Near-infrared light(NIR)-responsive nanoliposomes combining photodynamic therapy and chemotherapy for breast tumor control
Gungzho Lu,Xiqing Go,He Zhng,Yeye Zhng,Yun Yu,Zhiguo Sun,Wei Li,Wei Wu,Ying Lu,*,Ho Zou,*
a Department of Pharmaceutical Sciences,School of Pharmacy,Naval Medical University,Shanghai 200433,China
b Department of Pharmacy,Zhongshan Hospital,Fudan University,Shanghai 200032,China
c Laboratory of Nano Biomedicine &International Joint Cancer Institute,Naval Medical University,Shanghai 200433,China
d Key Laboratory of Smart Drug Delivery of MOE,School of Pharmacy,Fudan University,Shanghai 200032,China
1 These authors contributed equally to this work.
ABSTRACT Light-responsive carriers have been used for the controlled release of antitumor drugs in recent years.However,most light-responsive vectors require high-energy ultraviolet or visible light to achieve local drug release,and ultraviolet light would cause cellular damage.Near-infrared light has a deeper tissuepenetration depths and minimal harm to tissues,but it is difficult to cleave the chemical bond directly.The aim of this study is to develop a novel near-infrared light-responsive carrier for local release of antitumor drugs.Unsaturated phospholipids can be oxidized by singlet oxygen to achieve liposomal drug release,and singlet oxygen can be produced by photosensitizer under light irradiation.A new near-infrared light-responsive nanoliposome was designed that imparts light-triggered local drug release.Nanoliposomes,which were composed of matrix phospholipids and unsaturated phospholipids,were prepared by ammonium sulfate gradient method,and loaded with antitumor drug doxorubicin(DOX)and photosensitizer 1,4,8,11,15,18,22,25-octabutoxypalladium phthalocyanine.Under near-infrared light,photosensitizers could produce singlet oxygen and damage tumor cells by photodynamic therapy.Simultaneously,the unsaturated phospholipids were oxidized by singlet oxygen and result in DOX release,causing sustained cell damage by chemotherapy.Near-infrared light-responsive nanoliposomes exhibit enhanced anticancer activity owing to combined treatment of photodynamic therapy and chemotherapy.A new platform is thus offered for designing effective intracellular drug-release systems,holding great promise for future cancer therapy.
Keywords:Near-infrared light Unsaturated phospholipids Nanoliposomes Photosensitizer Singlet oxygen
Smart drug delivery systems are commonly applied pharmaceutical carriers because these activable delivery vehicles can control drug release with increased tumor selectivity and reduced toxicity[1].Smart drug delivery systems typically exhibit fast responses to a variety of stimuli,including temperature[2],light[3,4],pH[5],ultrasound[6],magnetic field[7],redox[8],and reactive oxygen species[9].Among these,light-triggered carriers have shown a significant advantage in tumor therapy,mainly due to their high spatiotemporal resolution in noninvasive manipulation[10].However,most light-responsive vectors require high-energy ultraviolet or visible light,which causes cellular damage and owns poor penetration depth[11–14].Therefore,near-infrared(NIR)light-responsive carriers become potential drug delivery systems because NIR with the range of wavelengths from 650 nm to 1350 nm owns deeper tissue-penetration depths,photothermal effect and minimal harm to tissues.Thus,NIR light was used in many responsive carriers to achieve drug local release and reduce side effects[15,16].
