L-Arginine based polyester amide/hyaluronic acid hybrid hydrogel with dual anti-inflammation and antioxidant functions for accelerated wound healing
Tong Liu,Guiting Liu,Jinhu Zhng,Zhngfn Ding,Yike Li,Krishn Sigdel,Xioyi Wng,*,Huixu Xie,*
a State Key Laboratory of Oral Diseases,National Clinical Research Center for Oral Diseases,Department of Head and Neck Oncology Surgery,West China Hospital of Stomatology,Sichuan University,Chengdu 610041,China
b The State Key Laboratory of Polymer Materials Engineering,Polymer Research Institute of Sichuan University,Chengdu 610065,China
c College of Polymer Science and Engineering,State Key Laboratory of Polymer Materials Engineering,Sichuan University,Chengdu 610065,China
1 These authors contributed equally to this work.
ABSTRACT Nowadays,there are still many challenges to skin regeneration.As a new type of skin substitute,hydrogel has emerging gradually with its excellent properties.However,it is still a challenge to combine with biological active agents to facilitate skin regeneration.Under the circumstance,we synthesized argininebased poly(ester amide)(Arg-PEA)and hyaluronic acid(HA-MA),and combined them into new hybrid hydrogels via photo-crosslinking.We found that the internal structure and physicochemical properties of hybrid hydrogels were greatly improved with the increase of content of Arg-PEA.Therefore,we designed hybrid hydrogels with 5 wt% and 10 wt% of Arg-PEA content,respectively.Besides,we selected the corresponding anti-inflammatory(CRP,TNF-α)indicators to detect the anti-inflammatory properties of the hybrid hydrogels at the protein level,and the corresponding antioxidant indicators(SOD,GSH/GSSG,MDA)were selected to investigate the antioxidant properties of hybrid hydrogels at the cellular level in vitro.In addition,we also selected relevant genes to test the effect of hybrid hydrogels on fibrosis and vascularization in the process of skin wound healing in vitro and verified them in vivo with a mouse dorsum wound model.The results confirmed that Arg-PEA/HA-MA(AH)hybrid hydrogel was a prospective scaffold material for skin regeneration.
Keywords:Hydrogel Skin wound healing Antioxidant Anti-inflammation
As the largest organ,skin plays an important role in preventing mechanical,chemical and pathological damage as well as dehydration and infection[1–5].Once the skin is damaged,it should be very urgent to achieve wound repair,otherwise it will lead to chronic wounds and even death.In the past few decades,autologous skin grafts have always been the most frequently utilized method to repair skin defects[6–8].However,there are still some significant clinical challenges and socioeconomic burdens in autologous skin grafts,such as the limitations of the autologous tissue sources,the difficulty of skin grafts,and the low success rate of operations[9,10].Recently,tissue engineering has received widespread attention as a new method of wound repair,as it could lead to a range of new and powerful artificial skin alternatives and overcome the limitations of skin transplantations to some extent.Scaffold plays a key role in tissue engineering,it should not only provide space and place to support tissue regeneration,but also possess the properties of biocompatibility and biodegradability[11–13].At present,hydrogel has become a prospective scaffold material.It has good biocompatibility and biodegradability,and could provide a three-dimensional polymer network to facilitate cell adhesion,proliferation,differentiation,migration and transport of nutrients,which is conducive to skin regeneration.Moreover,according to the physiological characteristics of wound tissue,hydrogels can be given corresponding biological activities to accelerate wound healing.
Generally,wound healing consists three stages:inflammatory and contractile responses,granulation tissue formation and tissue remodeling[14,15].Among them,appropriate inflammatory and contractile responses are crucial,which could play a role in hemostasis,protecting the body and preventing serious complications[15–17].However,continuous inflammatory response leads to the accumulation of a large number of reactive oxygen species(ROS).However,the antioxidant capacity of cells is limited,and a large number of ROS hinders the transition from the inflammatory stage to the proliferative stage of the wound[17–19].Many studies have shown that low levels of ROS could stimulate cell migration and angiogenesis,thus promoting wound healing,but high levels of ROS would hinder wound healing,especially in chronic wounds[20,21].Therefore,it is vital to promote effective antiinflammatory and anti-oxidation in the process of skin wound healing.L-Arginine,known as a protein amino acid and one of the most crucial nutrition supplies,is essential for wound healing[16,17].As numerous works demonstrated that L-arginine displays both anti-inflammatory and antioxidant activities,and can participate in metabolism and produce creatine,polyamines,agmatine and nitric oxide(NO),which are beneficial to tissue regeneration.However,in wound tissue,the supply of L-arginine is seriously insufficient and prolongs the inflammatory and contractile responses,hence sustained and targeted supply of L-arginine in wound site could be a potential and powerful strategy for wound repair.
