the lotus root-based scaffolds were prepared and investigated about their potential to serve as bone tissue engineering scaffolds.The lotus root-based scaffold possesses good natural porous structure,excellent biocompatibility,and bioactivities favorable for bone repairing,such as antioxidant capacity and angiogenesis promotion.Remarkably,after mineralization,the lotus root scaffolds showed further improved bone regeneration performancein vivo.All the results indicated that the lotus root-based scaffolds with natural porous architecture,robust bioactivities and low manufacturing cost possessed high potential for bone regeneration applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.51503129),Guangdong Innovative and Entrepreneurial Research Team Program(No.2016ZT06S029),Fundamental Research Funds for the Central Universities(No.191 gzd35),Shenzhen Basic Research Project(No.JCYJ20190807155801657)and Science and Technology Planning Project of Shenzhen(No.JCYJ20180307163534533),Guangdong Basic and Applied Basic Research Foundation(No.2019A1515110686).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.073.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