NIR-responsive carriers realize drug release by using photothermal responsive materials,singlet oxygen-labile materials and upconversion nanoparticles[17,18].Most vectors composed of singlet oxygen-labile materials usually were prepared as micelles,which could not maintain nano morphology for a long time due to critical micelle concentration.Herein,a novel NIR-responsive nanoliposome composed of singlet oxygen-labile materials unsaturated phospholipids was designed.Under NIR light irradiation,some photosensitizers could produce singlet oxygen,which could peroxide unsaturated phospholipids(such as egg phosphatidylcholine(EggPC),1,2-dioleoyl-sn–glycero-3-phosphocholine(DOPC)and 1,2-dilinoleoyl-sn–glycero-3-phosphocholine(DLPC))to form lipid peroxides[19].The peroxidation of phospholipids increased the hydrophilicity of phospholipids,resulting in fragmentation of nanoliposomes composed of unsaturated phospholipids[19–21].A higher reactivity with singlet oxygen can be achieved using lipids with biallylic hydrogens(from methylene groups adjacent to two double bonds)than lipids with singly allylic hydrogens(from methylene groups adjacent to one double bond)[20].
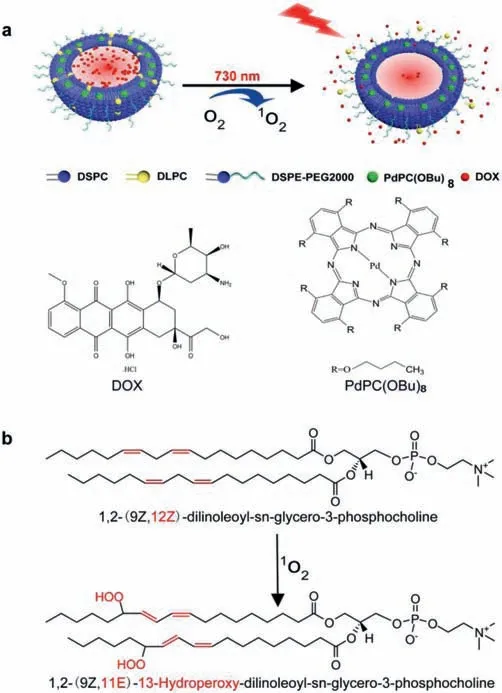
Fig.1.Schematic illustration showing the NIR-responsive drug release of LPD made of DSPC,DLPC,cholesterol and DSPE-PEG2000.(a)LPD constructed by encapsulating PdPC(OBu)8 and doxorubicin hydrochloride(DOX)exhibit a fast drug release under light irradiation(730 nm).(b)LPD realized drug release owing to the Peroxidation of DLPC.
Nanoliposomes that allow photosensitizers to effectively generate singlet oxygen from tissue oxygen under light irradiation,have shown great capacity to selectively damage cancer cells for enhanced therapeutic efficiency[4].In order to obtain phospholipids peroxides and increase the permeability of the phospholipids bilayer,photosensitizers showing high singlet oxygen production are required.1,4,8,11,15,18,22,25-Octabutoxypalladium phthalocyanine[PdPC(OBu)8]can produce a large amount of singlet oxygen under the NIR-light irradiation,it can meet the requirements of phospholipids peroxidation.What is more,it is a NIRabsorbing photosensitizer with absorption peak(730 nm),which owns deeper tissue-penetration depths[20].
Here,we introduced a novel NIR-responsive nanoliposomes that constructed by encapsulating PdPC(OBu)8and doxorubicin hydrochloride(DOX)(nanoliposomes loaded with PdPC(OBu)8and DOX,LPD)that imparted light-induced drug release(Fig.1a).DOX is one of the most widely used chemotherapeutic agents that can be used for the treatment of a wide range of cancers.The responsive release of DOX can reduce side effects.