In this work,we attempted to prepare a novel L-arginine based polyester amide/hyaluronic acid(AH)hybrid hydrogel with anti-inflammation and antioxidant dual-functions for accelerated wound healing.First,Arg-PEA was prepared by chemical bonding of dip-nitrophenyl ester of dicarboxylic acids(NF)and di-p-toluene sulfonic acid salts of bis-L-arginine(or bis-DL-2-allylglycine)esters(Arg-2-S),then AH hybrid hydrogel was fabricated by chemical reaction and electrostatic interaction between Arg-PEA and methacrylic anhydride modified hyaluronic acid(HA-MA)under UV irradiation.By this design,AH hydrogel could be used for in-situ gelation,suitable for irregular wounds,and the physical property of hydrogel could be adjusted by the addition of Arg-PEA,furthermore,L-arginine could be released sustainedly and targeted by the hydrolysis reaction of Arg-PEA in wound site to achieve antiinflammatory and antioxidant dual-effects.In the present study,we aimed to characterize the composition,physicochemical properties,biological properties,anti-inflammatory and antioxidant activityin vitro,meanwhile,we verified the effect of AH hybrid hydrogels in a mouse dorsal injury modelin vivo.
This experiment was approved by the Ethics Committee of West China Hospital of Stomatology,Sichuan University(WCHSIRBD-2017–263).In this experiment,we observed the internal microstructures of hydrogels by SEM.For the physical and chemical properties of hydrogels,we tested compressive mechanical property,swelling kinetics and degradation of hydrogels,respectively.The extracts of hydrogel samples were prepared by soaking the hydrogels in DMEM or MEM at a mass-to-volume ratio of 100 mg/mL respectively[15].Cell viability was quantitatively evaluated using CCK-8 colorimetric assay.To observe the morphology and distribution of cells under the influence of extracts of the biomaterials,the processed samples were placed under the CLSM.The protein expression of inflammatory factors(CRP,TNF-α)was measured by ELISA to detect the anti-inflammatory effect of hydrogels.To explore the anti-oxidant of HUVECs under the influence of extracts of hybrid hydrogels,we stimulated the cellular microenvironment by hydrogen peroxide(H2O2).To explore fibrosis and vascularization in skin regeneration,we tested Krt 10,Krt 14,Col 1α,Col 3α,EGF,TGF-βand VEGF by qRT-PCR,respectively.Sevenweek-old female Kunming(KM)mice weighing about 25 g were used for the experimentsin vivo.At the specific time points,the wound area and surrounding normal tissues were removed and fixed in 4% paraformaldehyde,and then these tissues were sectioned and carried out by histological and immunohistochemical staining.HE and Masson’s trichrome staining were used to assess morphology of the wound areas and collagen formation.Immunohistochemical analysis was performed with antibodies against Krt 10,Krt 14,Col 1α,Col 3α,CD 31 and CD 34 to evaluate fibrosis and vascularization of the wound areas.After all the treated sections were observed,representative locations were selected for further evaluation.Related experimental procedures are provided in Supporting information.
The hydrogel samples were shown in Fig.1A.We found that the color of hybrid hydrogels gradually turned dark yellow with the increase of the content of Arg-PEA.The FTIR spectra of hydrogels were shown in Fig.1B.We observed that the stretching positions of amide I and amide II corresponding to C=O in Arg-PEA were 1640 cm-1and 1560 cm-1,respectively,and the stretching position of C–OH in HA-MA was 1000 cm-1.The presented FTIR indicated the successful formation of hybrid hydrogels.The microstructure of the hydrogels was shown in Fig.1C.The pore size of the HA-MA hydrogel was about 100 μm,while the pore sizes of the hybrid hydrogel became denser and decreased to about 50 μm with the increase of the content of Arg-PEA.The hybrid hydrogels have good three-dimensional porous structures,which are beneficial to cell attachment and transport of nutrients and metabolites.Under the circumstance,AH hybrid hydrogel provides a feasible choice for obtaining adequate mechanical properties of skin regeneration.