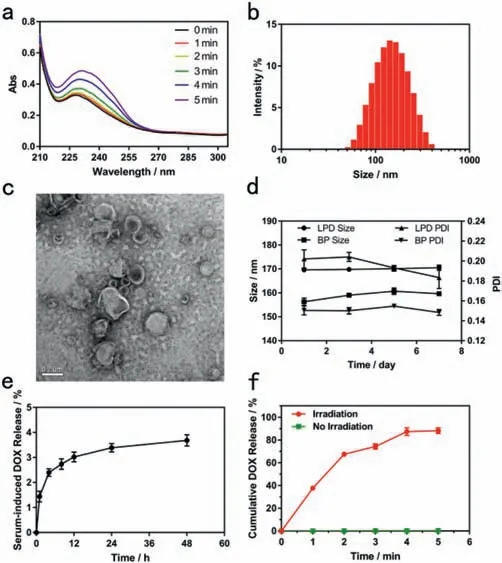
Fig.2.(a)Effect of irradiation on an ethanolic mixture of DLPC and PdPC(OBu)8.(b)Particle size distribution of LPD measured by using DLS.(c)Transmission electron microscopy(TEM)image of LPD.(d)The changes of size and PDI of LPD stored at 4 °C.(e)DOX leakage rate of LPD in 10% FBS at 37 °C.(f)Cumulative DOX release from the LPD under light irradiation(730 nm,300 mW/cm2).
The synthesis of PdPC(OBu)8was based on the previously reported method[22].PdPC(OBu)8was characterized by UV–vis absorption,the absorption peak at 729 nm in ethanol(Fig.S1 in Supporting information).To demonstrate the phototriggering mechanism,a mixture of DLPC and PdPC(OBu)8was irradiated in ethanol at 730 nm(300 mW/cm2).A new absorption peak(233 nm)suggested the formation of conjugated dienes,products of unsaturated phospholipids peroxidation(Figs.1b and 2a)[20].With the increase of illumination time,the peak value increased gradually.The results showed that DLPC was peroxidated with the increase of illumination time.
The nanoliposomes were composed of DLPC(18:2(cis))or DOPC(18:1(cis))PC)or hydrogenated soybean phospholipids(HSPC),1,2-distearoyl-sn–glycero-3-phosphocholine(DSPC),DSPEmPEG2000and cholesterol.We fabricated NIR-responsive nanoliposomes according to ammonium sulfate gradient method.We firstly demonstrated whether 18:2(cis)PC could result in faster release compared to 18:1(cis)PC.We found that DLPC(18:2(cis)PC)showed faster drug release compared to DOPC(18:1(cis)PC),consistent with previous studies[19,20,22].The optimal prescription of NIR-responsive nanoliposomes was investigated in terms of encapsulation efficiency,drug loading,NIR-response rate and the stability in serum.We found that the optimal prescriptions of nanoliposomes was[DLPC:cholesterol:DSPE-mPEG2000:DSPC],[5:40:5:50,mol%]with a drug to lipid molar ratio of 1:100 for PdPC(OBu)8and a drug to lipid molar ratio of 1:10 for DOX.
The distribution of particle size was analyzed using dynamic light scattering(DLS).LPD exhibited a spherical morphology with 169.3 ± 1.2 nm hydrodynamic diameter in aqueous solution(Fig.2b).To characterize self-assembled nanoliposomes,transmission electron microscopy(TEM)was used to observe their morphology.The diameter of the nanoliposomes is approximately 150 nm(Fig.2c),which was not significantly different from the particle size measured by DLS.Encapsulation efficiency(EE)and drug loading(DL)were measured by using HPLC or UV–vis respectively(Figs.S2 and S3 in Supporting information).The encapsulation efficiency of PdPC(OBu)8and DOX were 84.43% ± 3.12%and 91.03% ± 3.33%,respectively.The drug loading PdPC(OBu)8and DOX were 0.42% ± 0.01% and 9.84% ± 0.40%(Table S1 in Supporting information).
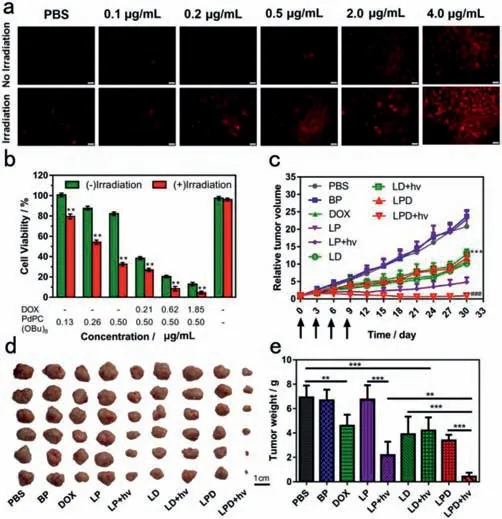
Fig.3.(a)Intracellular ROS detection of ROS-responsive liposome with DHE staining(scale bar = 100 μm).(b)Cell viability of MCF-7 cells incubated with LPD before or after laser irradiation(730 nm)at a power intensity of 1200 mW/cm2 for 3 min.**P <0.01,compared with the control group.(c)Tumor growth profile of the mice bearing MCF-7 tumor under 5.0 mg/kg of DOX and 1.0 mg/kg of PdPC(OBu)8.***P <0.001,compared with the PBS group.###P <0.001,compared with the LPD group.(d)Photograph of the tumors extracted from the mice bearing MCF-7 tumor.Scale bar = 1 cm.(e)The tumor weight of tumor-bearing mice administered with PBS,BP,DOX,LP,LD and LPD.**P <0.01,***P <0.001.
To evaluate storage stability of nanoliposomes,nanoliposomes were stored at 4 °C in closed amber vials without any other precautions and nanoliposomes were periodically removed for routine analysis.Size and polydispersity(PDI)were assessed every two days.The particle size of LPD remained close to 170 nm,together with a low PDI of close to 0.20,indicating LPD exhibited excellent storage stability.The particle size of blank nanoliposomes(BP)was about 160 nm and the PDI was about 0.15(Fig.2d).We also investigated the stability of LPD in 10% FBS at 37 °C.After 48-h incubation in 10% FBS,the DOX leakage rate of LPD was less than 5%(Fig.2e),indicating that LPD had good stability in 10% FBS.
To quantify the drug release,the nanoliposomes were subjected to 730 nm light irradiation and then the released drug was measured by using Fluorescence Spectrophotometer.Because PdPC(OBu)8owns absorption peak(730 nm),so 730 nm was used at following experiments.LPD almost did not release DOX without irradiation(Fig.2f),but it released DOX rapidly upon irradiation(730 nm,300 mW/cm2,5 min).After light irradiation for 5 min,95% DOX release was observed.The irradiation time required for 90% DOX release was less than 5 min.
To evaluate whether the nanoliposomes could generate intracellular ROS,DHE as a fluorescent probe was performed(Fig.3a).PBS as a control almost showed no red fluorescence before or after irradiation(730 nm,1200 mW/cm2,3 min),suggesting that PBS didn’t generate ROS before or after light irradiation.In the absence of light irradiation,the probe showed a dose-dependent increase in fluorescence.DHE exhibited significant red fluorescence when LPD under irradiation,indicating that LPD generate intracellular ROS upon irradiation.
To determine whether near-infrared light(730 nm)itself could kill MCF-7 cells,cell viability under different intensity illumination for 3 min was tested.It could be seen that the viability rate of MCF-7 cells treated with anyone intensity illumination was calculated to be more than 90%.It indicated that the near-infrared light(730 nm)illumination(light intensity even up to 1200 mW/cm2)was not toxic to MCF-7 cells(Fig.3b).Therefore,the light intensity of 1200 mW/cm2can be used as the illumination intensity on MCF-7 cells.As shown in Fig.3b,without light irradiation,as the concentration of photosensitizer gradually increases,cell viability decreases.When the concentration of PdPC(OBu)8in PdPC(OBu)8-loaded liposomes(LP)reached 0.5 μg/mL,upon light irradiation,the cells only maintained ~30% cell viability,while the cells maintained ~80% cell viability without light irradiation.By contrast,light irradiation alone did not cause any change in viability for MCF-7 cells.The result showed that LP produced singlet oxygen after light irradiation,thereby enhancing cytotoxicity.This is consistent with the detection result of ROS levelsin vitro.