The physical and chemical properties of hydrogels were shown in Fig.S1(Supporting information).We found that the compression modulus of HA-MA hydrogel was about 0.9 MPa.With the increase of content of Arg-PEA,the compression modulus and brittleness of hybrid hydrogels decreased.In the swelling kinetics experiment,the weight of the hydrogel samples increased rapidly in the initial 2 h,and the swelling rate tended to be stable with the passage of time.With the increase of Arg-PEA content,the equilibrium swelling ratio of hydrogels decreased from 10 to 3.In the hybrid hydrogel system,the electrostatic attraction between HAMA and Arg-PEA hinders the expansion of the network of hydrogels,which explains the low expansion rate of the hybrid hydrogel.From the perspective of molecular structure,Arg-PEA has a lot of ester bonds and amide bonds,which also means that Arg-PEA is hydrophilic and easy to hydrolysis.As a consequence,with the increase of the content of Arg-PEA,the degradation rate of the hydrogels increased gradually.At the later stage of the degradation kinetics experiment,the remaining hydrogel scaffolds were relatively stable,which also provided a basis for skin regenerationin vivo.
In live/dead assay,the CCK-8 showed that the hydrogel samples had an effect on the proliferation ability of HUVECs(Fig.S2A in Supporting information).Induced or overexpressed arginase promotes arginine metabolism,resulting in the production of polyamines,which contribute to endothelial cell proliferation to some extent.In addition,the groups of hydrogels showed no significant cytotoxicity to HUVECs.At the same time,through CLSM(Fig.S2B in Supporting information),cells in each group showed their proper color,morphology and distribution.It is proved that AH hybrid hydrogels have a good biocompatibility with HUVECs,and the result demonstrated the bio-safety of hydrogels for skin regenerationin vitro.
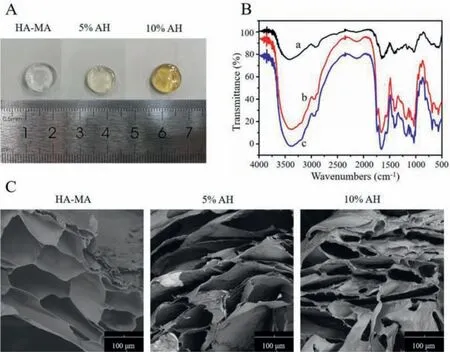
Fig.1.Characterization of the formation of obtained hybrid hydrogels:(A)An image of AH hydrogels with Arg-PEA contents of 0%,5%,10% from left to right.(B)FTIR spectra of precursors and hybrid hydrogels(a,HA-MA;b,5% AH;c,10% AH).(C)SEM images of freeze-dried pure HA hydrogel and AH hydrogels.Scale bar:100 μm.

Fig.2.Anti-oxidant,anti-inflammation,fibrosis and vascularization properties of hybrid hydrogels.(A)Image of ROS immunofluorescence in HUVECs photographed by fluorescence microscope.(B-D)Absorbance values of GSH/GSSG,SOD and MDA under the influence of hybrid hydrogels.(E)Concentration of CRP and TNF-α.(F-H)Krt 10,Krt 14,Col 1α,Col 3α,EGF,TGF-β and VEGF mRNA expression in skin regeneration treated with hydrogels.The quantitative analysis of gene transcript level,relative to β-Actin,was determined by qRT-PCR.Error bars indicate mean ± SD,*P <0.05,**P <0.01,***P <0.001.
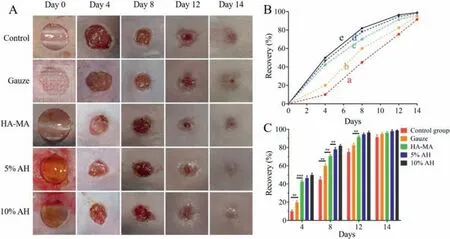
Fig.3.Healing trend chart of dorsal wound of mouse model.(A)Image of dorsal wound of mouse model.(B and C)During wound healing,every skin wound area was quantitatively measured at a specific time.(B)Represents the healing tendency.Data points represent mean recovery(%)(a,Control group;b,Gauze;c,HA-MA;d,5% AH;e,10% AH).(C)Represents the statistical significance between two groups,error bars indicate mean ± SD,**P <0.01,***P <0.001.