We further evaluated the anticancer activity of LPD using cell viability assay.LPD exhibited dose-dependent dark cytotoxicity against MCF-7 cells maybe because of the release of a small amount of photosensitizer and DOX in endolysosomes(Fig.3b).After irradiation(730 nm,1200 mW/cm2,3 min),LPD showed significant increased anti-cancer activity.When the concentration of DOX in LPD reached 1.85 μg/mL,it can be seen that the cells only maintained ~5% cell viability.It indicated that LPD could promote anticancer activity with irradiation.
To demonstrated thein vivoanti-tumor activity of LPD,the combination of chemotherapy and photodynamic therapy was evaluated,LPD were injected intravenously into the mice bearing MCF-7 tumor under 5.0 mg/kg of DOX and 1.0 mg/kg of PdPC(OBu)8,followed by the irradiation(730 nm,1200 mW/cm2,5 min)at the tumors at 24 h postinjection.Then,the tumor volumes were measured during subsequent 30 days and normalized against their original volumes(0 day)to evaluate the growth behaviors(Figs.3c-e).PBS as a control exhibited about the 26-fold increase of tumor volumes compared to their original volumes.Blank nanoliposomes(BP)and PdPC(OBu)8-loaded nanoliposomes(LP)exhibited the similar tumor growth behavior to those of PBS,suggesting that BP and LP lacked remarkable influence on tumor growth.Interestingly,LP exhibited 26-fold increase of tumor volume in the absence of irradiation,and exhibited 5-fold increase of tumor volume under irradiation,indicating that light promoted anti-cancer efficacy because of photodynamic therapy under irradiation.DOX-loaded nanoliposomes(LD)in the absence of irradiation exhibited the similar tumor growth behavior to those of LD under irradiation,suggesting that the light irradiation had a negligible influence on the tumor growth.LPD in the absence of irradiation exhibited the similar tumor growth behavior to those of LD,indicating that PdPC(OBu)8exhibited no anti-cancer efficacy without irradiation.LPD with irradiation was significantly more effective than LPD without irradiation on the tumor growth owing to synergistic anti-cancer efficacy of chemotherapy and photodynamic therapy.
We further demonstrate thein vivoanti-tumor activity of LPD,non-responsive nanoliposomes(HPD)and LPD were injected intravenously into the mice bearing MCF-7 tumor under 5.0 mg/kg of DOX and 1 mg/kg of PdPC(OBu)8,followed by the irradiation(730 nm,1.2 W/cm2,5 min)at the tumors at 24 h postinjection.Then,the relative tumor volumes were measured during subsequent 24 days(Figs.4a-d).PBS exhibited about the 15-fold increase of tumor volumes compared to their original volumes.LPD in the absence of irradiation exhibited about 8-fold increase of tumor volumes,whereas HPD in the absence of irradiation exhibited about 10-fold,indicating that LPD showed the enhanced chemotherapeutic efficacy owing to drug release triggered byin vivoROS.
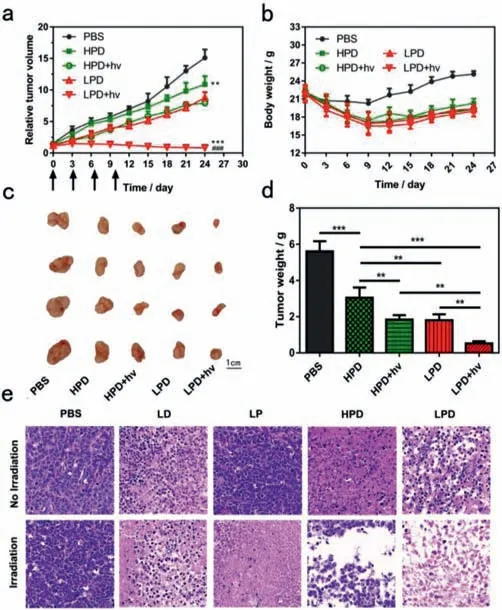
Fig.4.(a)Tumor growth inhibition of the mice bearing MCF-7 tumor.**P <0.01,***P <0.001,compared with the PBS group.###P <0.001,compared with the LPD group.(b)Weight changes of mice bearing MCF-7 tumor.(c)Photos of the tumors extracted from the mice bearing MCF-7 tumor.Scale bar = 1 cm.(d)The tumor weight of tumor-bearing mice administered with PBS,HPD and LPD.**P <0.01,***P <0.001.(e)Images of H&E-stained tumor sections.
Finally,the hematoxylin and eosin(H&E)staining of tumor sections validated that LPD resulted in more severe cancer necrosis and extensive hemorrhagic inflammation under irradiation as compared to non-irradiation group(Fig.4e),indicating that LPD showed significantly increased anticancer activity under light irradiation.In contrast,PBS showed no distinct tumor damage with or without irradiation.HPD only exhibited sporadic necrotic region,indicating that LPD were able to trigger more severe damages on tumors as compared to HPD,owning to efficient intracellular drug release.
In this study,we successfully fabricated NIR-responsive nanoliposomes that(1)was co-loaded with DOX and PdPC(OBu)8with high encapsulation efficiency;(2)maintained low leakage rate of DOXin vitroduring storage and in serum;(3)could rapidly release DOX upon NIR light.The light-responsive nanoliposomes mainly composed of DLPC and cholesterol.Increasing amount of DLPC enhanced NIR-triggered release of DOX,but reduced stability of nanoliposome.DLPC as an unsaturated phospholipid was easily oxidized.Increasing amount of cholesterol enhanced stability of nanoliposomes,but reduced NIR-triggered release of DOX.Both fast release of DOX and stability of nanoliposome were required.High amount of cholesterol and low amount of DLPC were used in this study.How DLPC and cholesterol effect NIR-triggered release of DOX and stability of nanoliposome is interesting and requires further mechanism study.
As a novel photosensitizer,PdPC(OBu)8owned high singlet oxygen yield which realized NIR-triggered release of DOX.The concentration of PdPC(OBu)8and light intensity effected singlet oxygen yield.Increasing concentration of PdPC(OBu)8enhanced release of DOX but reduced the loading of PdPC(OBu)8.From DHE results,we could find that high concentration of PdPC(OBu)8produced more singlet oxygen upon NIR irradiation.However,PdPC(OBu)8would produce excessive singlet oxygen with long time irradiation.We found that a few mice treated with LP or LPD were paralyzed after a few times NIR irradiation(730 nm,1200 mW/cm2,5 min),but mice treated with LD after irradiation did not exhibit the similar results.How PdPC(OBu)8leads to paralysis of rats is of interest and further elucidation of mechanistic aspects is required.
In summary,LPD was fabricated for NIR-responsive drug release.To ensure the fast response to light irradiation,18:2(cis)DLPC which contained biallylic hydrogens was used in this study.LPD could produce ROS under irradiation.Moreover,LPD could achieve drug release under irradiation.Meanwhile,LPD exhibited the collective characteristics including NIR-responsive drug release,remarkable photodynamic therapy,enhanced chemotherapeutic cell damage,and finally resulted in effective tumor control.In particular,LPD exhibitedin vivobifunctional effects of photodynamic therapy and chemotherapy under irradiation.Our NIRresponsive nanoliposomes represent a novel and versatile approach for cancer therapy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Nos.30801441 and 81773278).All the animal experiments were performed following the 3Rs principle with Guide for the Care and Use of Laboratory Animals published by Naval Medical University.The project was approved by the Animal Experimental Ethics Committee of Naval Medical University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.11.039.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