For anti-oxidant assay,the stained cells were observed under a fluorescence microscope and were photographed(Fig.2A).At the same time,we quantitatively detected the content of free radical(GSH/GSSG,SOD and MDA)(Figs.2B-D).SOD is one of major components of ROS.And it is the intermediate product of many biological processes and has certain cytotoxicity.Through co-cultured with hydrogels,with the increase of Arg-PEA content,stronger radical scavenging activities of superoxide were observed in HUVECs.Results showed that the generation of SOD was effectively inhibited to varying degrees by AH hybrid hydrogels.In addition,this study found that the accumulation of MDA was significantly negatively correlated with the content of Arg-PEA.These results indicated that with the increase of Arg-PEA content,the excess ROS in microenvironment could be effectively removed.In this study,electron transfer(ET)mechanism could explain the antioxidant effect of Arg-PEA[22–24].Arg-PEA could share one of its electrons to a free radical and react with it,thus terminating the chain reaction of free radicals.On this basis,high levels of Arg-PEA could be exposed to more proton/electron donors,and thus exhibiting stronger reductivity and antioxidant capacity[25,26].In addition,the electron cloud density of the functional group also has a significant effect on the activity of electron donor.High electron cloud density increases the activity of electron donor,thus increasing the ability to scavenging free radicals[27,28].Therefore,Arg-PEA promotes the availability of potential electron donors,thereby removing excess ROS in the microenvironment and ameliorating oxidative stress.Besides,an increased level of GSH was positively associated with the content of Arg-PEA.Among them,glutamate,which is contained in GSH,plays an important role in antioxidant activity.L-Arginine is a substrate for glutamate synthesis,and its supplementation may indirectly stimulate endogenous GSH synthesis.Therefore,Arg-PEA supplementation in this study could promote GSH synthesis and exert antioxidant properties.
ELISA assay was utilized to test the effect of hydrogel samples on the expression of inflammatory factors CRP and TNF-α(Fig.2E).The expression of CRP and TNF-αdecreased in the hydrogel groups compared with control group,and the higher the Arg-PEA content,the lower the factor expression(CRP:199.7,183.4,173.4,160.1 pg/mL;TNF-α:68.49,61.95,56.07,47.65 pg/mL).Therefore,it concluded that the three hydrogels could reduce the immune stress state of the body during wound healing,and it could provide a better basis for skin wound healing.
For the fibrosis and vascularization property of hydrogelsin vitro,we detected the expression of genes associated with skin wound healing(Krt 10,Krt 14,Col 1α,Col 3α,EGF,TGF-β,VEGF)by qRT-PCR(Figs.2F-H).The expressions of these seven genes were significantly up-regulated under the stimulation of different hydrogels(P <0.05).These results provided a basis for furtherin vivostudies.Keratin is the main skeleton protein in keratinocytes.During keratinocyte differentiation,the keratin expression profile changes due to the change of cell morphology and function.Therefore,the characterization of key keratins is the key to judge whether hydrogels could promote wound healing.As for vascular growth factors,thein vitroresults showed that the mRNA expression levels of EGF,TGF-βand VEGF were increased in different degrees in the experimental group,indicating that the hydrogel materials could promote cell proliferation during wound healing.It showed that hydrogel materials promote the synthesis of extracellular matrix,deposition of collagen and formation of granulation tissue during skin wound healing by regulating vascular growth factors,thus promoting skin wound healing.
The dorsum wound healing of each group was shown in Fig.3A.During this experiment,mice in each group were in good physical condition.Slight redness and swelling were observed in the process of the wound healing,and no obvious signs of infection was observed.Accordingly,we performed a quantitative analysis of the percentage of defect recovery based on the collected images of skin wounds throughout the experiment(Figs.3B and C).In the experimental groups,we found that the wound diameters decreased significantly from day 0 to day 4,the reduction rates of the wound diameters slowed down from day 4 to day 8,and the wounds were gradually closed until day 14.There was no significant difference between the three experimental groups.In the empty group and gauze group,the diameters of the defects also decreased over time,but the diameters of the wounds decreased significantly from day 4 to day 8.Up to day 14,there were scabs in the middle of the defect areas indicating incomplete closure.
By HE staining(Fig.S3A in Supporting information),the epidermal continuity of the wound area in the empty control group was interrupted in the early stage.While in the experimental group,there were more temporary matrix accumulated on the wound bed than the empty control group.As time went by,scab had formed over the wound of empty control group.Between the scab and the temporary matrix,it could be observed that the epidermal tongue gradually moved from the margin to the center of the wound.And the temporary matrix gradually covered the wound and the blue-stained collagen increased significantly.In the experimental groups,re-epithelialization of the wound bed was completed at day 8 post-surgery with a significant increase in the density of the new tissue due to collagen deposition of fibroblasts.At day 12 post-surgery,the process of re-epithelialization was still uncompleted in the empty group,while there was a more mature,layered new epidermis that was thicker than normal epidermal tissue around the wound in the experiment group.In addition,the density of collagen deposition during this time period was more uniform than before.Hybrid hydrogels facilitated the formation and maturation of new tissue in the wound.As mentioned above,the early granulation tissue formed by fibronectin and collagen,which was subsequently remodeled to the formation of the new dermis.Masson’s trichrome staining showed that in the empty control group,the collagen content in the wound was low despite an increased overall density of new tissue in the wound.While in the experimental group,collagen density significantly accumulated during wound recovery(Fig.S3B in Supporting information).
By immunohistochemical staining(Figs.S3C and D in Supporting information),at day 4 post-surgery,in the experimental groups,the expression of Krt 10 was widely performed in new epidermal tissue around the edge of the wound.For Krt 14,it was expressed in strongly stained basal cells in the experimental group and was widely dispersed from the apical to the basal layer in the dermal tongue that migrated from the edge of the wound to the center.Staining of the experimental group at 8–12 post-surgery showed that Krt 10 was widely distributed in the new epidermis tissue and gradually migrated to the differentiated spinous layer,and the expression of Krt 14 gradually tended to be evenly dispersed in the basal layer instead of the whole layer.It indicated that keratinocytes changed from the previously dedifferentiated state to the normal differentiated state,thus further remodeling the new epidermis and promoting skin healing.In this study,the hybrid hydrogels provided a temporary matrix by direct contact with the wound and activated keratinocytes,thereby accelerating re-epithelialization.It is not difficult to find that the expressions of Krt 10,Krt 14 by hydrogelsin vivois consistent with thosein vitro.
For vascular factors(Figs.S3E and F in Supporting information),we found that the immunofluorescence staining of CD 31 positive cells was widely distributed in the wound surfaces of each group.Moreover,the distribution of staining of CD 31 positive cells gradually spread and thickened from time goes on,indicating that CD 31 actively promoted wound healing.However,immunofluorescence staining of CD 34 positive cells was more frequent on the day 4 post-operation.As time went on,staining of CD 34 positive cells between day 8 and day 12 post-operation showed a downregulated trend,as CD 34 molecules would gradually weaken to disappear with the maturation of the cells,which was also consistent with its own function.In vivo,we focused on plateletendothelial adhesion factors(CD 31,CD 34).It could be found that although CD 31 and CD 34 play a role in wound healing process,there is no significant difference between the control group and the experimental groups.It could be seen that hybrid hydrogel materials have almost no regulated effect on CD 31 and CD 34 in the process of promoting wound healing.
In conclusion,we successfully synthesized Arg-PEA and HAMA,and prepared AH hybrid hydrogels with different feed ratios by photo-crosslinking to explore their roles in the process of skin wound healing.The results showed that the internal morphology,swelling,mechanical and biodegradation properties of the hybrid hydrogel system could be well adjusted by changing the content of Arg-PEA.More importantly,through detecting corresponding signal molecules,hybrid hydrogels were found to have antioxidant,antiinflammatory,fibrosis and vascularization effects in the process of skin wound healing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.52103039)and Sichuan University postdoctoral interdisciplinary Innovation Fund.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.022.
 Chinese Chemical Letters2022年4期
Chinese Chemical Letters2022年4期
- Chinese Chemical Letters的其它文章
- Key progresses of MOE key laboratory of macromolecular synthesis and functionalization in 2020
- Small nanoparticles bring big prospect:The synthesis,modification,photoluminescence and sensing applications of carbon dots
- Cell membrane-coated nanoparticles for immunotherapy
- Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics
- Exosome based miRNA delivery strategy for disease treatment
- Recent advances in targeted stimuli-responsive nano-based drug delivery systems combating atherosclerosis
